Abstract
To explore the in vivo and in vitro plant growth promoting activities, biocatalytic potential, and antimicrobial activity of salt tolerance rhizoactinobacteria, rhizospheric soil of a halotolerant plant Saueda maritima L. was collected from Rann of Tiker, near Little Rann of Kutch, Gujarat (India). The morphology analysis of the isolated strain TSm39 revealed that the strain belonged to the phylum actinobacteria, as it was stained Gram-positive, displayed filamentous growth, showed spore formation and red pigment production on starch casein agar (SCA). It was identified as Georgenia soli based on 16S rRNA gene sequencing. The Georgenia soli strain TSm39 secreted extracellular amylase, pectinase, and protease. It showed in vitro plant growth-promoting (PGP) activities such as indole acetic acid (IAA) production, siderophore production, ammonia production, and phosphate solubilization. In vivo plant growth-promoting traits of strain TSm39 revealed 30% seed germination on water agar and vigor index 374.4. Additionally, a significant increase (p ≤ 0.05) was found in growth parameters such as root length (16.1 ± 0.22), shoot length (15.2 ± 0.17), the fresh weight (g), and dry weight (g) of the roots (0.43 ± 0.42 and 0.32 ± 0.12), shoots (0.62 ± 0.41 and 0.13 ± 0.03), and leaves (0.42 ± 0.161 and 0.14 ± 0.42) in treated seeds of Vigna radiata L. plant with the strain TSm39 compared to control. The antibiotic susceptibility profile revealed resistance of the strain TSm39 to erythromycin, ampicillin, tetracycline, and oxacillin, while it displayed maximum sensitivity to vancomycin (40 ± 0.72), chloramphenicol (40 ± 0.61), clarithromycin (40 ± 1.30), azithromycin (39 ± 0.42), and least sensitivity to teicoplanin (15 ± 0.15). Moreover, the antimicrobial activity of the strain TSm39 was observed against Gram’s positive and Gram’s negative microorganisms such as Shigella, Proteus vulgaris, and Bacillus subtilis. These findings indicated that the Georgenia soli strain TSm39 has multiple plant-growth-promoting properties and biocatalytic potential that signifies its agricultural applications in the enhancement of crop yield and quality and would protect the plant against plant pathogens.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Actinobacteria are the most significant microbes present in the soil and are studied for the production of various biologically active substances, such as antibiotics and enzymes [1]. Plant growth-promoting rhizobacteria (PGPR) promote plant growth directly or indirectly through plant growth hormone production, nutrient acquisition, and plant disease suppression [2]. The PGPR are beneficial to plants as they increase the availability of macro and microelements such as nitrogen, phosphorus, iron, and zinc in the rhizosphere and produce plant growth-promoting substances such as indole [3]. The rhizobacteria can improve plant growth under unfavorable conditions such as chemically contaminated soil [4]. Currently, most attention has been paid to PGP rhizobacteria to replace chemical fertilizers and pesticides [5]. Moreover, general belonging to the phylum Actinobacteria such as Streptomyces, Thermobifida, and Microbispora help the plant by producing phytohormones such as indole acetic acid (IAA) as well as siderophores and various fungicidal compounds [6, 7]. Actinobacteria have been oppressed in the pharmaceutical industry since the 1940s; however, only a few products have been developed for their applications in agriculture [8].
Plants exhibit a relationship with the microbial populations colonizing around the rhizosphere [9]. Actinobacteria are a group of microbes among the total soil microbial community that display the ability to produce various secondary metabolic compounds. They can display the antagonistic and competitive effects on the other microbial communities and can also produce plant growth regulators (PGRs) [10, 11]; especially Streptomyces have biocontrol action against a range of phytopathogens [12].
Suaeda maritima L. is a dominant halotolerant plant generally found in the saline regions of Little Rann of Kutch, Gujarat (India), which is nominated as a biosphere reserve and is characterized as terrestrial and coastal ecosystems [13]. The PGP activities of rhizospheric microbes from this extreme habitat are still the least studied [14]. Therefore, the main objective of this study was to isolate actinobacteria from the rhizospheric soil of the medicinal plant Suaeda maritima L., followed by identification based on cultural, morphological, and molecular features. Moreover, this work focused on mining in vitro plant growth attributes by evaluating the potential of strain TSm39 to solubilize phosphate, produce IAA (indole acetic acid), siderophore, ammonia, and extracellular enzymes. Furthermore, the antimicrobial potential of salt-tolerant strain TSm39 was explored. Additionally, the strain TSm39 was studied for its ability to enhance the growth of the Vigna radiate L. plant under controlled conditions by pot experiment to evaluate its applications for sustainable agriculture.
Materials and methods
Study site and rhizospheric soil sample collection
The rhizospheric soil sample of the medicinal plant Suaeda maritima L. was collected from the Rann of Tiker nearby Little Rann of Kutch, Gujarat (India). The rhizospheric soil from a depth of 15 cm was collected with the help of a sterile spatula, packed in sterile disposable bags, and labeled. The sample was stored in an icebox and transported to the laboratory, where it was stored at − 20 °C until further analysis. The rhizospheric soil sample was analyzed for its physicochemical parameters such as electric conductivity, pH, and water holding capacity.
Isolation and morphological characterization of rhizospheric actinobacteria
The rhizospheric actinobacteria were isolated from rhizospheric soil samples by serial dilution and the spread plate method. The soil sample of 1 g was added to 10 mL sterile D/W and mixed well. The supernatant was spread on various isolation media such as a range of International Streptomyces Project (ISP) media (ISP1 to 7), nutrient agar, starch agar, starch casein agar, actinomycete isolation agar, followed by incubation at 28 °C for 7 days. After incubation, the isolates were characterized based on their morphology, Gram’s reaction, and cultural characteristics.
The salt and pH profile
The salt profile of actinobacteria strain TSm39 was studied in a starch agar medium embedded with 0–15% NaCl (w/v). The strain was spot inoculated on media and incubated at 28 °C for 7 days. Similarly, the pH profile of the actinobacteria strain TSm39 was studied at 7–12 pH.
