Abstract
Purpose
The aim of the study was to examine the changes in the rate of torque development (RTD) as indirect marker of muscle damage following a knee flexion exercise-induced muscle damage protocol in healthy individuals.
Methods
Ten participants (24.8 ± 5.3 years) performed 60 maximal knee flexion eccentric contractions and were evaluated before 0, 24, 48, and 72 h after exercise protocol for maximal isometric and concentric isokinetic strength, optimum angle, RTD, muscle soreness, range of motion (ROM) and biceps femoris and semitendinosus muscle thickness (MT), and echo intensity (EI). RTD was analyzed at 0–50 ms (RTD0–50), 0–100 ms (RTD0–100), 100–200 ms (RTD100–200) windowing, and peak RTD (RTDpeak).
Results
RTD0–50 was decreased (p < 0.05) after 24 h. RTD0–100, RTD100–200, and muscle soreness were decreased after 24, 48, and 72 h after exercise (p < 0.05). RTDpeak, maximal isometric and concentric isokinetic strength decreased and biceps femoris and semitendinosus MT increased (p < 0.05) at all time points after eccentric exercise. ROM was decreased (p < 0.05) 48 and 72 h after exercise. Semitendinosus EI was increased (p < 0.05) 72 h after exercise. Optimum angle was not changed after exercise.
Conclusion
The knee flexor muscle RTD measured at different intervals were changed after the eccentric exercise protocol and may be used as an indirect marker of muscle damage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Exercise-induced muscle damage (EIMD) is typically shown by changes in several indirect markers of muscle damage such as the loss of range of motion (ROM), delayed onset muscle soreness, muscle swelling, elevation of blood markers, and decrease in maximal isometric strength [1]. The prolonged decrease in the ability to produce maximal isometric strength is considered the most representative and reliable functional indirect marker of muscle damage in humans [2, 3]. Furthermore, the changes in maximal voluntary strength have been advocated as the main representative measure of structural muscle fiber damage [4] and neural [5] alteration after eccentric contractions. However, it has been suggested that peak isometric torque from maximal voluntary contraction may not fully describe neuromuscular changes after EIMD. Hence, more detailed assessments of force production capacity have been recently proposed to assess muscle impairments after eccentric contractions.
The assessment of changes in rate of torque development (RTD) at early and late phases after eccentric exercise has been recommended [6, 7]. Early RTD (≤ 75 ms from contraction onset) is thought to be influenced mostly by the neural drive, while the late phase (from 100 to 200 ms from contraction onset) is more related to maximal voluntary contraction (peak isometric torque) [8]. The use of the RTD as an indirect marker of muscle damage has been suggested by Jenkins et al. [7] and Penailillo et al. [6] for the forearm flexors and knee extensors, respectively, where both studies supported the use of RTD as a more sensitive and specific marker of EIMD than peak isometric torque. However, it is not clear whether the response of other muscle groups such as the hamstring can be assessed by RTD to identify changes related to EIMD.
Jenkins et al. [7] reported that RTD at the early phase intervals (0–10 and 0–50 ms) exhibited greater decrease and remained depressed until 48 h after eccentric exercise of the forearm flexors, but recovered faster than the later phase RTD (0–200 ms) and peak torque, which were not fully recovered until 72 h after exercise. However, Penailillo et al. [6] suggested that 100–200 ms interval is more sensitive than earlier intervals of RFD and might be used as an indirect marker of EIMD for the knee extensor muscles. It is possible that these discrepancies might be related to the differences between the sensitivity of different muscles to EIMD. As the knee flexors have smaller pennation angle and longer muscle length, these muscle groups are more susceptible to EIMD than the knee extensor muscles [9]. Furthermore, the knee flexors have great proportion of fibers type II than the quadriceps [10] and it has been evidenced that type II fiber type is more susceptible to muscle damage [11]. Thus, since fiber type distribution between muscles might determine RTD [12,13,14], alterations in RTD after eccentric exercise in the hamstring might be different from other previous studies muscle groups. Therefore, the aim of the present study was to evaluate the response of the RTD to an EIMD protocol in the hamstrings. We hypothesized that knee flexion RTD would be changed similarly to other indirect markers of muscle damage for hamstrings.
