Abstract
Purpose
Organophosphate pesticides (OPs) are among the most used insecticides in agriculture, causing the inhibition of esterases like acetylcholinesterase (AChE), butyrylcholinesterase (BChE) and carboxylesterase (CbE). Pesticides can reach the aquatic environment, posing risks to non-target organisms, including tadpoles.
Methods
In this work, we characterized the activities of AChE, BChE and CbE in tadpoles of the snouted treefrog Scinax fuscovarius, and verified their in vitro sensibility to different inhibitors [phenylmethane sulfonyl fluoride (PMSF), tetra-isopropylpyrophosphamide (iso-OMPA) and the OP diazinon]. In vivo effects of diazinon and esterase recovery after 2-pyridine-aldoxime (2-PAM) treatment of the protein extract were also studied in tadpoles with distinct stages of development exposed to 1 and 3 mg/l for 2 and 7 days.
Results
Optimal conditions were established for AChE and CbE; BChE activity was negligible. PMSF affected esterase activities and is not recommended for homogenization buffers. Iso-OMPA treatment caused no changes in AChE and CbE activities, but diazinon inhibited these enzymes in a dose-responsive manner. In vivo, CbE activity was insensitive to diazinon in younger tadpoles, but inhibited after 2 days of exposure in more developed tadpoles. AChE activity was inhibited after 2 and 7 days of exposure, in a dose-responsive manner. Esterase reactivation by 2-PAM was obtained both in vitro and in vivo.
Conclusions
(1) Tadpoles can be adequate sentinel organisms in biomonitoring studies of OP contamination; (2) AChE was more sensitive than CbE to diazinon; (3) tadpoles from earlier developmental stages seems to be less responsive to OPs; (4) AChE activity was sensitive to diazinon in both development stages, being a better OP biomarker.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Plagues and diseases are limiting factors in food production. To deal with this problem, pesticides are applied in agriculture, sometimes without any control (Sparling and Fellers 2007). The extensive use of pesticides after the Second World War allowed increases in food production, but also increased intoxication events, the contamination of soil, air and water, causing significant alterations in the characteristics of different environments.
Organophosphate pesticides (OPs) are one of the most extensively used classes of insecticides in agriculture. Their toxic effect involves the inhibition of esterases, such as acetylcholinesterase (AChE), butyrylcholinesterse (BChE) and carboxylesterase (CbE) (Vioque-Fernandez et al. 2007). AChE hydrolyses acetylcholine, an ester released to the synaptic cleft when a nervous impulse is transmitted from one cell to another (Bainy et al. 2006). When AChE is inhibited, acetylcholine accumulates leading to alterations in nervous function that can be lethal to organisms (Peakall 1996).
The physiological role of BChE is less known (Mason et al. 1995). It has been shown that this enzyme promotes the release of fatty acids from triacylglycerol and phospholipids, and can also act as a detoxification enzyme (Çokugras 2003). CbE catalyses the release of endogenous or exogenous short-chain fatty acids, which are involved in xenobiotic metabolism, promoting their detoxification (Maxwell 1992; Hyne and Maher 2003). Is has been proposed that CbEs are also involved in resistance and defense mechanisms of OP poisoning (Wheelock et al. 2008).
The inhibition of esterases in animals can be useful to indicate the presence of OPs and carbamates in the environment (Payne et al. 1996; Vioque-Fernandez et al. 2007; Galloway et al. 2002; Bonacci et al. 2004). It has been known that once inhibited by carbamates, the activity of esterases can be recovered by diluting protein extracts several times in homogenization buffer (Vioque-Fernandez et al. 2007). Nevertheless, those esterases inhibited by OPs can be recovered only after the treatment of protein extracts with 2-pyridine-aldoxime (2-PAM) (Sanz and Repetto 1994), since this compound has a higher affinity to the phosphate group of the OP than the enzyme, displacing it from the active site of the enzyme. Thus, the reversion of esterase inhibition after buffer dilution or 2-PAM treatment can be useful to distinguish between OP or carbamate exposure.
During rainy seasons, agricultural pesticides can reach aquatic environments, leading to toxic effects on non-target organisms. In tropical regions with marked seasons, the occurrence and reproduction of many species are restricted to the rainy periods, as in the case of anuran amphibians (Duellman and Trueb 1994; Aichinger 1987; Rossa-Feres and Jim 1994; Arzabe 1999; Eterovick and Sazima 2000; Prado et al. 2005). In our region (São José do Rio Preto, São Paulo, Brazil), the rainy period generally corresponds with the seasons between October and March (Rossa-Feres and Jim 2001). In the first life stages, anuran amphibians are exclusively gill breathing aquatic animals (tadpoles), and can be an example of non-target organisms subjected to the effects of pesticides in the environment, since occur in countless aquatic habitats and feed at many sites throughout the water column (Hoff et al. 1999).
The sensitivity of amphibians to different chemical substances has been demonstrated, including pesticides, herbicides, chemical fertilizers, organochloride compounds and others, but mechanisms underlying toxic events are not understood in this vertebrate group. The understanding of the effects of chemical contamination is quite relevant and urgent, faced with the global species loss and population declines among amphibians (Barinaga 1990; Blaustein and Wake 1995; Blaustein et al. 1994; Young et al. 2001). The global amphibian declines have been reported since the 1980s, as a consequence mostly of habitat destruction, pollution, global climate changes (Myers et al. 2000; Young et al. 2001; Bosch 2003), and of subtle factors acting in pristine habitats (Carey et al. 2001; Gardner 2001).
