Abstract
Background, aim, and scope
The focus of the present study is to know the potential of bacterial isolate for tannic acid degradation at low temperature. Also, we tried to evaluate the suitability of phytotoxicity testing protocol for the determination of tannic acid toxicity.
Methods
Screening for tannic acid degrading bacterial strains was carried out by using microbial isolation techniques. The 16S rDNA amplicon of the isolate was used to identify the isolate. The effect of different concentrations of tannic acid and its degradation products on germination of Vigna unguiculata was evaluated. The study was carried out to determine total sugar and starch content of the used seeds and even to check the presence of α-amylase activity during seed germination.
Results
The isolated bacterium was identified as Klebsiella sp NACASA1 and it showed degradation of tannic acid in 40 (±0.85***) h at 15°C and pH 7.0. A gradual decrease in root/shoot length was observed with increasing concentration of tannic acid. There was 95.11 (±0.24**)% inhibition in α-amylase activity at 20,000 ppm tannic acid, as compared to control. No such effects were observed on germination, root–shoot length, and α-amylase activity with tannic acid degradation products.
Conclusions
The results obtained confirmed that tannic acid may act as a toxic agent in plant cells. The simple biodegradation process presented in this study was found to be effective in reducing toxicity of tannic acid. Also, it reveals the potential of soil bacterium to degrade tannic acid at low temperature.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
As a result of the high catalytic efficiency of their enzymes and their unique specificity at low and moderate temperatures, cold-adapted microorganisms should be ideal for bioremediation purposes (Margesin and Schinner 1999). However, little is known about these microorganisms, and the optimum conditions for their use need to be carefully evaluated. The treatment of waste waters contaminated as a result of human activities would probably be the easiest way to start studying the potential applications of cold-adapted microorganisms in lowering the amount of toxic compounds, viz. nitrates, hydrocarbons, aromatic compounds, heavy metals, and biopolymers such as cellulose. These efforts have already begun (Margesin and Schinner 1998; Timmis and Pieper 1999).
Aromatic compounds such as polyphenols comprise the second largest group of natural products, in addition to a variety of xenobiotics that are manmade aromatic pollutants (Fuchs et al. 1994). Tannins are defined as naturally occurring water-soluble polyphenols of varying molecular weight (Bhat et al. 1998). Tannins are found to be occurring in vascular plant tissues of leaves, seeds, and flowers (Mingshu et al. 2006). Tannins are considered nutritionally undesirable because they inhibit digestive enzymes and affect the utilization of vitamins and minerals. Ingestion of large quantities of tannins may result in adverse health effects. However, the intake of a small quantity of the right kind of tannins may be beneficial to human health (Gu et al. 2003). They are divided into two classes: hydrolyzable and condensed (not hydrolyzable) (Haslam 1966). Hydrolyzable tannins are toxic to animals and cause poisoning if consumed by them in large amounts (Garg et al. 1992). Tannins inhibit the growth of a number of microorganisms, resist microbial attack, and are recalcitrant to biodegradation (Field and Lettinga 1992).
Despite the antimicrobial properties of tannins, many fungi, bacteria, and yeasts are quite resistant to tannins and can grow and develop on them (Bhat et al. 1998). The importance of tannin biodegradation in accordance to industrial and agricultural applications has been published earlier (Archambault et al. 1996; Lekha and Lonsane 1997). By considering these facts, an effort has been made to isolate and identify cold-adapted tannic acid degrading bacteria by optimizing various parameters of degradation. The identification of bacterial isolate was carried out. Various parameters for degradation were optimized.
Different kinds of phenolic compounds are known to inhibit seed germination due to their general phytotoxicity. Because of the widespread occurrence and distribution of phenolic compounds in plants and fruits, it has been suggested that these substances might act as natural germination inhibitors (Colpas et al. 2003). In general, phenolics have the property of altering mitochondria and chloroplasts membranes, hindering the energy transfer necessary to ion transport, as observed in spinach (Moreland and Novitzky 1987). In the present study, an attempt was made to evaluate the phytotoxic effect of tannic acid and its degradation products on germination of Vigna unguiculata seeds.
