Abstract
Purpose
This study correlated the heart rate (HR) dynamics and parasympathetic activity at rest, during and after a resistance exercise (RE) session with resting heart rate (RHR) in young men.
Methods
The RR-interval series was recorded at rest in the supine (sup) and following the postural change (from supine to orthostatic position) in the orthostatic (ort), as well as during and after 15 min of a RE session in 14 young men. We used RHR value in the supine position and the square root of the mean of the square of successive adjacent R-R intervals difference (rMSSD) as the parasympathetic indexes. The statistical analysis employed Pearson and Spearman correlation tests with a two-tailed p value ≤ 0.05.
Results
We observed that HRort, relative variation (∆%) of RHR, and HR and relative (%) heart rate recovery (HRR) at five, ten, and fifteen minutes were correlated with RHR (r = from − 0.80 to 0.89, p ≤ 0.02). At rest, rMSSDsup, rMSSDort, and absolute variation (∆) rMSSD were correlated with RHR (rs = from − 0.53 to − 0.82, p ≤ 0.049); during RE session, we observed a correlation between ∆rMSSD and ∆%rMSSD with RHR (rs = − 0.82 to − 0.68, p ≤ 0.01) and after a RE session, rMSSD5–15 min were correlated with RHR (rs = from − 0.75 to − 0.86, p ≤ 0.01).
Conclusion
We concluded that young men with lower RHR showed higher HR dynamics and parasympathetic reactivity at rest, higher parasympathetic withdrawal during the RE session, and higher HRR and parasympathetic reactivation after a RE session.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The resting heart rate (RHR), evaluated in the supine position, is a low-cost, noninvasive measure, and its recording feasibility across a large range of settings, with the technique offering a way to individually screen for risk factors with unfavorable prognoses, such as increased cardiovascular morbimortality and sudden death [1]. In addition, RHR is also often used in the clinical setting to assess the relative strength of parasympathetic activity on sympathovagal balance in the heart [2].
Traditionally, resistance exercise (RE) session consists of movements being performed against a load, ranging from body weight to external weighted equipment, comprising repetitions before muscle exhaustion [3]. In this scenario, during the RE session, the heart rate (HR) increases due to parasympathetic withdrawal and sympathetic activation [4]. However, following the exercise in the first five minutes (the fast phase), the short-term HR adjustment responds to rapid parasympathetic reactivation; from the five minutes onwards (the slow phase), it is more dependent on parasympathetic reactivation with simultaneous sympathetic deactivation [4]. Thus, an increase the HR and heart rate recovery (HRR) have been proposed as prognostic measures of cardiovascular morbimortality in individuals with distinct clinical and functional conditions [5].
Hence, considering a high RHR, a delayed increase in HR and a slow HRR are associated with reduced parasympathetic activity, withdrawal impairment, and parasympathetic reactivation, respectively [1, 5]. So, it is reasonable to expect that these chronotropic and parasympathetic adjustments during, and after exercise may be interrelated with a RHR measure.
Studies have investigated the association between resting parasympathetic activity by heart rate variability (HRV) with HRR [6,7,8,9,10,11,12,13,14,15,16,17]. Some studies have shown a significant association between resting parasympathetic activity with HRR after a maximal (MET) or submaximal exercise test (SET) [8, 10, 14,15,16, 18] and after aerobic exercise (AE) [7, 17]. Only one study showed an association between RHR with HRR after the SET [11]. On the other hand, other studies have shown no significant correlation between resting parasympathetic activity with HRR and parasympathetic activity after MET or AE [6, 9, 12, 13].
Indeed, due to the different approaches used in those studies above to evaluate resting status, inconsistent results may be produced because the chronotropic response and parasympathetic modulation are adaptative phenomena that are affected by body positions (supine, sitting, or standing), types of exercise (exercise test or aerobic exercise), exercise test protocols (maximal or submaximal), types of ergometer (bicycle, arm, or treadmill) and types of recovery protocols (active or passive) [4, 19].
So, the inconclusive results regarding the interaction between resting parasympathetic activity measurements and the chronotropic and parasympathetic responses during and after exercise remain, and new approaches are needed to expand prior investigations. To our knowledge, no studies have shown a relationship between HR dynamics and parasympathetic activity during, and after a RE session with RHR.
Furthermore, the hypothesis that RHR is correlated with HR dynamics and parasympathetic activity during and after a RE session opens the possibility of a new approach (or analysis) using RHR values, which may add helpful information as a preliminary tool for decision-making (i.e., stress management) for healthcare professionals bringing essential and complementary information related to individuals cardiac autonomic capacity without the expense of clinical exercise tests or maximal/near the maximal effort required for exercise and recovery analysis and to the adequate prescription of RE session for individuals with or without risk of cardiovascular disease.
Therefore, we hypothesized that physically active young men correlate RHR values in the supine position with HR dynamics and parasympathetic activity at rest, during, and after a RE session.