Moreover, tadpoles could be excellent bioindicator species for aquatic environmental monitoring, since they can be found in different freshwater environments including small or temporary ponds, where other aquatic animals, like fish, are not found. They have a low migration rate related to the spawning area, making these animals representative of the sampled area. Also, their physiology is well known, having great adaptative potential to stressing environments, and being relatively resistant to variations in abiotic factors like pH or low oxygen concentration (Semlitsch et al. 2000; Tejedo 2003).
Despite there having been several studies analyzing the effects and dose-response relationship of different chemical products on amphibians species (Tejedo 2003), studies related to the effects of OPs and carbamates on tadpole esterases are still scarce in the literature. Widder and Bidwell (2006) studied how chlorpyrifos exposure influenced cholinesterase (ChE) activity in R. sphenocephala tadpoles, verifying significant ChE inhibition after 12 days of exposure to 100 and 200 μg/l. They also observed a dose-dependent ChE decrease in four different tadpole species exposed to sublethal concentrations of chlorpyrifos (Widder and Bidwell 2008). Colombo et al. (2005) also observed a dose-dependent inhibition of AChE in Xenopus laevis tadpoles exposed to chlorpyrifos. These findings are consistent with the deleterious effects of carbamate pesticides (CPs) and OPs on cholinesterase activity; however, studies on other tadpole esterases, like CbE and BChE, are totally non-existent in the literature. A screening of how these enzymes respond to OPs and CPs could improve the knowledge of pesticide toxicity for tadpoles, so the aim of this work was to establish optimal conditions for measurement of different esterases in tadpoles of the snouted treefrog Scinax fuscovarius, and to verify how these enzymes respond to model inhibitors in vitro [phenylmethane sulfonyl fluoride (PMSF), tetra-isopropylpyrophosphamide (iso-OPMA), and the OP diazinon] and in vivo (diazinon exposure for 2 and 7 days).
2 Material and methods
2.1 Animal collection and exposure to diazinon
Scinax fuscovarius is widespread in southern, southeastern and central Brazil, as well as in eastern Bolivia, Paraguay, northern Argentina, and northern Uruguay, at 0–2,000 m above sea level (asl) (Aquino et al. 2004; Frost 2008). During breeding time, it can be found in standing water bodies in open habitats, such as pastures and other cultures, and presents tolerance of a broad range of habitats, being very common in urban areas, and inside houses (Aquino et al. 2004). Its tadpoles are very abundant through the rainy season (October to March), taking about 2 months to complete their larval development (Rossa-Feres and Jim 1996; Vasconcelos and Rossa-Feres 2005; Santos et al. 2007).
Two-hundred tadpoles of S. fuscovarius were collected in ponds near to São José do Rio Preto city (SP, Brazil) in January (summer) of 2008, and transported to the laboratory. The development stages of each animal at the beginning of the experiments were identified according to Gosner (1960), and the animals were placed in four plastic tanks containing 20 l of clear water, acclimating to the laboratory conditions for 1 week. Twenty animals from stages 25–29 were separated for the in vitro studies (establishment of optimal esterase assay conditions and inhibition-reactivation studies). Another 180 tadpoles were separated into two groups of 90 animals, being one group composed of animals from development stage 25 (here named “Stage 1”), and another group of animals from development stages 34–40 (“Stage 2”). Tadpoles grouped in Stage 1 did not start the formation of bud of hind limbs and those grouped in Stage 2 present different phases of growing of legs.
After the acclimatization period, the tadpoles were divided in groups of ten animals and transferred to 18 tanks containing 3 l of clear and de-chlorinated water from an artesian well. Thus, nine of the tanks contained just animals of Stage 1 and another nine tanks were composed by animals of Stage 2, and animals remained acclimating to this condition for 24 h. Before addition of chemicals to the exposure system, one tadpole from each tank was taken to serve as the time zero (T0) group, resulting in a remaining nine per exposure chamber. Three chambers for each Stage 1 and Stage 2 groups were designated as controls, receiving solvent only. For the remaining six experimental chambers, diazinon was added in two sublethal concentrations. So, for all the three treatments (control and two different diazinon concentrations), we had three replicates of nine animals. A previous study reported that the malathion (OP) concentration which killed 50% of the sample population (LC50) for Limnonectus limnocharis tadpoles was 13.3, 8.7, 6.3 and 5.4 mg/l, respectively, for 24, 48, 72 and 96 h exposure (Gurushankara et al. 2003). In addition, Relyea (2004) tested 1 and 2 mg/l of diazinon in different tadpole species, observing a 5 and 35% reduction, respectively, in survival after 16 days. Considering these previous data on pesticide toxicity to tadpoles, we chose the concentrations of 3 and 1 mg/l of diazinon for our exposure experiments. Thus, we had three replicates of treatments, exposed to 1 mg/l and exposed to 3 mg/l, for Stage 1 and Stage 2 animals.
The pesticide solution was prepared in a concentrated stock solution (10 mg/ml in ethanol) and added to the tanks in a final volume of 300 μl. A volume of 300 μl ethanol without diazinon was also added to the control tanks, in order to control for any potential effect due to solvent used. After 2 and 7 days of exposure, three animals were collected from each tank and directly frozen in liquid nitrogen (n = 9 for controls as well as for exposure to 1 mg/l and 3 mg/l, with three replicates for each treatment). During the whole experiment, animals were fed daily with commercial fish food offered ad libitum and the water was siphoned (500 ml) every 2 days in order to eliminate faeces and non-ingested food, being replaced by clear water containing the same treatment. Oxygenation was supplied by air pumps for all tanks, and the tanks were maintained at room temperature (∼25°C). No additional water quality parameter was measured during the experiment. All experiments were conducted in accordance with national and institutional guidelines for the protection of animal welfare.