16S rRNA gene amplification, sequencing and phylogeny analysis
The genomic DNA was extracted directly by colony PCR. The 16S rRNA gene amplification was carried out using a set of universal eubacterial primers (27F and 1492R). The amplified products were loaded on 0.8% agarose gel, and the final PCR product was observed under a UV transilluminator (Bangalore Genei, India). The isolate was identified based on 16S rRNA gene sequence similarity with that of the gene sequence of known organisms in the NCBI database. The 16S rRNA gene sequence was analyzed with a gapped BLAST (http://www.ncbi.nlm.nih.gov) search, and closely related sequences were aligned by CLC Genomics Workbench version 11. 1.0. The evolutionary distances were computed using the neighbor-joining method.
Screening of actinobacteria forin vitroplant growth-promoting activities
Ammonia production
To determine the ammonia production, the actinobacterial culture was inoculated into 10 mL of peptone water (Himedia, India) and incubated at 28 °C for 7–8 days with shaking at 150 rpm. After incubation, 1 mL of Nessler’s reagent was added. The development of brown color indicates a positive result for ammonia production [15].
IAA production
For IAA production, the spore culture of the isolate was inoculated into 1 mL tryptophan broth and incubated at 28 °C for 8–10 days at 150 rpm. After the completion of incubation time, the broth was centrifuged at 10,000 × g for 10 min. The Salkowski’s reagent (1 mL 0.5 M Ferric chloride; 50 mL 35% solution of perchloric acid) of 1 mL was added. The development of the pink color indicates a positive test [16].
Siderophore production
The chrome azurol S (CAS) agar plates were prepared and spot inoculated with actinobacterial strains and incubated at 28 °C for 8–10 days. The strains producing a yellow halo zone around the colony are considered positive for siderophore production [17].
Phosphate solubilization
The phosphate solubilization was detected on a minimal medium embedded with insoluble Ca3(PO4)2 and bromophenol blue dye. The plate was spot inoculated and incubated at 28 °C for 8–10 days. The isolates showing a clear zone around the colony were considered as positive [18].
Enzymatic profile
Protease
Protease secretion was detected in the gelatin agar plate (gelatin 30 g L−1; peptone 10 g L−1; yeast extract 5 g L−1; NaCl 30 g L−1; agar 30 g L−1). The strain TSm39 was inoculated by the spot inoculation method [19]. After an incubation for 4–5 days, the plate was flooded with Frazier’s reagent (HgCl2 150 g L−1; 200 mL HCl was dissolved in 1000 mL D/W) to determine protease secretion.
Amylase
For analysis of amylase secretion, starch agar plate (starch agar powder 30 g L−1; agar 30 g L−1) was spot inoculated and incubated at 28 °C for 7 days. After incubation, the plate was flooded with Gram’s iodine solution to detect the zone of the utilization of the substrate.
Cellulase
Detection of cellulase was performed on cellulose agar medium (KH2PO4 0.5 g L−1; MgSO4 0.25 g L−1; cellulose 2 g L−1; agar 30 g L−1; gelatine 2 g L−1). The pH of the medium was adjusted to 8 by adding separately autoclaved Na2CO3 (200 g/L). The pure isolate was spot inoculated on cellulose agar, followed by incubation for 7 days at 28 °C. Cellulase production was detected by flooding the plates with Gram’s iodine reagent. The clear zone surrounding the colony indicated the production of extracellular cellulase.
Pectinase
The pectinase activity was determined on pectin agar (pectin 1 g L−1; NH4Cl 0.3 g L−1; KH2PO4 0.2 g L−1; K2HPO4 0.3 g L−1; MgSO4 0.01 g L−1; agar 3 g L−1). The isolate was spot inoculated on pectin agar and incubated at 28 °C for 7 days. Pectinase production was detected by flooding the plates with 1% CTAB (cetyltrimethylammonium bromide) solution and incubating at room temperature for 15 min. The clear zone around the colony indicates hydrolysis of pectin.
Antibiotic sensitivity and antibacterial activity analysis
An antibiotic sensitivity test of selected actinobacteria was performed using the disc diffusion method in starch agar medium supplemented with 20 different antibiotics, including amoxyclav (30 mg), cephalothin (30 mg), novbiocin (5 mg), erythromycin (15 mg), co-trimoxazole (25 mg), penicillin-G (10 mg), ofloxacin (5 mg), clindamycin (2 mg), chloramphenicol (30 mg), ampicillin (10 mg), amikacin (30 mg), teicoplanin (10 mg), methicillin (5 mg), tetracycline (30 mg), oxacillin (1 mg), and gentamicin (10 mg). The isolate was also screened for antibacterial activity against Gram’s positive and Gram’s negative pathogens on the ISP-2 (yeast malt extract agar) medium.
Pot experiment
The selected isolate was analyzed for in vivo plant growth-promoting attributes using Vigna radiate L. (mung) test plant. The mung beans were surface sterilized with sodium hypochlorite (20 g L−1) solution and washed with sterile D/W several times. Furthermore, the test seeds were soaked in actinobacterial culture (108 CFU), while the control seeds were soaked in sterile D/W. Both test and control seeds were incubated for 2 h and dried. Then, the seeds were inoculated on water agar (20 g L−1) for seed germination rate and were sown in a polythene bag containing 1.5 kg sterile soil for the determination of growth parameters such as root length, shoot length, fresh weight, and dry weight of the test plant Vigna radiata L.
Statistical analysis
All the experiments were laid out in a completely randomized block system with three replicates of bacterial and control treatment. Statistical analysis was performed using R statistic software version 4.1.3 (2022) [20]. One-way ANOVA was performed to test the variations in shoot length, root length, fresh and dry weight of root, and shoots of the plant. Means of the bacterial treatment were compared with control using Tukey’s HSD test at considered significance at the p < 0.05 level; all the data presented in this study means ± standard deviations (SD) of independent replicates.