Accordingly, our objectives were: (a) to explore the correlation between HR dynamics and parasympathetic activity at rest, during and after a RE session with RHR in physically active young men; (b) to develop an explication regression based on the effect of RHR values on the HR dynamics at rest, during and after RE session in physically active young men; (c) to compare HR dynamics and parasympathetic activity at rest, during, and after a RE session.
Methods
Participants
Participants were eligible for inclusion if they were men, physically active (≥ 150 min of moderate-vigorous physical activity per week, International Physical Activity Questionnaire—IPAQ) [20], non-athletes, body mass index ≥ 18.5 ≤ 29.9 kg/m2, healthy (no medical restrictions nor known disease), not performing resistance training in the last 3 months, and aged between 19 and 40 years old. Thus, we conducted a cross-sectional study, enrolling 14 young, healthy, non-obese, untrained in RE session, non-athletes, and physically active males with a median (minimum–maximum) age of 26.5 (19.0–40.0) years with a normal electrocardiogram (ECG) and sinus rhythm. At all visits, volunteers underwent exercise two hours after breakfast, between 8:00 and 10:00 a.m., and were previously instructed to abstain from stimulants and alcoholic beverages and physical activity for at least 24 h before evaluation.
Study design
The participants performed three visits to the laboratory with 48 h intervals between visits. During the first visit, we collected anthropometrical measurements, a resting ECG, and information on lifestyle habits. Afterward, all participants first familiarized the leg press exercise to learn its purpose. After 48 h of the interval, in the second visit, the second familiarization in the leg press exercise was performed, and the participants performed one set for warm-up, then 60 s of the rest interval, and up to three attempts from 8 to 12 repetition maximum (RM) to find the weight for the resistance exercise session.
After 48 h of the interval, basic physiological data (RHR) were recorded in the third visit, and an RE session was performed. In a quiet exercise physiology laboratory room, at a temperature between 22 and 24 °C and relative humidity of 50–60%, continuous HR was recorded according to a standardized protocol previously described to obtain the R-R interval series [21, 22]. In summary, a valid five-minute R-R series of HR was first obtained following 10 min of rest in the supine position. After, participants were asked to actively adopt the orthostatic posture at the bedside. Two minutes after this postural change, the blood pressure was measured to verify the absence of significant postural hypotension, and an additional five minutes of RR series of HR was then recorded. Blood pressure was measured by using the auscultatory method [23] via a sphygmomanometer and stethoscope (Preminum®, Brazil).
The RE session was applied after the two 5-min R-R series of HR (at supine and orthostatic postures) had been recorded. The R-R series of HR was recorded during and after a RE session. So, participants completed three sets of 8–12 RM with 60 s of rest interval between sets. Soon after the interruption of the RE session, participants proceeded to a passive recovery period with the participants in the supine position on a padded stretcher for 15 min.
Heart rate and heart rate variability analysis
The HR and R-R interval series were recorded using a valid and reliable heart rate monitor Polar® (model, RS800CX, Polar™, Kempele, Finland) with a sample rate of 1000 Hz [24, 25]. Then, each R-R interval series file was transferred to a computer for offline data processing and analysis of HR and HRV of the R-R interval utilizing the Polar Pro Trainer 5 software and the Kubios HRV software (version 2.2, Kuopio, Finland), respectively [26].
All R-R segments were visually analyzed, and occasional artifacts were manually or automatically removed (< 1% of recording) [27]. The automated artifact identification and removal were performed using the threshold method, which consists of selecting R-R intervals that were larger or smaller than 0.45 s (very low), 0.35 s (low), 0.25 s (medium), 0.15 s (strong), or 0.05 s (very strong) compared to average R-R intervals [26]. We used the medium threshold that only removed the visually observed ectopic points if the tracing did not lose the physiological pattern and the removal did not exceed 1% of the recording.
The parasympathetic activity was evaluated by the square root of the mean of the square of successive adjacent R-R intervals difference (rMSSD), a time-domain index associated with respiratory sinus arrhythmia [28]. For better comprehension and visualization of parasympathetic activity magnitude, rMSSD was analyzed without logarithmic modification or corrections [29, 30].
At rest, during, and after the RE session, rMSSD was assessed using two different metrics: the short-term HRV measurement, i.e., 5 min, and the ultra-short-term HRV measurement, i.e., ≤ 1 min, which was analyzed at peak effort (30 s final). These quantitative analysis methods are because they do not require the stationarity of the HR series, allowing the analysis during and after the exercise [28, 31].
At rest, the rMSSD at supine (rMSSDsup) and orthostatic (rMSSDort) positions were recorded, and the absolute (∆absrMSSDres) and the relative (∆%rMSSDres) values of rMSSD were calculated by subtracting rMSSDort from rMSSDsup. During the RE session, the rMSSD was recorded when participants reached voluntary movement failure in the last repetition of the last set in the peak of exercise (rMSSDpeak) utilized a window of 30 s, and the absolute (∆absrMSSDexer) and the relative (∆%rMSSDexer) values of rMSSD were calculated by subtracting rMSSDpeak from rMSSDsup [21]. After the RE session, the rMSSD was obtained at five, ten, and fifteen minutes over the passive recovery phase.