2.2 Sample preparation and biochemical analyses
For esterase evaluation, tadpoles were entirely homogenized in 600 (Stage 1) or 1,000 (Stage 2) μl of 0.1 M Tris-HCl buffer, pH 8.0, and centrifuged for 30 min at 9,500 g. The supernatant fraction was then collected and frozen (−80°C) for later esterase evaluation. In order to verify the presence of membrane-bound esterases, extracts were also prepared with buffer containing 0.5% of the non-ionic detergent Triton X-100. Esterases were assayed according to Ellman et al. (1961). Acetyl- and butyryl-thiocholine and phenylthioacetate were used as substrates for AChE, BChE and CbE, respectively, and monitored by the release of thiol-derivatives at 412 nm. Assays were carried out in duplicate (exposure experiments) or triplicate (esterase characterization) in homogenization buffer with 1 mM DTNB [5-5′-dithiobis(2-nitrobenzoic acid)]. Blanks without substrates or samples were incubated at 25°C for 5 min to assess endogenous cross-reaction with DTNB. Assays were started by substrate addition. Specific activities were expressed as U/mg protein; where 1 U is the amount of enzyme hydrolyzing 1 μmol substrate per minute. Protein was determined according to Bradford (1976).
To test the effect of Triton X-100 on activity, an increasing concentration of this detergent was used in the assay buffer. Since the assay of a certain volume of extract yielded different activity depending on the enzyme concentration in each tissue, extract volumes were adjusted to obtain a change in absorbance (ΔAbs)/min at 412 nm of about 0.2–0.3 U, a range in which all assays were linear. Ten millimolar acetyl- or butyryl-thiocholine, and 4.5 mM phenylthioacetate was selected for routine assays of AChE, BChE and CbE, respectively. The pH and substrate concentration effect were also studied by varying the pH of the assay buffer from 6.0 to 10.0, and the respective substrate concentration from 0.5 to 10.0 mM. Esterase characterization was performed by using four pools of five Stage 1 tadpoles each, while data from exposure experiments were obtained by analyzing nine tadpoles individually.
2.3 Sensitivity of esterase assays to different compounds and 2-PAM reactivation
Extracts were incubated at room temperature in homogenization buffer containing 2–10 mM PMSF (a general esterase inhibitor), 0.5–2.5 mM iso-OMPA (a BChE inhibitor), or 0.001–10 mM diazinon. For the reactivation study, aliquots were incubated with 10 mM of diazinon for 15 min, and the esterase activities were measured. These aliquots were then ultrafiltered at 4°C in Millipore YM10 centricons at 12,000 g for 60 min to eliminate excess pesticide. The retentates were diluted to regain initial volume and incubated with 1 mM 2-PAM for 30 min. After the incubation period, esterases were measured again to access the recovery of the activity.
2.4 Statistical analyses
Statistical analyses were performed by means of the software Estatistic 6.0. Tests were performed to verify if the samples had normal distribution and similar variances, to apply analysis of variance (ANOVA). Data that did not present homogeneity in variance were submitted to the formula logn + 1, which converts values into normal and homogeneous data. So the ANOVA test for parametric data was applied, followed by the complimentary Fisher LSD test, comparing controls versus treatments. Data from the 2-PAM recovery experiment were statistically compared (with and without 2-PAM) by the t-test. Data are presented as mean ± standard deviation (SD). Significant differences between groups were detected considering p < 0.05.
3 Results
3.1 Characterization of esterase activities
The use of Triton X-100 detergent in the homogenization buffer did not accounted for differences in esterase activity compared with samples prepared without the detergent (Fig. 1). Optimum protein extract volumes for the assays of AChE, BChE and CbE were 20, 40 and 10 μl, respectively. The activities of AChE and CbE were similar, but BChE had ten- to 15-fold lower activity when compared with the other two esterases, even using large volumes of protein extracts. This indicates that BChE activity is negligible in tadpoles, so we excluded this enzyme in subsequent experiments. The presence of Triton X-100 did not cause variations in esterase activity, but this detergent was adopted for subsequent analyses because it caused a greater ΔAbs/min at 412 nm than values obtained without detergent. Although use of Triton X-100 resulted in the extraction of more protein (data not shown), the esterase activities were similar when normalized to protein levels.
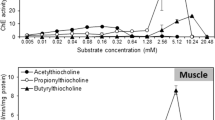
With regard to the effect of pH on the activities of AChE and CbE, both activities increased from pH 6.0 to 9.0, and decreased with pH > 9.0, as shown in Fig. 2a. Actually, the spectrophotometric measurement of AChE activity in pH > 9.0 was not possible, because the assay medium turned strongly yellowish. The effects of pH on substrate auto-hydrolysis are also shown in Fig. 2. Changing from pH 6.0 to 8.0 did not cause substrate hydrolysis, but auto-hydrolysis increased strongly with pH increasing from 8.0 to 10.0.