Results
Rhizospheric soil sample analysis
The rhizospheric soil of the Suaeda maritima L. plant was collected from Rann of Tiker (23; 13; 16.8300 E and 71; 6; 21.6199 N), Gujarat, India. The physicochemical parameters of the rhizospheric soil, such as electrical conductivity of 1.16 dS/m and pH 8.41, indicated the saline and slightly alkaline nature of rhizospheric soil. Additionally, the water holding capacity of rhizospheric soil was 46.46% which was very high compared to the water holding capacity of non-rhizospheric soil collected from Little Rann of Kutch.
Cultural and physiological characterization
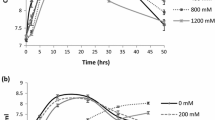
The actinobacterial strain TSm39 could grow on a range of International Streptomyces Project (ISP) media (ISP1 to 7), starch agar, starch casein agar, actinomycete isolation agar, except on nutrient agar at the optimum growth temperature of 28 °C. The actinobacterial strain TSm39 was Gram-positive, showed filamentous growth, and produced red-colored reverse side pigmentation on starch casein agar (SCA). Furthermore, the strain TSm39 grew in a starch agar medium supplemented with NaCl (0–200 g L−1). It could tolerate up to 100 g L−1 of NaCl containing starch agar medium at an optimum pH of 8 (Table 1), indicating the organism’s haloalkali tolerant nature. The cultural characteristics, physical properties, and biotechnological potential of rhizospheric actinobacteria isolated from various sites are provided in (Table 1).
16S rRNA gene sequencing and phylogenetic analysis
Colony PCR yielded approximately 1500 bp amplified product. The amplified product was sequenced further to identify the organism. The comparison of the 16S rRNA gene sequence of the strain TSm39 with 16S rRNA gene sequences from the NCBI GenBank database revealed a 99.56% similarity of the strain TSm39 with Georgenia soli. Thus, the isolated organism was identified as Georgenia soli strain TSm39. The phylogenetic analysis of the Georgenia soli strain TSm39 is shown in Fig. 1.
In vitroplant growth promoting activity
A Georgenia soli strain TSm39 produced ammonia, which can be detected by the formation of a yellow color after the addition of Nessler’s reagent in peptone water. Furthermore, to check the IAA production, 1 mL of Salkowski’s reagent was added to a tryptophan solution inoculated with strain TSm39. The pink color development indicated the usage of tryptophan and the production of indole acetic acid. The rhizospheric Georgenia soli strain TSm39 produced 51.27 μg/mL IAA when 500 mg/mL tryptophan was provided in the medium. The ability of Georgenia soli strain TSm39 to produce siderophores was found on chrome azurol S (CAS) agar. After 7 days of incubation, the strain produced a yellow halo zone around the colony, indicating a siderophore production. The Georgenia soli strain TSm39 was a phosphate solubilizer, as indicated by the clear zone around the colony on media containing inorganic phosphate. The rhizospheric Georgenia soli strain TSm39 exhibited a solubilization index of 3.5 on a Pikovskaya’s agar medium embedded with tricalcium phosphate.
Enzymatic profile of rhizospheric actinobacteria
The Georgenia soli strain TSm39 was screened to detect the secretion of extracellular enzymes such as protease, amylase, cellulase, and pectinase. A clear halo zone around the colonies confirmed the production of the enzymes. The Georgenia soli strain TSm39 used substrates such as gelatin, starch, and pectin and showed protease, amylase, and pectinase secretion in respective media (Fig. 2). The Georgenia soli strain TSm39 showed maximum amylase activity (zone ratio 6 ± 0.32) on starch agar, followed by pectinase activity (zone ratio 2.1 ± 0.11) on pectin agar, and protease activity (zone ratio 2 ± 0.16) on gelatin agar medium. However, the strain TSm39 did not show cellulase activity in the cellulose agar medium.
Antibiotic sensitivity and antibacterial activity profile
The antibiotic susceptibility test revealed that the Georgenia soli strain TSm39 was more sensitive to vancomycin (30 mg), chloramphenicol (30 mg), clarithromycin (15 mg), and azithromycin (15 mg) as indicated by zone ratios (mean ± SD) 40 ± 0.72, 40 ± 0.61, 40 ± 1.30, and 39 ± 0.42, respectively, while the strain was least sensitive to teicoplanin (10 mg), as indicated by zone ratio 15 ± 0.15. Moreover, the strain TSm39 showed resistance to erythromycin (15 mg), ampicillin (10 mg), methicillin (5 mg), tetracycline (30 mg), and oxacillin (1 mg) (Fig. 3a, b). Furthermore, the Georgenia soli strain TSm39 exhibited antibacterial activity against various test organisms. The strain TSm39 showed resistance to Staphylococcus aureus, Salmonella typhi, and Bacillus subtilis on yeast malt extract agar, as shown in Fig. 4. However, the strain was sensitive to Enterococcus faecalis, Bacillus cereus, and Escherichia coli, as indicated by the zones of inhibition 1.5 ± 0.12, 2 ± 0.46, 1.8 ± 0.39, respectively, on yeast malt extract agar.
In vivo plant growth promoting traits
The Georgenia soli strain TSm39 showed multiple in vitro plant growth-promoting activities, produced multienzymes, and displayed antimicrobial properties. Therefore, in vivo plant, growth-promoting potential was further checked by performing the pot experiment. The results of the pot experiment showed that the seed germination rate significantly (p ≤ 0.05) increased up to 30% in treated seeds compared to untreated seeds (control). The vigor index of 374.4 was calculated from the total plant length (Table 2). While various growth parameters were compared, significant differences (p ≤ 0.05) were observed between treated and untreated seeds of plant Vigna radiate L. (Table 3; Fig. 5a–c). The shoot length (12.0 ± 0.04) and root length (13.3 ± 0.18) of plant Vigna radiata L. increased significantly (p ≤ 0.05) by 32% in treated seeds compared to untreated seeds. Similarly, the fresh weight (g) and dry weight (g) of the roots (0.43 ± 0.42 and 0.32 ± 0.12), shoots (0.62 ± 0.41 and 0.13 ± 0.03), and leaves (0.42 ± 0.161 and 0.14 ± 0.42) also increased significantly in TSm39-treated seeds compared to untreated seeds (Fig. 6). The in vivo studies revealed that the Georgenia soli strain TSm39 produced indole acetic acid (IAA) and enhanced root and shoot elongation of plant Vigna radiata L.