The HR at supine (RHR) and orthostatic (HRort) positions were recorded as previously described at resting conditions, and absolute (∆absRHR) and relative (∆%RHR) variations were calculated by subtracting HRort from RHR. During the RE session, HR was recorded when participants reached voluntary movement failure in the last repetition of the last set at the peak of the exercise (HRpeak). The chronotropic reserve (∆absCR) was calculated by subtracting HRpeak from RHR [32]. After the RE session, HR was recorded at five, ten, and fifteen minutes during the passive recovery phase, and the absolute (HRR) and relative (%HRR) values of HRR were calculated by subtracting HR at 5, 10, and 15 min during the recovery phase from the HRpeak.
Resistance exercise session
All participants performed the two familiarization and one session of RE in the leg press exercise (Rotech® Fitness, Goiânia, GO, Brazil). This exercise was chosen due to safety, easy movement learning, and significant muscular involvement, and this exercise could reduce parasympathetic activity for 25 min after an acute RE [33].
In the first familiarization, all participants had familiarity with the leg press exercise. So, the range of motion and time under tension 1–2 s was standardized as the concentric and the eccentric phase, and the participants performed one set of 12 repetitions with 20 kg to learn the purpose.
After 48 h of rest, in the second familiarization, participants performed one set for warm-up, then 60 s of the rest interval, and up to three attempts were performed to find the weight for the RE session. Afterward, the participants were encouraged to perform one set of 8–12 RM without knowing the exercise load to avoid psychological influences on weight. If participants could not complete 8–12 RM, then the weight was adjusted on the next set with 60 s of the rest interval. The weight at the beginning of the RE session was when participants reached 8–12 RM in one of three attempts.
After 48 h of rest, participants performed one set for warm-up of 12 repetitions with 20 kg, then 60 s of the rest interval. After the 60 s of the rest interval, the participants completed three sets of 8–12 RM with 60 s rest interval between sets. If participants completed 8 RM, weight was reduced on the next set. When participants reached voluntary movement failure in the last repetition of the last set, a 15-min passive recovery period was immediately initiated with the participants in the supine position on a padded stretcher. We adopted this protocol to avoid the potential influence of movement on parasympathetic reactivation [19].
Statistical analysis
Statistical analysis employed the IBM SPSS Statistics 23 (SPSS Software, Inc., USA, 2015), and Prism® 8 for Windows software (GraphPad Software, Inc., USA, 2019) was used in the construction of the graphics.
The normality of the distribution of the variables was verified by the Shapiro–Wilk test, visual Q-Q plot analysis, frequency distribution histogram, and by Z-score of skewness and kurtosis if a value does not exceed ± 1.96 (a significance level of 0.05). So, the Z-score was obtained by dividing the skewness and kurtosis values by their standard errors (standard error of skewness and standard error of kurtosis). Also, scores greater than 1.5 times the interquartile range out of the box plot were considered outliers [34, 35]. We verified linearity by a scatter plot and homoscedasticity by plots of standardized residuals against predicted values. We used mean and standard deviation (SD) as descriptive statistics to present compliance with normality assumptions. Otherwise, data are presented as median and quartiles (25% and 75%).
Depending on the data distribution, inferential analyses were run either with the paired sample t-test or the Wilcoxon test in the paired comparative analysis. In the comparative analysis repeated, we used the one-way repeated measures ANOVA test in the variables that presented normality assumptions, homoscedasticity by Hartley's FMAX test, and sphericity by Mauchly's test. Analyses with significant main effects were followed up with the post hoc Bonferroni-adjusted pairwise comparisons, and Greenhouse–Geisser corrections were used when the assumption of sphericity was not met based on Mauchly's test. The Friedman’s ANOVA test was conducted when normality assumptions were not met, and the analyses with significant main effects were followed up with the post-hoc Dunn’s test with Bonferroni adjustment pairwise comparisons.
The effect size (ES) used for the one-way repeated measures ANOVA test was omega-squared (ω2), adopting the following parameters: ω2 < 0.02: no effect; ω2 ≥ 0.02 and < 0.13: small effect; ω2 ≥ 0.13 and < 0.26: medium effect and ω2 ≥ 0.26: large effect (Cohen, 1992; Tomczak & Tomczak, 2014). ES used for the Friedman’s ANOVA test was Kendall’s W coefficient (W), and we adopted the following criteria for interpreting: ≤ 0.3: weak agreement; > 0.3 ≤ 0.5: moderate agreement; > 0.5 ≤ 0.7: good agreement and > 0.7: strong agreement [36, 37].
The correlation analysis was performed using the Pearson correlation test when compliance with normality, linearity, homoscedasticity, and absence of outlier assumptions was verified. The Spearman correlation test was conducted when normality assumptions were not met. We calculated the determination coefficient for Pearson's r (R2) and the determination coefficient for Spearman's rho (Rs2) [38].
We adopted the following criteria for interpreting the correlation coefficient: < 0.1: trivial; ≥ 0.1 < 0.3: poor; ≥ 0.3 < 0.6: fair; ≥ 0.6 < 0.8: moderate; ≥ 0.8 ≤ 0.9: very strong; and = 1.0: perfect [39]. Pearson's coefficient's 95% confidence interval was calculated, while Spearman's one was estimated by approximation due to a sample size > 10 [40].
