Abstract
The exploration and production of shale gas technology provides a way for utilization of clean fuels. However, during the exploration process of shale gas, enormous amount of drilling cutting was generated and had to be solidified and landfilled. So the accumulation of shale gas drilling cutting solidified body (SGDS)causes severe land resource misuse and environmental complications. This study focuses on the utilization of SGDS as a raw material for the production of cement clinker, and the phase composition, microstructure, and environmental performance of the cement clinker was investigated by X-ray powder diffraction (XRD), scanning electronic microscopy (SEM), energy-dispersive X-ray spectrum analysis (EDX), and soaking test, respectively. The results show that the cement clinker obtained mainly constitutes of typical Portland cement mineral (C3S, C2S, C3A, and C4AF). The leaching test indicated that the concentration of heavy metal ions in leachate is within the limits allowed by the state “Technical specification for co-processing of solid wastes in cement kiln” (GB 30760-2014). This study therefore provides a benchmark on environmental effects resulting from drilling cuttings and utilization of resources.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the rapid development of China’s economy, demand for energy is constantly on the rise. This has made shale gas leading as the new energy source and predominantly the main motivation in China’s development economically. The straight hole and horizontal well technology for shale gas exploration is commonly used, and it is inevitable that enormous amount of drilling cuttings mixed with drilling fluid generated. Hardening agents, such as Portland cement and fly ash are added to act as a binder for purposes of stabilizing and solidifying it to reduce the contamination caused by the drilling cuttings within a shorter period (Leonard and Stegemann 2010; Kogbara et al. 2016; Kogbara 2014; Chao-qiang et al. 2017). The Shale gas drilling cuttings solidified body (SGDS) was generated and had to been landfilled. Since the SGDS are situated underground, the solid blocks undergo a serious of physical-chemical reaction, resulting in secondary-contamination, especially in heavy metal release and organic pollution (Antemir et al. 2010; Leonard and Stegemann 2010). And thus the need for safe disposal and recycling of SGDS is in urgent.
Previous studies of the physicochemical properties of drilling cuttings have indicated that SGDS can be used in place of sand and mineral addition for the production of building materials, such as the preparation of non-fired brick (Acchar and Marques 2016; Liu et al. 2018; Dmitriy V. Oreshkin et al. 2015), concrete block (Ablieieva and Leonid 2016; Mahmoud Kassem et al. 2018), road material (Tuncan et al. 2000; Chang-Seon Shon et al. 2016), concrete (N.M. Wasiuddin et al. 2002; Ehsan et al. 2015), cement (Wang et al. 2017; Bernardo et al. 2007; Al-Otoom 2006), sintered brick (Xiang-Guo Li et al. 2011), and other materials, such as lightweight ceramsite and cementitious material (Bamdad Ayati et al. 2019; M. Aboutabikh et al. 2016). Although, the research on resource utilization of drilling cuttings has a lot of aspects, but few of them could be put into practice. So, based on the results of above research, and according to the actual situations, the aim of this study is to evaluate the potential use of SGDS as a raw material for the production of Portland cement clinker on an industry scale, so as to make a resource utilization of SGDS and eliminate the potentially secondary-contamination caused by the landfill of SGDS. This study provides a way for the security and environmental disposal of SGDS.
Materials and methods
Raw materials
SGDS was obtained from a drilling plant in Chongqing. Slag and fly-ash were procured from a local coal-fired power plant. Cement raw meal used was obtained from a cement plant in Chongqing. The raw materials’ lixivium heavy metal pollutants were presented in Table 1.
Field experimentation and testing methods
Field experimentation
Figure 1 shows the process flow of the production of the Portland cement clinker using SGDS.
In reference to the preliminary experiments’ results, for preparing the cement raw meal, 83.6% limestone, 10.1% sandstone, 2.5% SGDS, 2.8% coal ash, and 1.0% sulfuric acid residue were used (in mass). All the raw materials were crushed, mixed, and ground to the required fineness, typically 20% of retained on an 80 micro sieve. The homogenized raw meal is introduced into the top of the cyclone preheater and it was heated and decarbonated. After decomposition of the raw meal, it was fed into a rotary kiln in which the raw meal is heated to 1500 °C. After firing, the clinker exits the kiln and is cooled from 1200 °C to 60 °C in a cooler. And then the clinker is ground together with slag, cinder, and desulfurization gypsum in a mill to produce Portland cement.