Pot experiment and growth parameters of Vigna radiata plant (a) Effect of the strain TSm39 on plant height — control (without inoculation of the strain TSm39) and test (treated with the strain TSm39) (b) Effect of the strain TSm39 on shoot length (□) and root length (■) per plant (c) Effect of TSm39 culture on root fresh weight (
 ) shoot fresh weight (
) shoot fresh weight (
 ), leaves fresh weight (
), leaves fresh weight (
 ), root dry weight (
), root dry weight (
 ), shoot dry weight (
), shoot dry weight (
 ), leaves fresh weight (
), leaves fresh weight (
 ) per plant
) per plant
Discussion
In this study, actinobacteria were isolated and screened for their plant growth-promoting attributes from rhizospheric soil with high salinity. Little Rann of Kutch is a saline ecosystem with sandy soil and contains 60% clay [31]. The pH value of the rhizospheric soil of the Suaeda maritime L. plant was 8.41, which is related to rhizospheric soil of Arnebia euchroma with pH values ranging from 7.97 to 8.43 that assessed for cultivable microbial diversity [32]. The electrical conductivity (EC) of the rhizospheric soil of Suaeda maritime L. was 7.81 dS/m. Electrical conductivity is generally associated with soil salinity [33]. Recently, the electrical conductivity of the rhizospheric soil of gray mangrove (Avicennia marina) was measured 4.81 dS/m, which is less compared to this study [34]. In contrast, the electrical conductivity of the rhizospheric soil of Durum plants from various areas of the Dead Sea region varied in the range of 3.32–17.7 dS/m [35]. The actinobacteria are abundantly distributed in rhizospheric habitats, where they promote plant growth by making nutrients available to the plant by dissolving the molecules surrounding the plant or inhibiting the activity of plant pathogens [34]. A Georgenia soli strain TSm39, belonging to the phylum Actinobacteria, showed maximum growth on isolation media including ISP-1 to 7, actinomycete isolation agar, starch casein agar, and starch agar at an optimum pH of 8 and 28 °C temperature, which is comparable to the reported actinobacteria isolated from rhizospheric soil of various regions (Table 1).
The Georgenia soli strain TSm39 showed various in vitro plant growth-promoting activities such as ammonia production, IAA production, phosphate solubilization, and siderophore production. Ammonia production plays a vital role in the suppression of plant disease. Overproduction of ammonia serves as a prompting factor for the virulence of opportunistic plant pathogens and satisfies the nitrogen demand of the plant [25, 36]. In this study, a Georgenia soli strain TSm39 produced 51.27 ± 0.25 μg mL−1 indole acetic acid (IAA) in the presence of tryptophan. The few genera belonging to the phylum Actinobacteria promote plant growth by producing IAA. These IAA producing actinobacteria such as Streptomyces viridis isolated from the rhizospheric soil of Thai medicinal plant and Streptomyces fradiae NKZ-259 isolated from Qinghai, China were produced IAA in the range of 6.44–42.34 μg mL−1 that enhanced the root and shoot development [37, 38]. Recently, an endophyte, Bacillus siamensis CNE6, isolated from Cicer arietinum L. produced 33.27 ± 2.16 μg mL−1 IAA (Gorai et al., 2021) which is comparatively less compared to Georgenia soli strain TSm39 that produced 51.27 ± 0.25 μg mL−1 IAA [39]. In comparison, Carlosrosaeavrieseae UFMG-CM-Y6724 showed a maximum IAA production 76.1 μg mL−1 in the presence of tryptophan [40].
Siderophores are ferric iron chelators secreted by bacteria to acquire iron from the surrounding. Siderophores significantly enhance plant growth and display antagonism against phytopathogens. Siderophores form a complex with iron (Fe3+) and make the iron available to the plant under iron-deficient conditions while making the iron unavailable to phytopathogens [27, 41]. Georgenia soli strain TSm39 under study showed siderophore production on CAS agar medium, indicated by the color change of the medium from blue to yellowish orange. Similarly, Streptomyces sp. isolated from various rhizospheric soils of different plants collected from diverse regions of Brazil were well studied for the production of siderophores [42]. Recently reported Azospirillum brasilense also produced siderophores in the CAS medium [43]. Besides, a Streptomyces sp. CLV45 isolated from rhizosphere soil of plants belonging to the family Fabaceae was studied for their in vitro plant growth promoting activities, including IAA and siderophore production [44].
Phosphate solubilization is a variable characteristic among bacteria and is very advantageous for their application as biofertilizers. Phosphate solubilization generally occurs through the secretion of organic acids. The phosphate solubilization index of the Georgenia soli strain TSm39 was 3.5 on Pikovskaya’s agar. It exhibited the highest solubilization index on a medium embedded with tricalcium phosphate. Similarly, the endophytic bacteria such as Bacillus cereus (BacDOB-E19) and Pseudomonas aeruginosa (RBacDOB-S24) showed phosphate solubilization activity on Pikovskya’s medium [45]. More recently, Streptomyces coelicoflavus showed phosphate solubilizing activity on Pikovskya’s medium [34].