2 Materials and methods
2.1 Screening, isolation of microorganism, and culture conditions
The garden soil collected from the botanical garden of N.A.C. & S. College, Ahmednagar, India was used for isolation of tannic acid degrading bacterial strains. One gram of soil was added to 100 mL Bushnell and Hans’s medium containing tannic acid (0.2%) and incubated at 15°C under shaking (120 rpm) as well as static condition. The culture showing tannic acid degrading activity at static condition was acclimatized by transferring 5-mL aliquots from the flask to the fresh tannic acid-containing medium with various concentrations of tannic acid increased from 0.2% to 1%. The tannic acid-containing agar plates were inoculated with 0.1 mL suspension from these flasks. The isolated colonies were transferred to the tannic acid-containing Bushnell and Hans’s medium and selected on the basis of high tannic acid degrading activity. Out of these isolates, one showing high tannic acid degrading activity under static condition was identified and used for further study. The pure culture was maintained on tannic acid-containing agar slants at 4°C.
2.2 16S rDNA sequencing
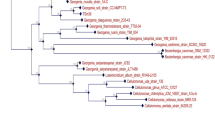
16S rDNA sequencing of isolated bacteria was carried out at “Molecular Diagnostic Center”, Pune, India. The nucleotide sequence analysis of the sequence was done at Blast-n site at NCBI server (http://www.ncbi.nlm.nih.gov/BLAST). The sequence was refined manually after crosschecking with the raw data to remove ambiguities and submitted to the NCBI, with accession number HM179099. The evolutionary history was inferred using the Neighbor-Joining method (Saitou and Nei 1987). The optimal tree with the sum of branch length = 0.91170207 is shown (Fig. 1). The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (500 replicates) is shown next to the branches (Felsenstein 1985). The evolutionary distances are computed using the Maximum Composite Likelihood method (Tamura et al. 2004) and are in the units of the number of base substitutions per site. All positions containing gaps and missing data were eliminated from the dataset (Complete deletion option). There were a total of 304 positions in the final dataset. Phylogenetic analyses were conducted in MEGA4 (Tamura et al. 2007).
2.3 Effect of incubation temperature and pH on degradation of tannic acid
Temperature and pH for maximum tannic acid degradation was standardized. Variable temperature (0 to 45°C) and pH range (1 to 10) were employed for degradation of tannic acid. The initial pH of Bushnell and Hans’s medium was adjusted using concentrated HCl/NaOH. To study the effect of temperature, the flasks were incubated at various temperatures.
2.4 Optimization of carbon sources for degradation of tannic acid
Different carbon sources (glucose, starch, lactose, maltose, and sucrose) were used at a concentration of 1.0% (w/v). These were used along with tannic acid in standard growth medium. The study was carried out at 15°C temperature.
2.5 Optimization of nitrogen sources for degradation of tannic acid
Different nitrogen sources (ammonium sulfate, peptone, ammonium persulfate, beef extract, malt extract, and tryptone) were used at a concentration of 1.0% (w/v). The study was carried out at 15°C temperature.
2.6 Total phenol estimation
Total phenol estimation of the control and treated samples was carried by the method of Bhakta and Ganjewal (2009). An amount of 0.1 mL of each of the test sample was taken and volume was made up to 7 mL in each test tube using distilled water, to which 0.5 mL of Folin–Ciocalteau was added in all the tubes and shaken for 3 min. One milliliter of 35% sodium carbonate was added and the tubes were allowed to stand for 1 h. The deep blue coloration developed was read for absorbance at 630 nm. The concentration of phenolics in the test solution was calculated by using standard tannic acid (1 mg mL−1) curve and expressed as milligrams of tannic acid equivalents (TAE) per milliliter of the sample. Phenol concentration of the control sample was taken as 100% and % phenol removal was calculated from the phenol remaining in the treated sample after each treatment.
2.7 Plant material
Healthy, dry, and mature seeds of V. unguiculata were procured from Mahatma Phule Agriculture University (MPKV, Rahuri), Ahmednagar, Maharashtra, India.