A simple linear regression was also performed on data that met the assumptions of normality, linearity of parameters, normality of residuals, independent values, homoscedasticity, and absence of autocorrelation of residuals (Durbin-Watson test), absence of multicollinearity, and absence of outliers. The two-tailed level of statistical significance was set at a p ≤ 0.05.
Results
The basic physiological data variables were within the normal range for all subjects. Medians and extreme (minimum–maximum) values of arterial blood pressure systolic and diastolic were 108.0/62.0 (100.0/58.0–128/74) mmHg in the supine position and 107.0/78.0 (90.0/60.0–132.0/90.0) mmHg in a standing position (p < 0.01) and body mass index was 24.9 (21.6–29.7) kg/m2. We observed an increase in HR and a decrease in rMSSD after changing posture (supine to orthostatic) at rest (p ≤ 0.01), as shown in Table 1.
Table 1 shows sample values of HR response and parasympathetic activity at rest, during, and after the RE session. The one-way repeated measures ANOVA test showed there is a factor effect of RE session on HR. Mauchly’s test indicated that the assumption of sphericity had been violated, χ2(9) = 33.09, p ≤ 0.01, therefore Greenhouse–Geisser corrected tests are reported (ε = 0.47). The results show that the HR was significantly affected by the condition, [F(44,965.67, 104.89) = 428.66, p ≤ 0.01] with a large effect (ω2 = 0.31). The post hoc Bonferroni showed the differences in RHR with HRpeak, HR5min, HR10min, and HR15min and differences between HRpeak with HR5min, HR10min, and HR15min.
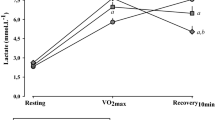
Friedman’s ANOVA test showed that rMSSD values are different during and after the RE session [χ2(4) = 34.91; p ≤ 0.01], and a good agreement (W = 0.62). The post-hoc Dunn’s test with Bonferroni adjustment showed the differences in rMSSDsup with rMSSDpeak, rMSSD5min, rMSSD10min, and rMSSD15min and no significance in rMSSDpeak with rMSSD5min, rMSSD10min, and rMSSD15min, as shown in Table 1.
Figure 1 shows sample values of the median and extremes (minimum–maximum) of comparative values between variables the overload control during the RE session. The one-way repeated measures ANOVA test showed there is a factor effect set on load. Mauchly’s test indicated that the assumption of sphericity had been violated, χ2(2) = 25.17, p ≤ 0.01, therefore Greenhouse–Geisser corrected tests are reported (ε = 0.53). The results show that the load was significantly affected by the set, [F(376.57, 9.54) = 39.46, p ≤ 0.01] with a medium effect (ω2 = 0.13) and the post hoc Bonferroni showed differences in load between sets. The Friedman’s ANOVA test showed that the number of repetitions did not significantly change between sets [χ2(2) = 1.47; p = 0.47] with a weak agreement (W = 0.05).
The one-way repeated measures ANOVA test showed there is no factor effect set on the time under tension. Mauchly’s test indicated that the assumption of sphericity had not been violated, χ2(2) = 0.26, p = 0.87. The results show that the time under tension was no significant difference in the set, [F(16.73, 9.76) = 1.71, p = 0.20] and no effect (ω2 = 0.002).
The one-way repeated measures ANOVA test showed there is a factor effect set on volume. Mauchly’s test indicated that the assumption of sphericity had been violated, χ2(2) = 9.75, p ≤ 0.01, therefore Greenhouse–Geisser corrected tests are reported (ε = 0.64). The results show that the volume was significantly affected by the set, [F(149,240.43, 10,765.68) = 13.86, p ≤ 0.01] with a small effect (ω2 = 0.028) and the post hoc Bonferroni showed differences in volume between sets.
Table 2 describes the correlation coefficient and 95% confidence interval (95% CI) between RHR and HR dynamics at rest, during, and after the RE session and Fig. 2 shows the regression line with confidence lines (95% confidence interval to slope) and correlations between RHR in the supine position and chronotropic response after a RE session. The ∆absRHR, HRpeak, CR, and all HRR (five, ten, and fifteen minutes) showed no correlation with RHR (p ≥ 0.05). However, HRort and HR at five, ten, and fifteen minutes were positively correlated (moderate to very strong) with RHR (r = 0.75—0.89, p ≤ 0.01), explaining 56% to 79% of the shared variance in HR on these conditions. We observed a negative correlation (fair to very strong) between ∆%RHR and %HRR at five, ten, and fifteen minutes with RHR (r = − 0.55 to − 0.80, p ≤ 0.02), which RHR explains shared variance from 30 to 64% of the ∆%RHR and %HRR (five, ten, and fifteen minutes).