Aa: Effect of pH on AChE and CbE activities. b: Auto-hidrolysis rate for the substrates of CbE (phenilthioacetate) and AChE (acetylthiocholine) in different pH. B Effect of increasing substrate concentration on AChE and CbE activities. Each value represents the mean ± SD of four pools of five animals, each done in triplicate
The effects of increasing substrate concentration on AChE and CbE activities are shown in Fig. 2b. AChE activity increased according to the substrate concentration and reached a steady state (Vmax) with high substrate concentration. These data allowed the calculation of apparent KM (0.68 mM), by Lineweaver-Burk plot. Nevertheless, CbE had a different behaviour, and no such Vmax was obtained. This could indicate that there are several enzymes with CbE activity with different KM values for the substrate. More than 10 mM of phenylthioacetate substrate rapidly turned the solution very yellowish, with absorbance values higher than 2 units, so it was impossible to test higher substrate concentration for CbE activity.
3.2 In vitro inhibition tests
The effects of PMSF, a general esterase inhibitor, and iso-OMPA, a BChE inhibitor, are depicted in Fig. 3. As shown, PMSF caused an increase in AChE activity, while CbE was decreased in the presence of different PMSF concentrations. On the other hand, iso-OMPA did not cause any interference with both esterases.
The in vitro inhibition of esterases by increasing concentrations of diazinon is shown in Fig. 4a. Both enzymes were inhibited in a dose-responsive manner, but AChE reached 100% inhibition at 1 mM diazinon, while CbE needed 10 mM of the pesticide to present total inhibition.
A Effect of increasing concentration of diazinon on CbE and AChE activities. B Reactivation profiles of CbE and AChE after diazinon incubation (10 mM) and 2-PAM treatment, in comparison with untreated protein extract (control). Each value represents the mean ± SD of four pools of five animals, each done in triplicate
3.3 Reactivation study
Data from the esterase reactivation study are described in Fig. 4b. Esterases were measured in protein extracts without pesticide and after incubation with 10 mM diazinon. As shown, 10 mM diazinon completely inhibited both esterases. These extracts were filtered to eliminate pesticide excess and incubated with 1 mM 2-PAM. AChE activity recovered by ~40% after 2-PAM treatment, while CbE activity had ~75% reactivation with the same treatment.
3.4 In vivo effects of diazinon on tadpole esterases
No mortality was observed during the experiments. The average length of tadpoles from Stage 1 and Stage 2 control groups at the beginning of the experiments were 1.33 ± 0.20 and 2.22 ± 0.36 cm, respectively. The average length of tadpoles from Stage 1 and Stage 2 exposed to 1 mg/l of diazinon at the beginning of the experiment were 1.35 ± 0.15 and 2.28 ± 0.34 cm, respectively, while for those tadpoles exposed to 3 mg/l, the length were 1.24 ± 0.13 and 2.38 ± 0.31 cm, respectively.
The activity of CbE in Stage 1 animals had no effects when exposed to 1 or 3 mg/l of diazinon for 2 or 7 days (Fig. 5). Nevertheless, the animals from Stage 2 presented a decrease in CbE activity after 2 days of 1 and 3 mg/l diazinon exposure. After 7 days, CbE activity was similar to control values.
With respect to AChE activity, diazinon was able to inhibit the enzyme in both groups of animals from Stage 1 and Stage 2. The inhibition of AChE was more pronounced and dose-dependent after 2 days of exposure. After 7 days, tadpoles presented a tendency to recover from inhibition, with those animals exposed to the lower doses presenting AChE activity similar to control values, and only those exposed to the higher dose remaining inhibited. This result could be an effect of the additional diazinon in chambers, considering that water containing diazinon was renewed (0.5 l) every 2 days along the experiment. It would also be speculated that the lack of AChE inhibition after 7 exposure days in animals exposed to the lower dose could be due to the absorbance of the pesticide to surface particles that were syphonated during the experiment, eliminating them from the chambers, and this should be considered for future experiments since it can limit the interpretability of the results.
When those extracts from Stage 2 animals presenting CbE inhibition were incubated with 1 mM 2-PAM for 30 min, CbE activity was recovered to control values (Fig. 6). Recovery was also observed for AChE activity, but to a lesser extent in comparison with CbE, except for animals from Stage 1, which had a higher recovery rate. Indeed, AChE recuperation was not obtained for Stage 2 animals exposed to 3 mg/l diazinon for 7 days.
CbE and AChE reactivation after 2-PAM treatment of protein extracts of tadpoles exposed in vivo to 1 and 3 mg/l of diazinon for 2 and/or 7 days; * indicates statistical difference (p < 0.05) between extracts treated with 2-PAM and the respective untreated extract. Only those tadpoles with a significant in vivo decrease on esterase activity were tested; n = 9 for each experimental group
4 Discussion
The presence of OPs and CPs in the environment can lead to esterase inhibition in living organisms, leading to adverse metabolic effects. So, the evaluation of esterase inhibition can be useful to indicate the presence of these compounds in the environment, and this approach is extensively used in biomonitoring studies of aquatic contamination, mainly in fishes (Fulton and Key 2001), bivalves (Galloway et al. 2002; Bonacci et al. 2004) and crustaceans (Barata et al. 2004; Vioque-Fernandez et al. 2007). Similar data for amphibians are scarce (e.g. Marco 2003), but promising in this context, thus we were interested in evaluating esterase inhibition in tadpoles of a Brazilian anuran species.