Test methods
The clear crystalline minerals of the clinker were identified by an X-rd (X-ray powder diffraction), conducted in a Panalytical (X’Pert PRO diffractometer) with a speed current of 60 mA and the voltage of 35 kV. The acquired spectrum was scrutinized in X’Pert High Score and software Plus MDI Jade 5.0.
The use of scanning electronic microscopy (SEM) and energy-dispersive X-ray spectrum analysis (EDX), the morphologies and elements of clinker were analyzed by scanning photo-electron microscope (ASTEREO SCAN440, Leica Cambridge Ltd).
According to the requirement standards for the production of lixivium which detects its admissible indexes and refers to Chinese national standard “Technical specification for co-processing of solid wastes in cement kiln” (GB 30760-2014) to determine the environmental performance of the product. The heavy metal content in leachates were analyzed by ICP-MS methods,
Results and discussion
Heavy metal analysis
According to the requirements of national quality, “Environmental protection technical specification for co-processing of solid wastes in cement kiln” (HJ 662-2013), the limiting value of heavy metal kiln feeding has specific requirements. Therefore, according to the article, the heavy metal content contained in solidified body cement clinkers is presented in Table 2. It is indicated that the heavy metal content contained in the clinker produced from SGDS is within the limits set by national standard.
XRF analysis
It is clear from the Table 3 that the chemical composition of the SGDS is similar with the composition of commercially available cement. However, the Cl, SO3, and K2O contained in SGDS were lower than commercially available cement, making the SGDS an idea raw material for the production of the Portland cement clinker.
Microstructure analysis
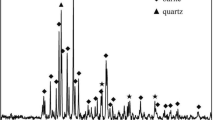
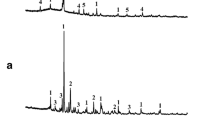
As shown in Fig. 2, that the mineral phases of the cement clinker produced from SGDS mainly consist of C3S, C2S, C3A, and C4AF, and the diffraction peak intensity and position were brought into correspondence with typical commercially available cement. In addition, Fig. 2 indicated that the major crystalline state of the cement clinker produced from SGDS is arranged in a lamellar fashion C3S (spot B and spot C) and near-spherical particles C2S (spot A) around the mineral phases. This may owe the addition of SGDS which can promote the formation of liquid phase in the raw meal. The early appearance of liquid phase reduces the burning temperature of cement clinker prepared with SGDS. The burnability was improved of the raw meal which contains SGDS, and is help for the synthesis of Belite. In addition, from Table 3, we can conclude that the calcium to silicon ratio of C2S and C3S unblended raw meal are 1.731 and 1.829, respectively; the cement clinkers produced from SGDS are 1.863 and 1.817, respectively, which is in accordance with the proposed theoretical analysis. Another cause could be the presence of alkaline liquid phase and valence bond strong AlO45− and FeO45− tetrahedron which are not conducive to the formation and growth of C3S crystals, so that the C3S mineral of clinker is close to C2S.
Environmental safety analysis
The results of leaching property of the cement clinker produced from SGDS are presented in the Table 4.
The dates in Table 4 clearly indicate all indexes within the required limit for the China standard “Technical specification for co-processing of solid wastes in cement kiln” (GB 30760-2014) (Liu et al. 2018; Shu et al. 2016). In conclusion, drilling cuttings solidified body-based cement clinker cannot pollute the environment.
Conclusions
This paper presents a case study of resource utilization of the SGDS in industrial scale. And it is indicated that the SGDS could be used as a raw material for the production of Portland cement clinker in a cement production line with a daily output of 10,000 tons using the ingredient ratio: 83.6% limestone, 10.1% sandstone, 2.5% solidified body, 2.8% coal ash, and 1.0% sulfuric-acid residue, resulting a daily consumption of about 250 tons of SGDS. The cement clinker obtained mainly constitutes of typical Portland cement minerals (C3S, C2S, C3A, and C4AF) and the leaching tests showed that the concentration of heavy metal ions in leachate of the cement clinker is within the limits allowed by the state national standard. Our strategy has provided a convenient reference for the controlling of the pollution induced by the SGDS and the resource utilization of it in a practical way.