Table 3 describes the correlation coefficient and 95% confidence interval (95% CI) between RHR and parasympathetic activity at rest, during, and after the RE session and Fig. 3 shows correlations between RHR in the supine position and rMSSD during, and after a RE session. The ∆%rMSSDres and rMSSDpeak showed no correlation with RHR (p ≥ 0.54). However, at rest, rMSSDsup, rMSSDort, and ∆rMSSDres were negatively correlated (fair to very strong) with RHR (rs = − 0.53 to − 0.82, p ≤ 0.049), explaining 28% to 67% of the proportion of variance in the ranks the parasympathetic activity on these conditions. We observed a negative correlation (moderate to very strong) between ∆rMSSDexer and ∆%rMSSDexer with RHR (rs = − 0.82 to − 0.68, p ≤ 0.01), which RHR explains 46 to 67% of the ∆rMSSDexer and ∆%rMSSDexer of the proportion of variance in the ranks, during the RE session. After the RE session, rMSSD at five, ten, and fifteen minutes were negatively correlated (moderate to very strong) with RHR (rs = − 0.75 to − 0.86, p ≤ 0.01), explaining 56–73% of the proportion of variance in the ranks the parasympathetic activity during recovery.
Table 4 shows the simple linear regression analysis derived from RHR in the supine position (predictor variable) with the HR dynamics (outcome variable) at rest and after the RE session and Fig. 2 shows the regression line with confidence lines (95% confidence interval to slope) and correlations between RHR in the supine position (predictor variable) and chronotropic response (outcome variable) after a RE session. The results demonstrated that the model significantly improves our ability to explain the behavior of the outcome variables (p ≤ 0.04). Thus, the behavior of the outcome variable can be explained by the predictor variable. In other words, for each unit increment (1 beat/min) in RHR, there is an increase of 0.8 bpm in HRort, 1.2 bpm in HR5min, and 1.3 bpm in HR10min and HR15min, which the RHR explains 51% to 79% on the variance of HRort and HR at five, ten and fifteen minutes after RE session. Also, for each unit increment (1 beat/min) in RHR, there is a decrease of 0.9% in ∆%RHR, 0.4% in %HRR5min, and 0.5% in %HRR10min and %HRR15min, in which, RHR explain from 50 to 64% of the variance of ∆%RHR, %HRR5min, %HRR10min, and %HRR15min.
Discussion
Our study observed new and relevant findings regarding the correlation between HR dynamics and parasympathetic activity during, and after a RE session with RHR in young men. We observed that HR dynamics and parasympathetic withdrawal during the RE session, and the capacity to re-establish HR and parasympathetic reactivation after the RE session are correlated with the RHR.
In other words, according to the strength and direction of correlations, physically active young men with lower RHR showed higher HR dynamics and parasympathetic reactivity after the postural change (from supine to orthostatic position) at rest and higher parasympathetic withdrawal during the RE session, and faster HRR and parasympathetic reactivation after the RE session. In addition, we observed the HR dynamics at rest and after the RE session can be explained by the RHR, in which RHR explains 50–79% of the variance of HRort, ∆%RHR, HR5–15 min, and %HRR5–15 min.
While evaluating HR dynamics and parasympathetic activity at rest, during and after an exercise is practical, straightforward, and feasible, a comprehensive understanding of the relationship between these measures could provide important clinical and functional implications for future research and exercise assessment [4, 41]. Some studies that have addressed the relationship between HR and parasympathetic activity after incremental exercise tests or AE with resting parasympathetic activity did not observe a significant correlation between these measures [6, 9, 12, 13]. In opposition, other studies have shown a significant association between resting parasympathetic activity with HRR after a MET or SET [8, 10, 14,15,16, 18], after AE [7, 17], and one study recently showed an association between RHR with HRR after the SET [11]. However, all the studies cited above carried out the association analysis (rest, exercise, and recovery) using an aerobic exercise session, submaximal, or maximum incremental exercise test approaches.
All the studies cited above carried out the association analysis (rest, exercise, and recovery) using an AE, SET, or MET approach and only one study showed that RHR < 60 bpm showed higher chronotropic and parasympathetic modulation at rest, higher chronotropic reserve, parasympathetic withdrawal during SET, and faster HRR and parasympathetic reactivation after effort in young physically active men [21]. Therefore, there is a lack of information regarding the relationship between RHR with HR dynamics and parasympathetic activity during and after a RE session. Thus, to our knowledge, the present study may be the first to show these associations using a RE session approach. Thus, our results have shown a significant correlation (from fair to very strong) between RHR (supine) with HR dynamics and parasympathetic activity at rest, parasympathetic withdrawal during the RE session, and HRR and parasympathetic reactivation after the RE session.
In this context, when considering the present outcomes, the novelty of the present study is that the results may open a new possibility to the RHR (supine) analysis which may add helpful information as a preliminary tool for decision-making (i.e., stress management) for healthcare professionals bringing essential and complementary information related to an individual's cardiac autonomic capacity without the expense of clinical exercise tests or maximal/near the maximal effort required for exercise and recovery analysis. Also, healthcare professionals may use RHR (supine) analysis to adequately prescribe RE session for individuals with or without risk of cardiovascular disease, highlighting the importance of investigating the flexibility of each individual’s cardiac autonomic capacity before a RE session. However, this should be investigated in future research.