2.8 Phytotoxicity study
The experiment was set up to study the effect of different concentrations of tannic acid on germination of V. unguiculata. The seeds were germinated in sterile 10-cm Petri dishes, layered with germination paper. Seeds were sterilized according to Somasegaram and Hoben (1985) before transferring to the surface of the paper in the Petri dishes (10 seeds per plate). The phytotoxicity bioassay was evaluated using the seed germination technique (Zucconi et al. 1981a, b). This method involves incubating the tannic acid at various concentrations with seeds at 25°C for 5 days in the dark, and then measuring the number of seeds germinated and root growth thereafter (Eqs. 1 and 2, respectively). After 5 days of incubation in the dark, the number of seed germination (%) and root length of V. unguiculata in the tannic acid solution were determined. The seed germination percentage and root elongation of the plants in distilled water were measured as control. Along with tannic acid, the degradation products of tannic acid were also analyzed for their effect on seed germination. Seeds were considered germinated when the radical and hypocotyl together appeared. The percent seed germination and root/shoot ratio was recorded at a regular interval of 24 h for 5 days at the same time. The germination index was determined by using the values of relative seed germination and relative root elongation (GI, the product of relative seed germination and relative root elongation; Eq. 3) (Tiquia 2010):
2.9 Determination of total sugar and total starch content
The embryo was removed from the seeds presoaked for 4 h. Now the seed without embryo were ground, from which 0.5 g of powder was used for homogenization in 10% ethanol. This homogenate was centrifuged at 2,000 rpm for 10 min at room temperature. The supernatant was collected and used for estimation of total sugar. Total sugar content was determined by Phenol-H2SO4 method (Dubios et al. 1951).
The residue collected from the above method was subjected for the determination of total starch content. The residue was dissolved in 5 mL distilled water, to which 6.5 mL of 52% perchloric acid was added. It was centrifuged at 2,000 rpm for 10 min at room temperature. The process was repeated thrice and then total volume was made up to 100 mL with distilled water. From this solution, a 1-mL aliquot was taken. To this 1 mL of 5% phenol and 5 mL of 96% concentrated H2SO4 was added. The tubes were incubated in boiling water bath for 30 min and then absorbance was measured at 470 nm (McCready et al. 1950).
2.10 Assay for alpha amylase enzyme activity
Amylase activity was determined by detecting the amount of reducing sugars liberated. The reaction mixture was prepared to 1 mL by adding 0.25 mL of 1% soluble starch, 0.25 mL of 0.4 M Tris–HCl buffer (8.0), and 0.5 mL of enzyme. The reaction was terminated by addition of 2 mL of 3,5-dinitrosalycylic acid reagent after incubation at 35°C for 30 min (Sengupta et al. 2000). The protein concentration was measured with bovine serum albumin as a standard (Lowry et al. 1951).
2.11 Determination of percent inhibition of amylase activity
The amylase activity was determined in control seeds as well as in seeds treated with tannic acid (10,000 and 20,000 ppm) during seed germination. Activity of amylase in control seed was considered as 100% activity and residual activity in treated sample was measured, which was subtracted from control activity to get percent inhibition. The percent inhibition of amylase activity was calculated by using following formula (Eq. 4)
2.12 Statistical analysis
The data was analyzed by one-way analysis of variance (ANOVA) with Tukey–Kramer multiple comparisons by taking the values which are significantly different from control (i.e., **P was <0.01, and ***P <0.001 were only considered). For the statistical analysis, Graphpad software was used.