The haloalkaliphilic organisms can survive in both saline and alkaline conditions [46]. Haloalkaliphilic actinobacteria can produce multiple enzymes, including amylase, cellulase, pectinase, protease, and chitinase. Bacterial strains that produce multiple enzymes under various stress conditions hold enormous ecological and industrial significance. Protease is essential in the detergent, food, pharmaceutical, cosmetic, and photographic industries. Similarly mylases are used in the food, textiles, paper, detergent, and biofuel industries [47]. Pectinases and cellulases are used in the food industry for extracting and clarifying wines, juices, and in the textile industry for preparing linen fabrics and hemp manufacture [48]. The Georgenia soli strain TSm39 tolerated both saline and alkaline conditions and produced multiple extracellular enzymes such as protease, amylase, and pectinase, signifying its industrial importance. Recently, Bacillus isolates from Brassica napus L. roots have produced hydrolytic enzymes, including protease, amylase, xanthanase, and cellulase [48]. According to the literature, above 60% of salt-tolerant alkaliphilic actinobacteria could produce extracellular enzymes such as proteases, cellulases, and amylases at higher salt and alkaline pH [49]. The Halomonas meridian showed optimum amylase production in 10% NaCl (w/v) [50], which is quite similar to the salt tolerance of Georgenia soli strain TSm39 [(10% NaCl (w/v)]. The protease and cellulase are cell wall degrading enzymes; thus, the isolates producing these enzymes protect the plant by breaking the cell wall of oomycete pathogens such as Pythium sp. [51]. Previously, the Nocardiopsis alba strain OK-5 was studied for alkali stable proteases isolated from Okha, the coastal region of Gujarat, India [52]. The cellulases from Bacillus sp., Pseudomonas sp., and Serratia sp. were isolated and screened from municipal solid wastes and rice straw wastes using carboxy methyl cellulose (CMC) agar medium [53]. Overall, the results showed that the Georgenia soli strain TSm39 tolerated both saline and alkaline conditions and produced multiple extracellular enzymes such as protease, amylase, and pectinase, signifying its industrial importance.
The actinobacterial genera, particularly Streptomyces, are renowned for antibiotic resistance. Earlier, the resistance of Streptomyces sp. to tetracycline (30 mg) and oxacillin (5 mg), and sensitivity to vancomycin (30 mg) and chloramphenicol (15 mg) were revealed together with the antibacterial activity of Streptomyces sp. Against Bacillus subtilis and Xanthomonas oryzae [54]. Similarly, the strain TSm 39 in this study was sensitive to vancomycin (30 mg) and chloramphenicol (30 mg) but displayed resistance to tetracycline (30 mg) and oxacillin (1 mg) antibiotics. Previously, the novel antibiotic tunicamycin was discovered with anti-complement properties based on the genome analysis of the marine actinobacteria Streptomyces sp. DUT11 [55]. Streptomyces is the most potential genera well studied for antimicrobial metabolites [56, 57]. Similarly, the very least explored Georgenia soli strain TSm39 in this study showed antimicrobial activity against Staphylococcus aureus, Salmonella typhi, and Bacillus subtilis on yeast malt extract agar. Similarly, a novel marine actinobacterium, Streptomyces variabilis RD-5, with antibacterial activity against Escherichia coli, Klebsiella pneumoniae, Enterobacter cloacae, Pseudomonas sp. and Salmonella enteritidis, Bacillus subtilis, and Staphylococcus aureus, was isolated from sea sediments of the Gulf of Khambhat, Gujarat [58]. More recently, the strains Acinetobacter lwoffii Bac109 and Pantoea agglomerans Bac131 were studied for their in vitro and ex vitro plant growth-promoting and antifungal activities [59].
In this study, a pot experiment was performed using mung beans treated with Georgenia soli strain TSm39. A significant increase (p ≤ 0.05) in shoot length (12.0 ± 0.04) and root length (13.3 ± 0.18) of plant Vigna radiate L. was observed in treated seeds compared to untreated seeds. Similarly, a significant increase was observed in the fresh weight (g) and dry weight (g) of the roots (0.43 ± 0.42 and 0.32 ± 0.12), shoots (0.62 ± 0.41 and 0.13 ± 0.03), and leaves (0.42 ± 0.161 and 0.14 ± 0.42). The Georgenia soli strain TSm39 enhanced the root and shoot length of the Vigna radiate L. plant significantly (p ≤ 0.05) compared to the control due to indole acetic acid and siderophores produced by the strain TSm39, as these two parameters direct in vitro PGP mechanisms to promote plant growth. Similarly, the plant growth-promoting Bacillus strains (V62 and V39) and Arthrobacter strains (V84 and V54) contain multiple plant growth-promoting traits and show their potential effect on maize growth [60]. Recently, Pseudomonas aeruginosa, the rhizospheric strain MK513745 significantly enhanced the shoot length (37 ± 0.88) of Vigna radiata L. plant compared to control (20 ± 0.06) [61]. Moreover, plant growth-promoting bacterium Azospirillum brasilense strain 2A1 increased the root length (17.7 ± 2.9) of petunia plant compared to control (14.07 ± 2.9) [43].
Overall, the Georgenia soli strain TSm39 grew under alkaline pH and at high salinity. Furthermore, the Georgenia soli strain TSm39 showed various in vitro plant growth-promoting attributes, produced various extracellular enzymes, and displayed antibiotic and antimicrobial potential. Additionally, the strain TSm39 significantly enhanced the plant growth parameters of Vigna radiata L. under natural environmental conditions. The results indicate that the Georgenia soli strain TSm39 would enhance plant growth and crop quality. Moreover, it would protect plants from phytopathogens in the agricultural field.