Hence, our data preclude a causal relationship, but our analysis demonstrated the direction and magnitude of the relationship between these variables. It is well-established that RHR and HRR after exercise have clear associations with cardiovascular health [1, 5]. Studies have shown that subtle variations in the HR, like 10 beats/min increase in RHR and a delay in HRR after the exercise test, may reflect a decreased parasympathetic activity [1, 5]. Therefore, this decreased parasympathetic activity is associated with unfavorable prognoses such as increased cardiovascular morbimortality and diagnostics of overtraining syndrome and impaired cardiorespiratory fitness [28, 41, 42].
In addition, our findings may add important information based on a novel approach toward developing an explication regression based on the effect of RHR on the HR response during these functional conditions (rest, exercise, and recovery) for a better understanding of these complex interactions.
Thus, the present study opens a new possibility to assess HR dynamics at rest and after a RE session in young men. Yet, the RHR could explain the response of HR dynamic at rest and after the RE session. For each unit of increment in RHR was observed an increase in 0.8 bpm in HRort, 1.2 bpm in HR5min, and 1.3 bpm in HR1 0–15 min, and the RHR explains 51–79% of the variance of these variables. In addition, for each unit increment in RHR, there is a decrease of 0.9% in ∆%RHR, 0.4% in %HRR5min, and 0.5% in %HRR10–15min, which RHR explains from 50 to 64% of the variance of these variables.
The protocol utilized during a RE session was the range of repetitions maximum (8-12RM) for controlling the intensity, three sets, and one minute of rest interval between sets in leg press exercise. It is expected that to maintain the same number of repetitions between sets, there is a decrease of a load of around 5–15% in lower limb resistance exercise with one minute of rest interval [43, 44]. So, this study's data agree with those presented in the literature. We observed a mean decrease in load of 5.7% between the first and second set, 11.4% between the first and third set, and 6.2% between the second and third set to the same range of repetitions and same time under tension between sets.
Thus, the choice of this number of sets, rest interval, and exercise was due to three or more sets, and less than 2 min of rest interval in lower limbs resistance exercise may cause a decrease in parasympathetic activity for until 30 min concerning rest [45,46,47]. It was not the main objective of this study, but we observed an increase in HR with a simultaneous decrease in parasympathetic activity at the end of a resistance exercise session, in which HR and parasympathetic activity stayed changed compared to rest for 15 min after a RE session. So, the protocol used in this study could alter HR and parasympathetic activity at the end of the exercise, and this disturbance remains for 15 min after a RE session, corroborating with the literature above.
Importantly, our findings resulted from a RE session, which has been less used in clinical practice but has important practical implications in the sports field and research protocols considering its safety. Nevertheless, our findings cannot be extrapolated for other workload exercises such as aerobic exercise or cardiopulmonary tests or protocols using treadmill or cycle or arm ergometry or upper resistance exercise limbs and analysis of HR in a sitting position during rest. Also, the participants performed the passive recovery phase in the supine position. Thus, the results cannot be extrapolated for other recovery protocols like passive recovery in an orthostatic or sitting position or active cool-down.
Some limitations of this study include the sample size, which may restrict the analyses. However, the fact that several correlations were significant with a power greater than 80% mitigated the possibility of a type I error. Also, the characteristics of the results cannot be extrapolated to women, athletes, and older adults. Although our results cannot be precisely extrapolated to men in general, we choose to prioritize the study's internal validity, aiming to overcome some of the common heterogeneity that may explain the controversy in the field. Consequently, we selected a sample composed only of young, physically active, and healthy men in a narrow range of age and BMI. So, even though this homogeneity between individuals may represent a limitation, it is, on the other side, one of our strengths since it reinforces our findings due to its strong internal validity.
Conclusion
In conclusion, physically active young men with lower RHR in a supine position show higher HR dynamics and parasympathetic activity at rest, higher parasympathetic withdrawal during the RE session, and higher recovery of HR and parasympathetic reactivation after the RE session. The RHR (supine) explains around 30–79% of the variance in the HR and around 28–73% of the proportion of variance in the ranks of the parasympathetic activity at rest, during, and after the RE session. Lastly, for each unit increment (1 beat/min) in RHR (supine), there is an increase in HRort, HR5–15 min, and a decrease in ∆%RHR, %HRR5–15 min.
Data availability
No datasets were generated or analysed during the current study.