3 Results
3.1 Isolation and characterization of tannic acid degrading bacterium
Bacterial strains isolated from the soil samples were screened on tannic acid agar plates. Seven bacterial colonies were scored (designated NACASA1 to NACASA7) and were grown individually in minimal liquid medium containing tannic acid (1% w/v) as the sole carbon source. It was found that isolate NACASA1 could degrade about 98 (±0.16)% tannic acid in 40 (±0.85***) h. The other strains could degrade about 80% to 97% of the tannic acid, but the time requirement is more which is clearly evident in Table 1. By considering these results, the isolated bacterial strain viz. NACASA1 was selected for further study. The morphological features of the colonies of isolated bacterial strain NACASA1 on tannic acid agar were 1 mm in diameter, white colored, smooth, flat, and opaque with entire margin. The cells of the isolate were Gram negative, non-motile, and rod shaped. Additionally, the isolate was catalase positive while indole negative. The isolate showed citrate positive and methyl red negative test. The isolate NACASA1 was able to utilize most of the sugars, except l-sorbose which was not used by NACASA1 (Table 2). Furthermore, the 16S rDNA amplicon of the isolate was used to determine the sequence of the nucleotides. The isolate NACSA1 is closely related to species belonging to the genus Klebsiella (Fig. 1). Therefore, the name to this strain is designated as Klebsiella sp NACASA1. The time course of tannic acid degradation by Klebsiella sp NACASA1 at 15°C and pH 7.0 is shown in Fig. 2. Klebsiella sp NACASA1 was able to degrade a major percentage (65%) of tannic acid in the first 25 h, while it took 40 h to degrade 98% tannic acid.
3.2 Effect of temperature and pH on tannic acid degradation
The growth temperature extension of isolated Klebsiella sp NACASA1 was 15–45°C, and 35°C was the optimal temperature for growth. Similar to the cell growth, degradation of tannic acid was determined at specific temperature, i.e., 15–45°C. Time required for complete degradation of tannic acid was decreased with the increasing temperature. Klebsiella sp NACASA1 showed degradation of tannic acid (1%) in 40 (±0.85***) and 30 (±0.71**) h at 15 and 35°C, respectively. The pH of medium was 7.0. Further increase in temperature above 35°C resulted in a marginal decrease in degradation activity. Klebsiella sp NACASA1 required 52 (±0.38***) h for tannic acid degradation at 45°C (Fig. 3). Though the optimum temperature for tannic acid degradation was 35°C, our interest was to evaluate the psychrotrophic nature of Klebsiella sp NACASA1. Klebsiella sp NACASA1 showed its potential to degrade tannic acid at 15°C in 40 h. To the authors' knowledge, the present study is the first description of cold-adapted biodegradation of tannic acid by bacteria. The indigenous microorganism adapted its metabolism to the presence of tannic acid and to the low temperatures. Klebsiella sp NACASA1 can degrade tannic acid at a concentration as high as 2.0% (data not shown). The requirement of less time for tannic acid degradation and the ability to sustain higher concentrations of tannic acid explores the potential of Klebsiella sp NACASA1. pH of the medium plays a vital role in most of the microbial processes. The effect of pH and optimization of it was studied by varying the pH from 3 to 7 for degradation of tannic acid. Klebsiella sp NACASA1 required 49 (±0.65**), 42 (±0.28**), and 40 (±0.85**) h time for tannic acid degradation at pH 3, 5, and 7, respectively. The optimum pH found was (neutral) 7.0 at 15°C. No tannic acid degradation was observed at pH higher than 7.0. No tannic acid degradation was observed at pH higher than 7.0 (i.e., in alkaline conditions) by the isolated Klebsiella strain.
3.3 Effect of carbon and nitrogen source on tannic acid degradation
Growth of the Klebsiella sp NACASA1 was determined in Bushnell and Hans’s medium with tannic acid as the sole carbon source. Also, the influence of the carbon source on tannic acid degradation by Klebsiella sp NACASA1 was investigated using different carbon sources. Based on sugar utilization results (Table 2), the screening for appropriate carbon source was done. Various carbon sources including glucose, starch, lactose, maltose, and sucrose were added separately at a fixed concentration of 1.0% to broth medium before the biodegradation extent of the tannic acid was examined. Figure 4 shows that almost all tested carbon sources were able to enhance the biodegradation of tannic acid. A great advantage for this degradation ability was particularly observed in presence of glucose carbon source. Klebsiella sp NACASA1 showed tannic acid degradation in 24 (±0.45**) h when glucose was supplemented along with tannic acid at 15°C. The most immediate inference that can be drawn is that an additional supply of readily available carbon, as energy source, helped in increasing the degradation of tannic acid. Galactose, xylose, and fructose did not show any supporting effect on tannic acid degradation as like glucose.