It has been shown that the presence of pesticides in the aquatic environment is responsible for significant alterations in tadpole metabolism as well. The decline of many amphibian populations has been associated with pesticide exposure, although little is known about pesticide toxicity in amphibians (Relyea 2004). Gurushankara et al. (2003) observed a decrease in food consumption, growth and development in Limnonectus limnocharis tadpoles exposed to increasing concentrations of the OP malathion. Widder and Bidwell (2006) also reported lower body mass for Rana sphenocephala tadpoles, after chlorpyrifos (OP) exposure. According to Relyea (2004), malathion was moderately toxic for six tadpole species, with LC50 values ranging from 1.25–5.90 mg/l according to the exposure period, but some species can be more susceptible than others to this compound. When exposed to CPs, tadpoles also present great variations in growth, body mass, time for metamorphosis, survival, and other parameters (Boone and Bridges 2003). Despite the relevance of changes in these parameters, they were not evaluated in the present work as the primary focus was on the development and validation of esterases in this species.
Both AChE and CbE were very active in tadpoles independent on development stage. AChE values found for S. fuscovarius are in accordance to values reported for tadpoles of other species, like Xenopus laevis, Hyla chrysoscelis, Rana sphenocephala (= Lithobates sphenocephalus), Acris crepitans, and Gastrophryne olivacea, where activities ranged from 0.2 to 5.0 U/mg of protein (Widder and Bidwell 2008; Colombo et al. 2005). On the other hand, BChE activity was almost undetectable in S. fuscovarius, even using great amounts of protein extracts in the assay. These data indicate that this enzyme is not significantly active in tadpoles, and thus unsuitable as an environmental biomarker for pesticide exposure. The absence of iso-OMPA effect on AChE activity also indicates that there is no interference of BChE, so we can assume that the ChE activity for acetylthiocholine substrate is exclusively due to AChE. Due to its negligible activity, BChE was not measured in subsequent experiments.
Enzyme kinetics showed that AChE activity is due to the presence of a single protein enzyme with apparent KM of 0.68 mM for acetylthiocholine, calculated by the Lineweaver-Burk plot. This value was higher than the apparent KM calculated for Xenopus laevis (0.3 mM) by Gindi and Knowland (1979), which indicates that AChE kinetics can variate significantly among different species. The calculation of KM value for CbE was not possible because enzyme did not reach a steady state, even at the highest substrate concentration (10 mM). However, enzyme activity levelled out at a substrate concentration of between 3 and 6 mM, indicating that CbE activity could be characterized by multiple enzyme isoforms, each one with a different KM value for the substrate used. In fact, a zymogram of S. fuscovarius esterases after staining with α- and β-naftyl acetate reveals at least five esterasic bands (data not shown).
Buffer pH variations indicated that pH 9.0 would be optimum for both AChE and CbE measurements. However, the substrate auto-hydrolysis increased significantly from pH 8.0 to 10.0, indicating that the higher esterase activity at pH 9.0 could be attributed to the high substrate auto-hydrolysis rate. So, the ideal pH for both enzymes was considered 8.0, with less substrate auto-hydrolysis interference.
PMSF has been described as a general esterase inhibitor, despite some works having reported increases or no effect on esterase in different model animals. Vioque-Fernandez et al. (2007) observed that PMSF caused a dose-dependent AChE and BChE inhibition in muscle, nervous tissue and digestive gland of the red crayfish, Procambarus clarkii. However, CbE was only inhibited in the digestive gland. In the present work, PMSF has a increasing and a decreasing effect, respectively, for AChE and CbE activities of tadpoles. So the use of this compound as protease inhibitor during extract preparation should be avoided.
When tadpole protein extracts were incubated with increasing concentrations of the OP diazinon, it was clear that CbE was less responsive than AChE for this pesticide. While AChE was completely inhibited by 1 mM of the pesticide, CbE was totally inhibited just by 10 mM diazinon. So, it is possible that events with low OP contamination can affect AChE while CbE remains non-responsive, which indicates AChE activity as a better OP biomarker than CbE in tadpoles. It is possible that the greater CbE resistance to inhibition compared with AChE could be due to the presence of multiple CbE isoforms, as previously commented.
Also interesting, was that both the inhibited AChE and CbE recovered after 2-PAM treatment, with CbE presenting a better recovery rate than AChE. This result could be related to the greater CbE resistance to diazinon. As the enzymes were previously inhibited with 10 mM diazinon, and AChE was totally inhibited by 1 mM diazinon while CbE was not, AChE recovery would be more difficult after 2-PAM treatment. Evidently, 2-PAM has a higher affinity for the phosphate group of the OP, displacing it from the active site of the enzyme. It would be necessary for a higher amount of 2-PAM in the assay to reduce OP below 1 mM (when the enzyme is totally inhibited) in order to re-activate AChE, but this was not tested. Concentrations of 2-PAM higher than 1 mM in the reaction media could interfere with the assay.
The experiments of in vivo tadpole exposure to diazinon corroborated the greater resistance of CbE to diazinon in comparison with AChE, since inhibition was only observed in animals from Stage 2 exposed for 2 days to 1 and 3 mg/l diazinon. Also, the absence of CbE inhibition in animals of Stage 1 indicates that CbE susceptibility to this OP can vary along development, so the observation of developmental stages in field studies on the evaluation of esterases in tadpoles as pesticide biomarkers is greatly recommended.
The greater resistance of CbE to diazinon related to AChE should not be taken as a rule for all pesticides. Vioque-Fernandez et al. (2007) observed that AChE was more resistant to chlorpyrifos than CbE in red crayfish, but malathion (another OP) inhibited AChE completely, while no effects were observed in CbE. So, the potential inhibition effect of different OPs on tadpole esterases should be tested, to predict the effect of specific OPs in field animals.