References
Park D-H, Jeon JY (2020) The prognostic value of resting heart rate for health status. Exerc Sci 29:24–33
Buchheit M (2014) Monitoring training status with HR measures: do all roads lead to Rome? Front Physiol 5:1–19
Lavin KM, Coen PM, Baptista LC, Bell MB, Drummer D, Harper SA, Lixandrão ME, McAdam JS, O’Bryan SM, Ramos S (2022) State of knowledge on molecular adaptations to exercise in humans: historical perspectives and future directions. Compr Physiol 12:3193–3279
Michael S, Graham KS, Davis GM (2017) Cardiac autonomic responses during exercise and post-exercise recovery using heart rate variability and systolic time intervals—a review. Front Physiol 8:1–19
Kurl S, Jae SY, Voutilainen A, Hagnäs M, Laukkanen JA (2021) Exercise heart rate reserve and recovery as risk factors for sudden cardiac death. Prog Cardiovasc Dis 68:7–11
Bosquet L, Gamelin F-X, Berthoin S (2007) Is aerobic endurance a determinant of cardiac autonomic regulation? Eur J Appl Physiol 100:363–369
Buchheit M, Laursen PB, Ahmaidi S (2007) Parasympathetic reactivation after repeated sprint exercise. Am J Physiol-Heart Circu Physiol 293:H133–H141
Danieli A, Lusa L, Potočnik N, Meglič B, Grad A, Bajrović FF (2014) Resting heart rate variability and heart rate recovery after submaximal exercise. Clin Auton Res 24:53–61
Dewland TA, Androne AS, Lee FA, Lampert RJ, Katz SD (2007) Effect of acetylcholinesterase inhibition with pyridostigmine on cardiac parasympathetic function in sedentary adults and trained athletes. Am J Physiol-Heart Circ Physiol 293:H86–H92
Evrengul H, Tanriverdi H, Kose S, Amasyali B, Kilic A, Celik T, Turhan H (2006) The relationship between heart rate recovery and heart rate variability in coronary artery disease. Ann Noninvasive Electrocardiol 11:154–162
Garcia GL, Da Cruz CJG, Soares EMKvK, Porto LGG, Molina GE (in press) (2024) Postexercise cardiac autonomic recovery is associated with resting chronotropic response on different body positions in men. Motriz J Phys Educ 30:1–10
Javorka M, Zila I, Balharek T, Javorka K (2002) Heart rate recovery after exercise: relations to heart rate variability and complexity. Braz J Med Biol Res 35:991–1000
Lee CM, Mendoza A (2012) Dissociation of heart rate variability and heart rate recovery in well-trained athletes. Eur J Appl Physiol 112:2757–2766
Molina GE, da Cruz CJ, Fontana KE, Soares EM, Porto LGG, Junqueira LF Jr (2021) Post-exercise heart rate recovery and its speed are associated with cardiac autonomic responsiveness following orthostatic stress test in men. Scand Cardiovasc J 55:220–226
Molina GE, Fontana KE, Porto LGG, Junqueira LF (2016) Post-exercise heart-rate recovery correlates to resting heart-rate variability in healthy men. Clin Auton Res 26:415–421
Nunan D, Jakovljevic DG, Donovan G, Singleton LD, Sandercock GR, Brodie DA (2010) Resting autonomic modulations and the heart rate response to exercise. Clin Auton Res 20:213–221
Tulppo MP, Kiviniemi AM, Hautala AJ, Kallio M, Seppänen T, Tiinanen S, Mäkikallio TH, Huikuri HV (2011) Sympatho-vagal interaction in the recovery phase of exercise. Clin Physiol Funct Imaging 31:272–281
da Fonseca RX, Gomes da Cruz CJ, Soares EdMKVK, Garcia GL, Porto LGG, Molina GE (2024) Post-exercise heart rate recovery and its speed are associated with resting-reactivity cardiovagal modulation in healthy women. Sci Rep 14:5526
Garcia GL, Porto LGG, Fontana KE, Gomes CJ, Junqueira LF, Molina GE (2017) Efeito de diferentes protocolos de recuperação sobre a função autonômica cardíaca. Revista Brasileira de Medicina do Esporte 23:16–20
Matsudo S, Araújo T, Marsudo V, Andrade D, Andrade E, Braggion G (2001) Questinário internacional de atividade física (IPAQ): estudo de validade e reprodutibilidade no Brasil. Rev bras ativ fís saúde 6:05–18
Garcia GL, Porto LGG, da Cruz CJG, Molina GE (2022) Can resting heart rate explain the heart rate and parasympathetic responses during rest, exercise, and recovery? PLoS ONE 17:1–13
Molina GE, Porto LGG, Fontana KE, Junqueira LF (2013) Unaltered R-R interval variability and bradycardia in cyclists as compared with non-athletes. Clin Auton Res 23:141–148
Barroso WKS, Rodrigues CIS, Bortolotto LA, Mota-Gomes MA, Brandão AA, Feitosa ADdM, Machado CA, Poli-de-Figueiredo CE, Amodeo C, Mion Júnior D (2021) Diretrizes brasileiras de hipertensão arterial–2020. Arq Bras Cardiol 116:516–658
Hernando D, Garatachea N, Almeida R, Casajús JA, Bailón R (2018) Validation of heart rate monitor Polar RS800 for heart rate variability analysis during exercise. J Strength Cond Res 32:716–725
Wallén MB, Hasson D, Theorell T, Canlon B, Osika W (2012) Possibilities and limitations of the Polar RS800 in measuring heart rate variability at rest. Eur J Appl Physiol 112:1153–1165
Tarvainen MP, Niskanen J-P, Lipponen JA, Ranta-Aho PO, Karjalainen PA (2014) Kubios HRV—heart rate variability analysis software. Comput Methods Programs Biomed 113:210–220