The effect of the nitrogen source on tannic acid degradation by Klebsiella sp NACASA1 was studied using various nitrogen sources having tannic acid and glucose as the carbon and energy sources at 15°C. Ammonium sulfate, peptone, ammonium persulfate, beef extract, malt extract, and tryptone were the common nitrogen sources routinely used in microbiological research. All nitrogen sources produced improvements in tannic acid biodegradation (Fig. 5). Ammonium sulfate in particular produced maximum stimulation of degradation that reached almost 98% within 20 (±0.65**) h.
3.4 Phytotoxicity analysis
Seed germination results showed a highly inhibitory activity of the tannic acid. There was 100% germination of seeds in distilled water (control). The germination index (GI) at 1,000 ppm of tannic acid was 41.55 (±0.45***), and at 5,000 and 15,000 ppm the GI values were 7.57 (±0.43***) and 0.53 (±0.01***), respectively. The GI tended to decrease with increasing concentration of tannic acid. The germination of seeds was completely inhibited at 20,000 ppm concentration. At the same time, there was no such adverse effect of 20,000 ppm degradation products of tannic acid on germination. The GI value for 20,000 ppm degradation products of tannic acid was 95.18 (±0.11**) (Fig. 6).
Competitive chemical effects not only operated on seed germination but on all aspects of growth. After germination of the seeds, the root and shoot development in the seeds was studied. For control seeds, the root length was 37.7 (±1.70) mm and the shoot length was 53.1 (±1.61) mm. The root lengths at 100, 1,000, 3,000, 5,000, 10,000, and 15,000 ppm concentrations were 28.1 (±2.16), 15.5 (±1.4**), 8.7 (±1.6**), 3.2 (±1.28**), 1.6 (±0.24***), and 0.66 (±0.16***) mm, respectively. The shoot lengths at 100, 1,000, 3,000, 5,000, 10,000, and 15,000 ppm concentrations were 39.4 (±1.90), 19.3 (±1.55***), 16.9 (±1.36***), 12.8 (±1.05***), 10.4 (±1.02***), and 0.9 (±0.28***), respectively. A gradual decrease in root/shoot length was observed with increasing concentration of tannic acid (Fig. 7).
At 20,000 ppm, no development of root and shoot was observed. There was no adverse effect on root/shoot elongation by tannic acid degradation products (data not shown). The normal development of root/shoot was observed similar to control.
3.5 Determination of total sugar, total starch, and α-amylase activity
The study was carried out to determine total sugar and starch content of the used seeds. The total sugar present was 950 μg. The total starch present in the same seeds was 351 μg. The study was also carried out to check the presence of α-amylase activity during seed germination. Seeds treated with 10,000 ppm tannic acid showed 43.42 (±0.39***)% inhibition in α-amylase activity. At 20,000 ppm, there was 95.11 (±0.24**)% inhibition of activity (Table 3). No α-amylase enzyme inhibition was observed in the seeds treated with tannic acid degradation products (data not shown).
4 Discussion
Antimicrobial activities of tannins has been the focus of many fields of research viz. food science, wood science, soil science, plant pathology, pharmacology, as well as animal and human nutrition (Scalbert 1991). Despite this fact, many authors reported microorganisms which are able to degrade tannins and to use them as carbon and energy source (Bhat et al. 1998; Franco et al. 2005; Monier and Lindow 2005). In the present study, all the seven isolated strains were able to degrade tannic acid, but NACASA1 was more efficient among all the isolates. The isolate NACASA1 was able to degrade 98% of tannic acid in 40 h as against 80% to 97% of degradation by the others when they were grown on 1% tannic acid at 15°C. The degradation of tannic acid by Klebsiella pneumonia was reported by several authors (Deschamps et al. 1983; Gandhi 1990). This is a first attempt to study the degradation of tannic acid at lower temperatures. In the present study, we reported the isolation and characterization of psychrotrophic tannic acid degrading bacterium Klebsiella sp NACASA1. Deschamps et al. (1980) isolated a mesophilic bacterium designated M24 that degraded 83% tannic acid in 5 days. The utilization of tannins by Pseudomonas fluorescens, Escherichia coli, and Azotobacter vinelandii (tannin at 0.65%, w/v) (Basaraba 1966) as sole carbon source was reported. Degradation of 1% (w/v) gallotannin by Bacillus pumilus, Bacillus polymyxa, Corynebacterium, and K. pneumoniae was reported by Deschamps et al. (1983). A maximum of 1% tannic acid was utilized by K. pneumonia (Gandhi 1990). As compared to the references mentioned above, Klebsiella sp NACASA1 seems to be a more potential bacterium since it degraded more percentage of tannic acid in less time and can withstand higher concentrations of tannic acid. The unique ability of Klebsiella sp NACASA1 to utilize high concentrations of tannic acid at low temperature sets it apart from all related bacteria.