Also, it should be considered that OPs exist in thionic or oxonic forms, and only the oxonic form is capable of inhibiting esterase (Sanz and Repetto 1994). Thionic OPs could be converted to the oxonic form by biotransformation enzymes, and then be able to inhibit esterases. Sparling and Fellers (2007) demonstrated that oxon derivatives of thionic OPs present higher toxicity to larval Rana boylii. It is possible that less-developed tadpoles had not yet developed the enzymes needed to produce the oxonic form of diazinon, which could explain their lesser responsiveness to this pesticide compared with more developed tadpoles. Jung et al. (2004) demonstrated that tadpoles of the green frog (Rana clamitans melanota) have about a tenfold lower EROD activity (indicative of cytochrome P4501A) compared with metamorphs, supporting our hypothesis. It is possible that other CYP isoforms responsible for the conversion of the thionic form of diazinon into the toxic oxonic form are not fully induced in early stages of tadpole development, but this remains to be clarified.
The dose-dependent inhibition of AChE of tadpoles from Stages 1 and 2 could indicate that this enzyme is a better exposure biomarker for diazinon and other OPs than CbE. However, it should be considered that AChE inhibition was more pronounced after 2 days of exposure, as tadpoles presented a tendency for recovery after 7 days of exposure. Same recuperation was observed for CbE activity of Stage 2 tadpoles. As the diazinon treatment was renewed every 2 days, this recovery seems to be more related to a de novo synthesis of the enzymes to deal with the initial inhibition. It is possible that the diazinon sub-lethal concentrations tested allowed animals to recover esterase activity over time, which would be not possible with doses higher than LC50 values, but this remains to be tested.
Widder and Bidwell (2006) tested lower OP doses (100 and 200 µg/l chlorpyrifos) for 4 and 12 days in R. sphenocephala (L. sphenocephalus) tadpoles, and also observed a dose-responsive AChE inhibition. With respect to CbE, there are no reports on this enzyme in tadpoles, so comparisons can not be done. On the other hand, our results indicate that this enzyme has great potential as an exposure biomarker in field studies with tadpoles.
Interestingly, esterase activity was recovered after 2-PAM treatment in those animals exposed to diazinon. It has been shown that, despite in vitro reactivation generally obtained in different model animals, in vivo the same profile of 2-PAM reactivation is difficult to observe. It seems that the irreversible esterase inhibition leads animal to eliminate the enzymes from cells, so reactivation is not obtained. In the case of tadpoles, reactivation was successfully obtained for all animals presenting esterase inhibition, except those that presented AChE inhibition after 7 days of exposure to 3 mg/l of diazinon, which would indicate an initial process of enzyme degradation. These data indicate the use of 2-PAM treatment of protein tadpole extracts as a potential tool in field biomonitoring studies, to attribute the esterase inhibition to OP exposure. Although CP-inhibited esterases can be recovered after extract dilution, they can not be recovered with 2-PAM treatment. However, it should be mentioned that this tool would function only for animals exposed for short periods, since it is possible that esterase inhibition due to OP exposure longer than 7 days causes enzyme degradation or aging, making recovery studies impossible.
Considering that there are very scarce data on pollution biomarker evaluation in tadpoles, and that this work represents the first approach in these organisms by our group, we were not concerned initially about the effects of this pesticide on morphological or physiological parameters of the animals, but in esterase responses. However, some slight discrepancies could be observed at the end of exposure experiments with respect to the growth and development of the animals, which were not recorded in the present work. Despite using animals of the same developmental stages in same experimental group at the beginning of the experiment, this did not guarantee that animals would develop equally over time within the same group, especially in those groups exposed to the contaminant. It has been shown, for example, that population density can affect development rate in tadpoles, and a more pronounced effect could be expected in animals exposed to environmental contaminants. These aspects are important and should be considered in future pollutant exposure experiments with tadpoles.
Finally, although our results indicated that the OP diazinon promotes a dose-responsive inhibition of esterases in tadpoles over time, it should be considered that only part of the water (0.5 l) containing the contaminant at the proper concentration for the experimental group was changed every 2 days during the experiment. Probably, this is not the best procedure for water renewal, since the correct dose at each exposure time is not assured, considering diazinon degradation could occur in water not renewed in the time versus new pesticide added every 2 days in a lesser volume that will be diluted in the remaining water. We just adopted a procedure concerning the elimination of uneaten food and faeces in water avoiding stress and death of tadpoles, caused by bacteria and fungi that can grow in leftover food and feces deposited at the bottom of the chambers (Rossa-Feres, personal observation; Mcdiarmid and Altig 1999). It might be suggested that the whole of the water should be changed every 2 days, but this could be a stressing condition for tadpoles, and in such a case a single-dose effect should not be considered, also interfering with the results. Another possibility is not to change the water during experiment, but in this case it is strongly suggested that water quality parameters should be measured, considering that variations in pH and ammonium levels, for example, can be expected due to the accumulation of excretion products from the animals and decomposition of uneaten food, which in this work were also not measured. However, although an ideal experimental design has not yet been reached, the apparent dose-response esterase inhibition seen in our results provides some interesting new information that is likely to be of value to other scientists working in the area of amphibian toxicology.