Tulppo MP, Makikallio TH, Takala T, Seppanen T, Huikuri HV (1996) Quantitative beat-to-beat analysis of heart rate dynamics during exercise. Am J Physiol-Heart Circ Physiol 271:H244–H252
Camm AJ, Malik M, Bigger JT, Breithardt G, Cerutti S, Cohen RJ, Coumel P, Fallen EL, Kennedy HL, Kleiger RE (1996) Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Ann Noninvasive Electrocardiol 1:151–181
Cruz CJGd, Rolim PdS, Pires DdS, Mendes CMO, Paula GMd, Porto LGG, Garcia GL, Molina GE (2017) Reliability of heart rate variability threshold and parasympathetic reactivation after a submaximal exercise test. Motriz Revista de Educação Física 23:65–70
de Geus EJ, Gianaros PJ, Brindle RC, Jennings JR, Berntson GG (2019) Should heart rate variability be “corrected” for heart rate? Biological, quantitative, and interpretive considerations. Psychophysiology 56:e13287
Shaffer F, Ginsberg JP (2017) An overview of heart rate variability metrics and norms. Front Public Health 5:1–17
Jouven X, Empana J-P, Schwartz PJ, Desnos M, Courbon D, Ducimetière P (2005) Heart-rate profile during exercise as a predictor of sudden death. N Engl J Med 352:1951–1958
Kingsley JD, Figueroa A (2016) Acute and training effects of resistance exercise on heart rate variability. Clin Physiol Funct Imaging 36:179–187
Ghasemi A, Zahediasl S (2012) Normality tests for statistical analysis: a guide for non-statisticians. Int J Endocrinol Metab 10:486–489
Razali NM, Wah YB (2011) Power comparisons of Shapiro-Wilk, Kolmogorov-Smirnov, Lilliefors and Anderson-Darling tests. J Stat Model Anal 2:21–33
Cafiso S, Di Graziano A, Pappalardo G (2013) Using the Delphi method to evaluate opinions of public transport managers on bus safety. Saf Sci 57:254–263
Tomczak M, Tomczak E (2014) The need to report effect size estimates revisited. An overview of some recommended measures of effect size. Trends Sport Sci 1:19–25
Strahan RF (1982) Assessing magnitude of effect from rank-order correlation coefficients. Educ Psychol Meas 42:763–765
Akoglu H (2018) User’s guide to correlation coefficients. Turk J Emerg Med 18:91–93
Zar JH (1999) Biological statistics. Prentice Hall, Upper Saddle River
Bosquet L, Merkari S, Arvisais D, Aubert AE (2008) Is heart rate a convenient tool to monitor over-reaching? A systematic review of the literature. Br J Sports Med 42:709–714
Gonzales TI, Westgate K, Hollidge S, Lindsay T, Jeon J, Brage S (2020) Estimating maximal oxygen consumption from heart rate response to submaximal ramped treadmill test. MedRxiv 2020:1–22
Kraemer WJ (1997) A series of studies—the physiological basis for strength training in American football: fact over philosophy. J Strength Cond Res 11:131–142
Willardson JM, Kattenbraker MS, Khairallah M, Fontana FE (2010) Research note: effect of load reductions over consecutive sets on repetition performance. Journal Strength Cond Res 24:879–884
Cruz CJGd, Porto LGG, Pires DdS, Amorim RFBd, Santana FSd, Molina GE (2020) Does the number of sets in a resistance exercise session affect the fast and slow phases of post-exercise cardiac autonomic recovery? Motriz: Revista de Educação Física 26:1–8
Kingsley JD, McMillan V, Figueroa A (2010) The effects of 12 weeks of resistance exercise training on disease severity and autonomic modulation at rest and after acute leg resistance exercise in women with fibromyalgia. Arch Phys Med Rehabil 91:1551–1557
Marasingha-Arachchige SU, Rubio-Arias JA, Alcaraz PE, Chung LH (2022) Factors that affect heart rate variability following acute resistance exercise: a systematic review and meta-analysis. J Sport Health Sci 11:376–392
Funding
This work was supported by Decanato de Pós-Graduação – DPG under Grant 11/2022. The funders had no role in the study design, data collection, analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
GLG, KEF, and GEM have made contributions. to the design of the protocol. GLG collected the experimental data. GLG and GEM analyzed the data. GLG wrote the first draft of the manuscript. All authors reviewed the manuscript. GLG, CJC, KEF, and GEM contributed to the design of the final article and approved the submitted version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethicla approval, Human and animal rights
The studies involving human participants were reviewed and approved by the Local Ethical Committee on Human Research in agreement with the Declaration of Helsinki.
Informed consent
All participants signed informed written consent to participate in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Garcia, G.L., da Cruz, C.J.G., Fontana, K.E. et al. Association between resting heart rate with cardiac autonomic modulation during and after a resistance exercise. Sport Sci Health (2024). https://doi.org/10.1007/s11332-024-01220-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11332-024-01220-w