References
Franco-Correa M, Quintana A, Duque C, Suarez C, Rodríguez MX, Barea JM (2010) Evaluation of actinomycete strains for key traits related with plant growth promotion and mycorrhiza helping activities. Appl Soil Ecol 45:209–217. https://doi.org/10.1016/j.apsoil.2010.04.007
Franco-Correa M, Chavarro-Anzola V (2016) Actinobacteria as plant growth promoting rhizobacteria. Actinobacteria-Basis and Biotechnological Application 249–270. https://doi.org/10.5772/61291
Alekhya G, Gopalakrishnan S (2016) Plant growth-promotion by Streptomyces spp. in sorghum (Sorghum bicolor L.). Afr J Biotechnol 15:1781–1788. https://doi.org/10.5897/AJB2016.15423
Kong Z, Glick BR (2017) The role of plant growth-promoting bacteria in metal phytoremediation. In: Poole RK (ed) Adv MicrobPhysiol 71:97–132. https://doi.org/10.1016/bs.ampbs.2017.04.001
Gupta G, Parihar SS, Ahirwar NK, Snehi SK, Singh V (2015) Plant growth promoting rhizobacteria (PGPR): current and future prospects for development of sustainable agriculture. J MicrobBiochem Technol 7:096–102. https://doi.org/10.4172/1948-5948.1000188
Jog R, Nareshkumar G, Rajkumar S (2012) Plant growth promoting potential and soil enzyme production of the most abundant Streptomyces spp. from wheat rhizosphere. J appl Microbiol 113:1154–1164. https://doi.org/10.1111/j.1365-2672.2012.05417.x
Majeed A, Abbasi MK, Hameed S, Imran A, Rahim N (2015) Isolation and characterization of plant growth-promoting rhizobacteria from wheat rhizosphere and their effect on plant growth promotion. Front Microbiol 6:198. https://doi.org/10.3389/fmicb.2015.00198
Minuto A, Spadaro D, Garibaldi A, Gullino ML (2006) Control of soil borne pathogens of tomato using a commercial formulation of Streptomyces griseoviridis and solarization. Crop Prot 25:468–475. https://doi.org/10.1016/j.cropro.2005.08.001
Qin S, Xing K, Jiang JH, Xu LH, Li WJ (2011) Biodiversity, bioactive natural products and biotechnological potential of plant-associated endophytic actinobacteria. Appl Microbiol Biot 89:457–473. https://doi.org/10.1007/s00253-010-2923-6
Suzuki SI, Yamamoto K, Okuda T, Nishio M, Nakanishi N, Komatsubara S (2000) Selective isolation and distribution of Actinomadurarugatobispora strains in soil. Actinomycetologica 14:27–33. https://doi.org/10.3209/saj.14_27
Ilic SB, Konstantinovic SS, Todorovic ZB, Lazic ML, Veljkovic VB, Jokovic N, Radovanovic BC (2007) Characterization and antimicrobial activity of the bioactive metabolites in Streptomycete isolates. Microbiol 76:421–428. https://doi.org/10.1134/S0026261707040066
Wang C, Wang Z, Qiao X, Li Z, Li F, Chen M, Wang Y, Huang Y, Cui H (2013) Antifungal activity of volatile organic compounds from Streptomyces alboflavus TD-1. FEMS Microbiol Lett 341:45–51.https://doi.org/10.1111/1574-6968.12088
Kalpesh I, I LK (2011) Ecologically important and life supporting plants of little Rann of Kachchh, Gujarat. J Ecol Nat 3:33–38
Goswami D, Dhandhukia P, Patel P, Thakker JN (2014) Screening of PGPR from saline desert of Kutch: growth promotion in Arachis hypogea by Bacillus licheniformis A2. Microbiol Res 169:66–75. https://doi.org/10.1016/j.micres.2013.07.004
Cappuccino JG, Sherman N (2005) Microbiology: a laboratory manual. Pearson/Benjamin Cummings, San Francisco 507
Gordon SA, Weber RP (1951) Colorimetric estimation of indole acetic acid. Plant Physiol 26:192. https://doi.org/10.1104/2Fpp.26.1.192
Schwyn B, Neilands JB (1987) Universal chemical assay for the detection and determination of siderophores. Anal Biochem 160:47–56. https://doi.org/10.1016/0003-2697
Mehta S, Nautiyal CS (2001) An efficient method for qualitative screening of phosphate-solubilizing bacteria. Curr Microbiol 43:51–56. https://doi.org/10.1007/s002840010259
Sandhu SS, Kanojiya A, Mishra PK and Vikrant P (2004) Antifungal activity of Bacillus thuringiensis var kurstaki on Trichoderma viridae. Proc Natl Acad Sci 74:303–304
Giorgi FM, Ceraolo C, Mercatelli D (2022) The R language: an engine for bioinformatics and data science. Life 12:648. https://doi.org/10.3390/life12050648
Han D, Wang L, Luo Y (2018) Isolation, identification, and the growth promoting effects of two antagonistic actinomycete strains from the rhizosphere of Mikania micranthaKunth. Microbiol Res 208:1–11. https://doi.org/10.1016/j.micres.2018.01.003
Radha TK, Rao DL, Sreeramulu KR (2017) Actinobacteria of arid and semi-arid soils: antagonism to fungal pathogens and plant growth promoting potential. J Pure Appl Microbiol 11:1045–1053. https://doi.org/10.22207/JPAM.11.2.47
Shrivastava P, Kumar R, Yandigeri MS (2017) In vitro biocontrol activity of halotolerant Streptomyces aureofaciens K20: a potent antagonist against Macrophominaphaseolina (Tassi) Goid. Saudi J Biol Sci 24:192–199. https://doi.org/10.1016/j.sjbs.2015.12.004
Laid B, Kamel K, Mouloud G, Manel S, Walid S, Amar B, Faiçal B (2016) Effects of plant growth promoting rhizobacteria (PGPR) on in vitro bread wheat (Triticum aestivum L.) growth parameters and biological control mechanisms. Adv Microbiol 6:677–690. https://doi.org/10.4236/aim.2016.69067
Anwar S, Ali B, Sajid I (2016) Screening of rhizospheric actinomycetes for various in-vitro and in-vivo plant growth promoting (PGP) traits and for agroactive compounds. Front Microbiol 29:1334. https://doi.org/10.3389/fmicb.2016.01334