References
Ablieieva I, Leonid P (2016) The immobilization of heavy metals during drilling sludge utilization. Environ Technol Innov 6:123–131
Acchar W, Marques SKJ (2016) Using oil drilling waste and sugarcane bags ash in soil-cement formulations. In: Ecological Soil-Cement Bricks from Waste Materials. Springer Briefs in Applied Sciences and Technology. Springer, Cham
Antemir A, Hills CD, Carey PJ, Magnie MC, Polettini A (2010) Investigation of 4-year-old stabilised/solidied and accelerated carbonated contaminated soil. J Hazard Mater 181:543–555
Al-Otoom AY (2006) Utilization of oil shale in the production of Portland clinker. Cem Concr Compos 28:3–11
Ayati B, Molineux C, Newport D, Cheeseman C (2019) Manufacture and performance of lightweight aggregate from waste drill cuttings. J Clean Prod 208:252–260
Bernardo G, Marroccoli M, Nobili M, Telesca A, Valenti GL (2007) The use of oil well-derived drilling waste and electric arc furnace slag as alternative raw materials in clinker production. Resour Conserv Recycl 52:95–102
Shon C-S, Estakhri CK, Lee D, Zhang D (2016) Evaluating feasibility of modified drilling waste materials in flexible base course construction. Constr Build Mater 116:79–86
Oreshkin DV, Chebotaev AN, Perfilov VA (2015) Disposal of drilling sludge in the production of building materials. Procedia Eng 111:607–611
Ehsan M, Asadi S, Ugochukwu E (2015) Feasibility study of the potential use of drill cuttings in concrete. Procedia Engineering 1181:1015–1023
Kogbara RB (2014) A review of the mechanical and leaching performance of sta-bilized/solidied contaminated soils. Environ Rev 22:66–86
Kogbara RB, Ayotamuno JM, Ikechukwu O, Ehio V, Damka TGD (2016) Stabilisation/solidi cation and bioaugmentation treatment of petroleum drill cuttings. Appl Geochem 71:1–8
Leonard SA, Stegemann JA (2010) Stabilization/solidification of petroleum drill cuttings: Leaching studies. J Hazard Mater 174:484–491
Liu D-s, Wang C-q, Mei X-d, Zhang C (2018) Environmental performance, mechanical and microstructure analysis of non-fired bricks containing water-based drilling cuttings of shale gas. Constr Build Mater 183:215–225
Aboutabikh M, Soliman AM, El Naggar MH (2016) Properties of cementitious material incorporating treated oil sands drill cuttings waste. Constr Build Mater 111:751–757
Kassem M, Soliman A, Naggar HEI (2018) Sustainable approach for recycling treated oil sand waste in concrete: engineering properties and potential applications. J Clean Prod 204:50–59
N.M. Wasiuddin, N. Ali, M.R. Islam (2002) Use of offshore drilling waste in hot mix asphalt (HMA) concrete as aggregate replacement, in: Proceedings of ETCE 2002 on ASME Engineering Technology Conference on Energy, Houston, TX,USA .
Shu J, Liu R, Liu Z, Chen H, Du J, Tao C (2016) Solidification/stabilization of electrolytic manganese residue using phosphate resource and low-grade MgO/CaO. J Hazard Mater 317:267–274
Tuncan A, Tuncan M, Koyuncu H (2000) Use of petroleum contaminated drilling wastes as sub-base material for road construction. Waste Manag Res 18:489–505
Wang C-q, Lin X-y, Jin J-z, Xiong D-m, Mei X-d (2017) A study on the oil-based drilling cuttings pyrolysis residues resource utilization by the exploration and development of shale gas. Environ Sci Pollut R (21):17816–17828
Chao-qiang W, Xiao-yan L, Chun Z, Xu-dong M (2017) Heavy metals environmental security control of resource utilization of drilling cuttings of shale gas. Environ Sci Pollut Res 27:21973–21983
Li X-G, Lv Y, Ma B-G, Jian S-W, Tan H-B (2011) Influence of sintering temperature on the characteristics of shale brick containing oil well-derived drilling waste. Environ Sci Pollut Res 18(9):1617–1622
Acknowledgments
We especially wish to thank Deng-tao Yu, whose suggestions have given us depth to improve the quality of the paper.
Funding
This work was financially supported by social program of Chongqing Science and Technology Committee in (cstc2017shmsA90012) and Technology program of Chongqing education committee (KJQN201801438).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, Ds., Wang, Cq., Mei, Xd. et al. An effective treatment method for shale gas drilling cuttings solidified body. Environ Sci Pollut Res 26, 17853–17857 (2019). https://doi.org/10.1007/s11356-019-05273-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-05273-0