Greatly varying data for the time course of biodegradation of tannic acid have been published, but all these studies were performed at 25–40°C temperature (Basaraba 1966; Deschamps et al. 1980; Gandhi 1990). However, the pollution problem is often more serious at low temperatures (Lee et al. 1995; Geerdink et al. 1996; Marchesi et al. 1997). Our isolate was able to degrade tannic acid efficiently at 15°C. Cold-adapted biodegraders could be of particular importance for in situ bioremediation treatments in deep soil horizons, where low temperatures prevail. These temperatures are significantly below the optimum for mesophilic microorganisms. Thus, a special cold adaptation of the microorganisms is necessary to achieve relevant substance conversions. The degradation of tannic acid achieved in the present study at 15°C was similar to that found at mesophilic temperature (Deschamps et al. 1980), which shows that, even at low temperatures, cold-adapted indigenous soil microorganisms may contribute to a great extent in tannic acid degradation. The knowledge of substrate specificity, growth temperature profiles as determined in this study, is needed in order to select strains with the required properties for efficient low temperature bioremediation. Klebsiella sp NACASA1 as characterized in this study could be useful as inoculums for the acceleration of low-energy wastewater treatment. The results obtained in this study may lead to a stronger consideration of cold-adapted bacteria for bioremediation techniques in environments exhibiting generally low temperatures, such as groundwater and subsoils. In order to implement bioremediation technologies at low temperatures, further studies are needed. Our results of pH optimum for tannic acid degradation are in agreement with those of Ilori et al. (2007). The supplementation of glucose and ammonium sulfate in the medium enhanced the degradation of tannic acid by Klebsiella sp NACASA1.
The ecotoxicity of the phenolic compounds and their possible hazard to human health were frequently reported. High phenol and organic acid concentrations in olive mill waste were shown to increase phytotoxicity under certain conditions (Chiacchierini et al. 2004). Present investigations also revealed that tannic acid exhibit phytotoxicity. The toxic effluent from these industries eventually reach the water channels and to the agricultural lands. Since V. unguiculata is an important crop from Ahmednagar (India) region, the present work attempts to assess the toxicity of tannic acid and its degradation products. Seed germination and plant growth bioassays are the most common techniques used to evaluate phytotoxicity (Kapanen and Itavaara 2001). In the present study, a significant reduction in root and shoot length was observed with increasing concentration of tannic acid. These results suggest the toxic nature of tannic acid towards V. unguiculata at higher concentrations. Our results are in good agreement with previous published literature indicating reduction in root–shoot length with increasing concentration of phenolic compounds (Yamamoto and Fujii 1997; Colpas et al. 2003). The psychrotrophic isolate characterized in the present work was isolated for the first time from garden soil, providing novel insights for the role it plays in degradation of tannic acid in nontoxic metabolites.
During seed germination, α-amylase in the aleurone layer plays an important role in hydrolyzing the endosperm starch into metabolizable sugars, which provide the energy for the growth of roots and shoots (Beck and Ziegler 1989; Fincher 1989; Gubler et al. 1995). These studies inspired us to check the α-amylase enzyme status during seed germination. We found that the increasing concentration of tannic acid inhibited α-amylase activity. Further study is needed to find out whether tannic acid affecting α-amylase transcription in aleurone layer or inhibiting the hydrating reaction of stored starch of α-amylase into the endosperm.