Goudjal Y, Zamoum M, Sabaou N, Mathieu F, Zitouni A (2016) Potential of endophytic Streptomyces spp. for biocontrol of Fusarium root rot disease and growth promotion of tomato seedlings. Biocontrol Sci Tech 26:1691–1705. https://doi.org/10.1080/09583157.2016.1234584
Passari AK, Mishra VK, Gupta VK, Yadav MK, Saikia R, Singh BP (2015) In vitro and in vivo plant growth promoting activities and DNA fingerprinting of antagonistic endophytic actinomycetes associates with medicinal plants. PLoS One 10:0139468. https://doi.org/10.1371/journal.pone.0139468
Dinesh R, Anandaraj M, Kumar A, Bini YK, Subila KP, Aravind R (2015) Isolation, characterization, and evaluation of multi-trait plant growth promoting rhizobacteria for their growth promoting and disease suppressing effects on ginger. Microbiol Res 173:34–43. https://doi.org/10.1016/j.micres.2015.01.014
Qin S, Miao Q, Feng WW, Wang Y, Zhu X, Xing K, Jiang JH (2015) Biodiversity and plant growth promoting traits of culturable endophytic actinobacteria associated with Jatropha curcas L. growing in Panxi dry-hot valley soil. Appl Soil Ecol 93:47–55. https://doi.org/10.1016/j.apsoil.2015.04.004
Srivastava S, Patel JS, Singh HB, Sinha A, Sarma BK (2015) Streptomyces rochei SM 3 induces stress tolerance in chickpea against Sclerotinia sclerotiorum and NaCl. J Phytopathol 163:583–592. https://doi.org/10.1111/jph.12358
Gupta V and Ansari AA (2014) Geomorphic portrait of the Little Rann of Kutch. Arab. J Geosci 7:527–536. https://doi.org/10.1007/s12517-012-0743-y
Devi S, Kaundal K (2017) Assessment of culturable microbial diversity associated with Arnebiaeuchroma: a critically endangered plant growing in Trans-Himalayas of Himachal Pradesh, India. Int J Curr Microbiol Appl Sci 6:2953–2968. https://doi.org/10.20546/ijcmas.2017.609.363
Lozupone CA, Knight R (2007) Global patterns in bacterial diversity. Proc Natl Acad Sci 104:11436–11440. https://doi.org/10.1073/pnas.0611525104
El-Tarabily KA, Sham A, Elbadawi AA, Hassan AH, Alhosani BK, El-Esawi MA, AlKhajeh AS and AbuQamar SF (2021) A consortium of rhizosphere- competent actinobacteria exhibiting multiple plant growth-promoting traits improves the growth of Avicennia marina in the United Arab Emirates ront. Mar Sci 8:715123. https://doi.org/10.3389/fmars.2021.715123
Albdaiwi RN, Khyami-Horani H, Ayad JY, Alananbeh KM and Al-Sayaydeh R (2019) Isolation and characterization of halotolerant plant growth promoting rhizobacteria from durum wheat (Triticum turgidums ubsp. durum) cultivated in saline areas of the dead sea region. Front Microbiol 10:1639
Mbai FN, Magiri EN, Matiru VN, Nganga J, Nyambati VC (2013) Isolation and characterization of bacterial root endophytes with potential to enhance plant growth from Kenyan Basmati rice. Am Int J Contemp Res 3:25–40
Khamna S, Yokota A, Peberdy JF, Lumyong S (2010) Indole-3-acetic acid production by Streptomyces sp. isolated from some Thai medicinal plant rhizosphere soils. Eurasia J Biosci 4(1):23–32. https://doi.org/10.5053/ejobios.2010.4.0.4
Myo EM, GeB, Ma J, Cui H, Liu B, Shi L, Jiang M and Zhang K (2019) Indole-3-acetic acid production by Streptomyces fradiae NKZ-259 and its formulation to enhance plant growth. BMC Microbiol 19:1–14. https://doi.org/10.1186/s12866-019-1528-1
Gorai PS, Ghosh R, Mandal S, Ghosh S, Chatterjee S, Gond SK and Mandal NC (2021) Bacillus siamensis CNE6-a multifaceted plant growth promoting endophyte of Cicer arietinum L. having broad spectrum antifungal activities and host colonizing potential. Microbiol Res 252:126859. https://doi.org/10.1016/j.micres.2021.126859
Marques AR, Resende AA, Gomes FC, Santos ARO, Rosa CA, Duarte AA, de Lemos-Filho JP and Dos Santos VL (2021) Plant growth–promoting traits of yeasts isolated from the tank bromeliad Vrieseaminarum LB Smith and the effectiveness of Carlosrosaeavrieseae for promoting bromeliad growth. Braz. J. Microbiol 52:1417–1429. https://doi.org/10.1007/s42770-021-00496-1
Vessey JK (2003) Plant growth promoting rhizobacteria as biofertilizers. Plant Soil 255:571–586. https://doi.org/10.1023/A:1026037216893
Nozari RM, Ortolan F, Astarita LV and Santarém ER (2021) Streptomyces spp. enhance vegetative growth of maize plants under saline stress. Braz J Microbiol 52:1371–1383. https://doi.org/10.1007/s42770-021-00480-9
Toffoli LM, Martínez-Zamora MG, Medrano NN, Fontana CA, Lovaisa NC, Delaporte-Quintana P, Elias JM, Salazar SM, Pedraza RO (2021) Natural occurrence of Azospirillum brasilense in petunia with capacity to improve plant growth and flowering. J Basic Microbiol 61:662–673. https://doi.org/10.1002/jobm.202100064
Horstmann JL, Dias MP, Ortolan F, Medina-Silva R, Astarita LV, Santarém ER (2020) Streptomyces sp. CLV45 from Fabaceae rhizosphere benefits growth of soybean plants. Braz J Microbiol 51:1861–2187. https://doi.org/10.1007/s42770-020-00301-5
Vinayarani G, Prakash HS (2018) Growth promoting rhizospheric and endophytic bacteria from Curcuma longa L. as biocontrol agents against rhizome rot and leaf blight diseases. Plant Pathol J 34:218. https://doi.org/10.5423/PPJ.OA.11.2017.0225
Grant WD (1991) General view of halophiles. Superbugs. Microorganisms in extreme environments. Japan Scientific Societies Press, Tokyo, Japan 15–37