5 Conclusions
In this work, we characterized the kinetics of different esterases in S. fuscovarius tadpoles, obtaining optimal conditions for AChE and CbE measurements; BChE was negligible. The in vitro inhibition tests indicated that AChE is more sensitive to inhibition than CbE, and would be more indicative of OP exposure, which was also confirmed in animals exposed in vivo to diazinon. Indeed, animals from earlier developmental stages seem to be more resistant to diazinon exposure. Treatment with 2-PAM was able to recover diazinon-inhibited esterases, both in vivo and in vitro, indicating this procedure to be a potential tool to attribute esterase inhibition due to OP exposure. Taking all these data into account, tadpoles appear to be excellent sentinel organisms in environmental studies for the presence of OPs in the aquatic environment.
References
Aichinger M (1987) Annual activity patterns of anurans in a seasonal neotropical environment. Oecologia 71:583–592
Aquino L, Bastos R, Reichle S, Silvano D, Baldo D, Langone J (2004) Scinax fuscovarius. In: IUCN 2008, 2004. 2008 IUCN Red List of Threatened Species. <www.iucnredlist.org>. Downloaded on 26 January 2009
Arzabe C (1999) Reproductive activity patterns of anurans in two different altitudinal sites within the Brazilian Caatinga. Rev Bras Zool 16(3):851–864
Bainy ACD, Medeiros MHG, Di Mascio P, Almeida EA (2006) In vivo effects of metals on the acetylcholinesterase activity of the Perna perna mussel’s digestive gland. Biotemas 19:35–39
Barata C, Solayan A, Porte C (2004) Role of B-esterases in assessing toxicity of organophosphorus (chlorpyrifos, malathion) and carbamate (carbofuran) pesticides to Daphnia magna. Aquat Toxicol 66:125–139
Barinaga M (1990) Where have all the froggies gone? Science 247:1033–1034
Blaustein AR, Wake DB (1995) The puzzle of declining amphibian populations. Sci Am 272:52–57
Blaustein AR, Wake DB, Sousa WP (1994) Amphibian declines: judging stability, persistence, and susceptibility of populations to local and global extinctions. Conserv Biol 8:60–71
Bonacci S, Browne MA, Dissanayake A, Hagger JA, Corsi I, Focardi S, Galloway TS (2004) Esterase atcivities in the bivalve mollusc Adamussium colberki as a biomarker for pollution monitoring in the Antartic marine environment. Mar Pollut Bull 49:445–455
Boone MD, Bridges CM (2003) Effects of carbaryl on green frog (Rana clamitans) tadpoles: timing of exposure versus multiple exposures. Environ Toxicol Chem 22(11):2695–2702
Bosch J (2003) Nuevas amenazas para los anfibios: enfermedades emergentes. Munibe, Suplement (16):56–73
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Carey C, Heyer WR, Wilkinson J, Alford RA, Arntzen JW, Halliday T, Hungerford L, Lips KR, Middleton EM, Orchard SA, Rand AS (2001) Amphibian declines an environmental change: use of remote-sensing data to identify environmental correlates. Conserv Biol 15:903–913
Çokugras NA (2003) Butyrylcholinesterase: Structure and Physiological Importance. Turk J Biochem 28(2):54–61
Colombo A, Orsi F, Bonfanti P (2005) Exposure to the organophosphate pesticide chlorpyrifos inhibits acetylcholinesterase activity and affects muscular integrity in Xenopus laevis larvae. Chemosphere 61:1665–1671
Duellman WE, Trueb L (1994) Biology of amphibians. Johns Hopkins University Press, Baltimore
Ellman GL, Courtney KO, Andress V, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Eterovick PC, Sazima I (2000) Structure of an anuran community in a montane meadow in southeastern Brazil: effects of seasonality, habitat, and predation. Amphib-Reptil 21:439–461
Frost DR (2008) Amphibian Species of the World: an Online Reference. Version 5.2 (15 July, 2008). Electronic Database accessible at http://research.amnh.org/herpetology/amphibia/index.php. American Museum of Natural History, New York. Downloaded on 26 January 2009
Fulton MH, Key PB (2001) Acetylcholinesterase inhibition in estuarine fish and invertebrates as an indicator of organophosphorus insecticide exposure and effects. Environ Toxicol Chem 20(1):37–45
Galloway TS, Millward N, Browne MA, Depledge MH (2002) Rapid assessment of organophosphorous/carbamate exposure in the bivalve mollusc Mytilus edulis using combined esterase activities as bimarkers. Aquat Toxicol 61:169–180
Gardner T (2001) Declining amphibian populations: a global phenomenon in conservation biology. Anim Biodiv Conserv 24(2):25–44
Gindi T, Knowland J (1979) The activity of cholinesterase during the development of Xenopus laevis. J Embryol Exp Morphol 51:209–215
Gosner KL (1960) A simplified table for staging anuran embryos and larvae with notes on identication. Herpetologica 16:183–190
Gurushankara HP, Vasudev V, Krishnamurthy SV (2003) Estimation of acute toxicity of malathion insecticide on tadpoles and adults of Rana (Limnonectus) limnocharis. Indian J Comp Anim Physiol 21:48–54
Hoff KS, Blaustein AR, McDiarmid RW, Altig R (1999) Behavior. Interactions and their consequences. In: McDiarmid RW, Altig R (eds) Tadpoles – the biology of anuran larvae. Chicago, The University of Chicago Press, pp 215–239
Hyne RV, Maher WA (2003) Invertebrate biomarkers: links to toxicosis that predict population decline. Ecotoxicol Environ Saf 54:366–374
Jung RE, Karasov WH, Melancon MJ (2004) Cytochrome P450 Activity in green frogs (Rana clamitans melanota) exposed to water and sediments in the Fox River and Green Bay, Wisconsin, USA. Bull Environ Contam Toxicol 73:955–962
Marco A (2003) Impacto de radiación ultravioleta y contaminación em anfíbios. Munibe Suplement (16):44–55
Mason RP, Reinfelder JR, Morell FMM (1995) Bioaccumulation of mercury and methylmercury. Water Air Soil Pollut 80:915–921
Maxwell DM (1992) Detoxification of organophosphorus compounds by carboxylesterase. In: Chambers JE, Levi PE (eds) Organophosphates: chemistry, fate and effects. Academic Press, San Diego, p 183
McDiarmid R, Alti, R. (1999) Research. Material and Techniques. In: McDiarmid RW, Altig R 756 (eds) Tadpoles – the biology of anuran larvae. Chicago, The 757 University of Chicago Press, p 7–23.