5 Conclusions
Klebsiella sp NACASA1 isolated from the garden soil was able to rapidly degrade tannic acid at 15°C, showing such feature for the first time. The use of Klebsiella sp NACASA1 will facilitate improved industrial and livestock production. This work is in an incipient stage and further studies have to be carried out to exploit the potential of Klebsiella sp NACASA1 for pilot and large-scale applications. The present results confirm that tannic acid may act as a toxic agent in plant cells. This implies that exposure to an environment contaminated by tannic acid may be a potential risk for damage in living organisms. The results also suggest that the phytotoxicity bioassay can be used as one of the efficient toxicity tests for tannic acid as well as an indicator of environmental contamination. Also, the simple biodegradation process presented here was found to be effective in reducing the toxicity of tannic acid.
References
Archambault J, Lacki K, Duvnjak Z (1996) Conversion of catechin and tannic acid by an enzyme preparation from Trametes versicolor. Biotechnol Lett 18:771–774
Basaraba J (1966) Effects of vegetable tannins on glucose oxidation by various microorganisms. Can J Microbiol 12:787–794
Beck E, Ziegler P (1989) Biosynthesis and degradation of starch in higher plants. Annu Rev Plant Physiol Plant Mol Biol 40:95–117
Bhakta Dipita and Ganjewala Deepak (2009) Effect of Leaf Positions on Total Phenolics, Flavonoids and Proantho-cyanidins Content and Antioxidant Activities in Lantana Camara (L) J. Sci. Res. 1 (2),363-369
Bhat T, Singh B, Sharma O (1998) Microbial degradation of tannins—a current perspective. Biodegradation 9:343–357
Chiacchierini E, Restuccia D, Vinci G (2004) Bioremediation of food industry effluents: recent applications of free and immobilised polyphenoloxidases. Food Sci Technol Int 10:373–382
Colpas F, Ono E, Rodrigues J, Passos J (2003) Effects of some phenolic compounds on soybean seed germination and on seed-borne fungi. Braz Arch Biol Technol 46:155–161
Deschamps A, Mahoudeau G, Conti M, Lebeault J (1980) Bacteria degrading tannic acid and related compounds. J Ferment Technol 58:93–97
Deschamps A, Otuk G, Lebeault J (1983) Production of tannase and degradation of chestnut tannins by bacteria. J Ferment Technol 61:55–59
Dubios M, Gilles J, Robers P, Smith F (1951) Colorimetric determination of sugar and related substances. Anal Chem 26:351–356
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791
Field J, Lettinga G (1992) Biodegradation of tannins. In: Sigel H (ed) Metal ions in biological systems volume 28. Degradation of environmental pollutants by microorganisms and their metalloenzymes. Marcel Dekker, New York, pp 61–97
Fincher G (1989) Molecular and cellular biology associated with endosperm mobilization in germinating cereal grains. Annu Rev Plant Physiol Plant Mol Biol 40:305–345
Franco A, Calheiros C, Pacheco C, De-Marco P, Manaia C, Castro P (2005) Isolation and characterization of polymeric galloyl-ester-degrading bacteria from a tannery discharge place. Microb Ecol 50:550–556
Fuchs G, Mohamed M, Altenschmidt U, Koch J, Lack A, Brackmann R, Lochmeyer C, Oswald B (1994) Biochemistry of anaerobic biodegradation of aromatic compounds. In: Ratledge C (ed) Biochemistry of microbial degradation. Kluwer, Dordrecht
Gandhi P (1990) Microbial degradation with special reference to tannery. Doctoral thesis, Madurai Kamaraj University, Madurai, India
Garg S, Makkar H, Nagal K, Sharma S, Wadhwa D, Singh B (1992) Toxicological investigations into oak (Quercus incana) leaf poisoning in cattle. Vet Hum Toxicol 34:161–164
Geerdink M, Loosdrecht M, Luyben K (1996) Biodegradability of diesel oil. Biodegradation 7:73–81
Gu L, Kelm M, Hammerstone J, Beecher G, Holden J, Haytowitz D, Prior R (2003) Screening of foods containing proanthocyanidins and their structural characterization using LC-MS/MS and thiolytic degradation. J Agric Food Chem 51:7513–7521