Mobini-Dehkordi M, Javan FA (2012) Application of alpha-amylase in biotechnology. J Biol Today’s World 1:39–50
Ribeiro IDA, BachE, da Silva Moreira F, Müller AR, Rangel CP, Wilhelm CM, Barth AL and Passaglia LMP (2021) Antifungal potential against Sclerotinia sclerotiorum (Lib.) de Bary and plant growth promoting abilities of Bacillus isolates from canola (Brassica napus L.) roots. Microbiol Res 248:126754. https://doi.org/10.1016/j.micres.2021.126754
Thumar JT, Singh SP (2007) Secretion of an alkaline protease from a salt-tolerant and alkaliphilic, Streptomyces clavuligerus strain MIT-1. Braz J Microbiol 38:766–772. https://doi.org/10.1590/S1517-83822007000400033
Maria-Jose C, Carmen V, Jurgen H, Antonio V, Joaquin JN (2000) Production and biochemical characterization of a K-amylase from the moderate halophile Halomonas meridian. FEMS Microbiol Lett 183:67–71. https://doi.org/10.1111/j.1574-6968.2000.tb08935.x
Geethu C, Resna AK, Nair RA (2013) Characterization of major hydrolytic enzymes secreted by Pythium myriotylum, causative agent for soft rot disease. Antonie Van Leeuwenhoek 104:749–757. https://doi.org/10.1007/s10482-013-9983-4
Gohel SD, Singh SP (2015) Thermodynamics of a Ca2+dependent highly thermostable alkaline protease from a haloalkliphilic actinomycete. Int J Biol macromol 72:421–429. https://doi.org/10.1016/j.ijbiomac.2014.08.008
Khatiwada P, Ahmed J, Sohag MH, Islam K, Azad AK (2016) Isolation, screening and characterization of cellulase producing bacterial isolates from municipal solid wastes and rice straw wastes. J Bioprocess Biotech 6:2–5. https://doi.org/10.4172/2155-9821.1000280
Arunachalam PS, Yang SH, Damodharan K, Suh JW (2013) Genetic and functional characterization of culturable plant-beneficial actinobacteria associated with yam rhizosphere. J Basic Microbiol 53:985–995. https://doi.org/10.1002/jobm.201200531
Xu XN, Chen LY, Chen C, Tang YJ, Bai FW, Su C, Zhao XQ (2018) Genome mining of the marine actinomycete Streptomyces sp. DUT11 and discovery of tunicamycins as anti-complement agents. Front Microbiol 9:1318. https://doi.org/10.3389/fmicb.2018.01318
Vu THN, Nguyen QH, Dinh TML, Quach NT, Khieu TN, Hoang H, Son C-K, Vu TT, Chu HH and Lee J (2020) Endophytic actinomycetes associated with Cinnamomum cassia Presl in HoaBinh province, Vietnam: distribution, antimicrobial activity and genetic features. J Gen Appl Microbiol 66:24–31. https://doi.org/10.2323/jgam.2019.04.004
Salam N, Khieu TN, Liu MJ, Vu TT, Chu-Ky S, Quach NT, Phi QT, Rao N, Prabhu M and Fontana A (2017) Endophytic actinobacteria associated with Dracaena cochinchinensis Lour.: isolation, diversity, and their cytotoxic activities. BioMed Res Intern 2017. https://doi.org/10.1155/2017/1308563
Dholakiya RN, Kumar R, Mishra A, Mody KH, Jha B (2017) Antibacterial and antioxidant activities of novel actinobacteria strain isolated from Gulf of Khambhat, Gujarat. Front Microbiol 8:2420. https://doi.org/10.3389/fmicb.2017.02420
Faria PSA, de Oliveira Marques V, Selari PJRG, Martins PF, Silva FG and de Fátima Sales J (2021) Multifunctional potential of endophytic bacteria from Anacardium othonianum Rizzini in promoting in vitro and ex vitro plant growth. Microbiol Res 242:126600. https://doi.org/10.1016/j.micres.2020.126600
Tchakounté GV, Berger B, Patz S, Fankem H, Ruppel S (2018) Community structure and plant growth-promoting potential of cultivable bacteria isolated from Cameroon soil. Microbiol Res 214:47–59. https://doi.org/10.1016/j.micres.2018.05.008
Uzma M, Iqbal A and Hasnain S (2022) Drought tolerance induction and growth promotion by indole acetic acid producing Pseudomonas aeruginosa in Vigna radiata. PloS One 17:0262932. https://doi.org/10.1371/journal.pone.0262932
Acknowledgements
JC gratefully acknowledges the DST-SERB (Department of Science and Technology — Science and Engineering Research Board) New Delhi, India, for the Junior & Senior Research Fellowship. The authors also acknowledged the infrastructural and financial support from Saurashtra University, Rajkot, Gujarat, India.
Funding
This work was supported by the DST-SERB (Department of Science and Technology — Science and Engineering Research Board), New Delhi, India (Sanction order no. ECR/2016/000928 Dated: 20.03.2017).
Author information
Authors and Affiliations
Contributions
JC carried out the laboratory work and drafted the first manuscript; SD conceptualized the study design, supervised the study, edited, and reviewed the manuscript. All the authors approved the submission of the final version of the manuscript for publication.
Corresponding author
Ethics declarations
Ethical approval
This article does not contain any studies with human participants or animals performed by any authors.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Fernando Andreote
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chauhan, J., Gohel, S. Exploring plant growth-promoting, biocatalytic, and antimicrobial potential of salt tolerant rhizospheric Georgenia soli strain TSm39 for sustainable agriculture. Braz J Microbiol 53, 1817–1828 (2022). https://doi.org/10.1007/s42770-022-00794-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-022-00794-2