Myers N, Mittermeier RA, Mittermeier CG, Fonseca GAB, Kent J (2000) Biodiversity hotspots for conservation priorities. Nature 403:853–858
Payne JF, Mathieu A, Melvin W, Francey LL (1996) Acetylcholinesterase, an old biomarker with new future? Field trials in association with two urban rivers and paper mill in Newfoundland. Mar Pollut Bull 32:225–231
Peakall DB (1996) Disrupted patterns of behavior in natural populations as an index of ecotoxicity. Environ Health Perspect 104(2):331–335
Prado CPA, Uetanabaro M, Haddad CFB (2005) Breeding activity patterns, reproductive modes, and habitat use by anurans (Amphibia) in a seasonal environment in the Pantanal, Brazil. Amphib-Reptil 26(2):211–221
Relyea RA (2004) Synergistic impacts of malathion and predatory stress on six species of North American tadpoles. Environ Toxicol Chem 23(4):1080–1084
Rossa-Feres DC, Jim J (1994) Distribuição sazonal em comunidades de anfíbios anuros na região de Botucatu, São Paulo. Rev Bras Biol 54(2):323–334
Rossa-Feres DC, Jim J (1996) Distribuição espacial em comunidade de girinos na região de Botucatu, São Paulo (Amphibia, Anura). Rev Bras Biol 56(2):309–316
Rossa-Feres DC, Jim J (2001) Similaridade no sítio de vocalização em uma comunidade de anfíbios anuros na região noroeste do Estado de São Paulo, Brasil. Rev Bras Zool 18(2):439–454
Santos TG, Rossa-Feres DC, Casatti L (2007) Diversidade e distribuição espaço-temporal de anuros em região com pronunciada estação seca no sudeste do Brasil. Iheringia Sér Zool 97:37–49
Sanz P, Repetto M (1994) Toxicology. Díaz de Santos, Madrid
Semlitsch RD, Bridges CM, Welch AM (2000) Genetic variation and a fitness tradeoff in the tolerance of gray treefrog (Hyla versicolor) tadpoles to the insecticide carbaryl. Oecologia 125:179–185
Sparling DW, Fellers G (2007) Comparative toxicity of chlorpyrifos, diazinon, malathion and their oxon derivatives to larval Rana boylii. Environ Pollut 147:535–539
Tejedo M (2003) El declive de los anfibios. La dificuldad de separar las variaciones naturales del cambio global. Munibe Suplement (16):20–43
Vasconcelos TS, Rossa-Feres DC (2005) Diversidade, distribuição espacial e temporal de anfíbios anuros (Amphibia, Anura) na região noroeste do Estado de São Paulo. Biota Neotropica 5(2): http://www.biotaneotropica.org.br/v5n2/pt/abstract?article+BN01705022005
Vioque-Fernandez A, Almeida EA, López-Barea J (2007) Esterases as pesticide biomarkers in crayfish (Procambarus clarkii, Crustacea): tissue distribution, sensitivity to model compounds and recovery from inactivation. Comp Biochem Physiol C 145:404–412
Wheelock CE, Phillips BM, Anderson BS, Miller JL, Miller MJ, Hammock BD (2008) Applications of carboxylesterase activity in environmental monitoring and toxicity identification evaluations (TIEs). Rev Environ Contam Toxicol 195:117–178
Widder PD, Bidwell JR (2006) Cholinesterase activity and behavior in chlorpyriphos-exposed Rana sphenocephala tadpoles. Environ Toxicol Chem 25(9):2446–2454
Widder PD, Bidwell JR (2008) Tadpole size, cholinesterase activity, and swim speed in four frog species after exposure to sub-lethal concentration of chlorpyrifos. Aquat Toxicol 88:9–18
Young BE, Lips KR, Reaser JK, Ibánez R, Salas AW, Cedeno JR, Coloma LA, Santiago R, La Marca E, Meyer JR, Munoz A, Bolanos F, Chaves G, Romo D (2001) Population declines and priorities for amphibian conservation in Latin América. Conserv Biol 15:1213–1223
Acknowledgements
This work had the financial support of the “Fundação de Amparo à Pesquisa do Estado de São Paulo” (FAPESP, Brazil, 2006/03873-1; 2004/04820-3).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Markus Hecker
Rights and permissions
About this article
Cite this article
Leite, P.Z., Margarido, T.C.S., de Lima, D. et al. Esterase inhibition in tadpoles of Scinax fuscovarius (Anura, Hylidae) as a biomarker for exposure to organophosphate pesticides. Environ Sci Pollut Res 17, 1411–1421 (2010). https://doi.org/10.1007/s11356-010-0326-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-010-0326-y