Gubler F, Kalla R, Roberts J, Jacobsen J (1995) Gibberellin-regulated expression of a myb gene in barley aleurone cells: evidence for Myb transactivation of a high-pI alpha-amylase gene promoter. Plant Cell 7:1879–1891
Haslam E (1966) The scope of vegetable tannin chemistry. Chemistry of vegetable tannins. Academic, London, pp 1–13
Ilori M, Adebusoye S, Amund O, Oyetoran B (2007) A study of tannic acid degradation by soil bacteria. Pak J Biol Sci 10:3224–3227
Kapanen A, Itavaara M (2001) Ecotoxicity tests for compost applications. Ecotoxicol Environ Saf 49:1–16
Lee C, Russell N, White G (1995) Rapid screening for bacterial phenotypes capable of biodegrading anionic surfactants: development and validation of a microtitre plate method. Microbiol 141:2801–2810
Lekha P, Lonsane B (1997) Production and application of tannin acyl hydrolase: state of the art. Adv Appl Microbiol 44:215–260
Lowry O, Rosebrough N, Farr A, Randall R (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Marchesi J, White G, Russell N, House W (1997) Effect of river sediment on the biodegradation kinetics of surfactant and non-surfactant compounds. FEMS Microbiol Ecol 23:55–63
Margesin R, Schinner F (1998) Low-temperature bioremediation of a waste water contaminated with anionic surfactant and fuel oil. Appl Microbiol Biotechnol 49:482–486
Margesin R, Schinner F (1999) Biodegradation of organic pollutants at low temperatures. In: Margesin R, Schinner F (eds) Biotechnological applications of cold-adapted organisms. Springer, Berlin, pp 271–289
McCready R, Guggolz J, Silviera V, Owens H (1950) Determination of starch and amylase in vegetables. Anal Chem 22:1156–1158
Mingshu L, Kai Y, Qiang H, Dongying J (2006) Biodegradation of gallotanins and ellagitannins. J Basic Microbiol 46:68–84
Monier J, Lindow S (2005) Aggregates of resident bacteria facilitate survival of immigrant bacteria on leaf surfaces. Microb Ecol 49:343–352
Moreland D, Novitzky W (1987) Effects of phenolic acids, coumarins, and flavonoids on isolated chloroplasts and mitochondria. In: Waller GR (ed) Allelochemicals: role in agriculture and forestry. American Chemical Society, Washington, pp 247–261
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Scalbert A (1991) Antimicrobial properties of tannins. Phytochem 30:3875–3883
Sengupta S, Jana M, Sengupta D, Naskar A (2000) A note on the estimation of microbial glycosidase activities by dinitrosalicylic acid reagent. Appl Microbiol Biotechnol 53:732–735
Somasegaram P, Hoben H (1985) Methods in legume Rhizobium technology. NifTAL, Paia Maui
Tamura K, Nei M, Kumar S (2004) Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc Natl Acad Sci USA 101:11030–11035
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Timmis K, Pieper D (1999) Bacteria designed for bioremediation. Trends Biotechnol 17:201–204
Tiquia S (2010) Reduction of compost phytotoxicity during the process of decomposition. Chemosphere 79:506–512
Yamamoto Y, Fujii Y (1997) Exudation of allelopathic compound from plant roots of sweet vernalgrass (Anthoxanthum odoratum). J Weed Sci Technol 42:31–35
Zucconi F, Forte M, Monaco A, De-Bertoldi M (1981a) Biological evaluation of compost maturity. BioCycle 22:27–29
Zucconi F, Pera A, Forte M, De-Bertoldi M (1981b) Evaluating toxicity of immature compost. BioCycle 22:54–57
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Elena Maestri
Rights and permissions
About this article
Cite this article
Jadhav, U., Kadu, S., Thokal, N. et al. Degradation of tannic acid by cold-adapted Klebsiella sp NACASA1 and phytotoxicity assessment of tannic acid and its degradation products. Environ Sci Pollut Res 18, 1129–1138 (2011). https://doi.org/10.1007/s11356-011-0468-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-011-0468-6