Materials and methods
Participants
Ten healthy untrained men (age: 24.8 ± 5.3 years; height: 177.2 ± 4.4 cm; body mass: 76.9 ± 8.5 kg) participated in this study. As the symptoms of EIMD may be influenced by muscle compliance, and thus, levels of flexibility [15] each participant were expected to have limited hamstring flexibility, defined as maximum of 80° hip flexion in passive straight-leg raise test [16]. None of the participants had performed lower limb resistance training for at least 6 months prior to the study, and had musculoskeletal or neurological injuries of the lower limbs. They were instructed to avoid any changes in their diet and physical activity levels (e.g., walking, jogging, and biking) or consume any anti-inflammatory medication and apply any treatment (e.g., massage, cryotherapy) during the course of the study. The participants were carefully informed of the purpose, procedures, and risks of study participation, and written informed consent was obtained from all participants before commence of the study. All procedures were approved by the University Institutional Review Board.
Experimental design
The participants attended the laboratory on five occasions. During the first visit, a familiarization with the testing procedures was performed at least 1 week prior to the eccentric exercise protocol. On the second visit, the participants performed the maximal eccentric exercise isokinetic protocol of the hamstrings. Measurements of indirect markers of muscle damage were performed before, immediately after (0 h), 24, 48, and 72 h after eccentric exercise protocol. The following measurements were performed in this order: (a) biceps femoris and semitendinosus muscle thickness and echo intensity assessed by ultrasonography, (b) hip flexion range of motion, (c) muscle soreness, (d) isometric and concentric isokinetic peak torque, and (e) RTD of the knee flexion. The measurements were performed on the right leg for all participants at the same time of the day (± 2 h). The same experienced investigator conducted all measurements.
Eccentric exercise protocol
The eccentric exercise protocol was performed in the isokinetic dynamometer (Cybex Norm; Ronkonkoma, NY, USA) in the seated position with trunk flexed at 85°. The knee joint of the right leg was aligned with the rotation axis of the dynamometer, and the participants’ thigh, torso, and pelvis were secured by straps for stabilization. Participants performed six sets of 10 maximal voluntary isokinetic eccentric contractions of the knee flexor muscles at an angular velocity of 60°·s−1 with 1 minute rest period between sets. The movement started with the knee joint flexed (90° of knee flexion) and ended with the knee extended (10° of knee flexion) and the participants were instructed to resisted with maximal effort the knee-extending action. Strong verbal encouragement to generate maximal force was given at all times in addition to real-time visual feedback by means of a computerized visual display.
Measurements
Muscle thickness and echo intensity
Images of the biceps femoris and semitendinosus were obtained by B-mode ultrasonography (Toshiba, VMI, Japan) using a high-resolution linear probe of 9.0 MHz with 55 mm field of view probe width, 70 mm of depth, and 90 dB. Ultrasound probe was positioned at 50% of the distance between the greater trochanter of the femur and the lateral (i.e., biceps femoris) and medial (i.e., semitendinosus) knee joint line [17] for image acquisition. Probe position was carefully marked on the skin with a demographic pen to ensure replication position at all testing days. Measurements were obtained with the participants laying in the prone position with the muscles relaxed after resting in a supine position for at least 10 min to allow fluid shifts to stabilize [18]. A water-based gel was used to promote acoustic contact and provide clear images of the superficial and the depth muscle interfaces without causing excessive probe pressure on the skin. Three images were obtained for each muscle and the same images were used for analyses of muscle thickness and echo intensity (i.e., echogenicity). As shown in Fig. 1, muscle thicknesses were determined as the distance between the interfaces of the superficial and deep aponeuroses and the ultrasound echo intensity was determined using the maximal muscle area, avoiding bone, and subcutaneous fat tissue, based on a histogram of gray scale (0 = black, 255 = white). Image analyses were performed using ImageJ software (version1.37, National Institutes of Health, Washington, D.C., USA).
Range of motion
Range of motion was measured using a maximum passive straight-leg raise test, which was also used as inclusion criteria of the present study. The participants were placed in the supine position with legs straight, the non-evaluated leg was stabilized with inelastic straps positioned in the ankle and above the knee joint to avoid compensatory movements. Participants were instructed to remain relaxed and to not offer any resistance, while one examiner passively and slowly lifted the participant’s leg into hip flexion until maximum tolerated amplitude, informed by the participant, was reached. A second examiner positioned the manual goniometer aligned in the center of the hip joint (great trochanter) to measure hip range of motion [16], and the goniometer’s stationary arm was positioned along the lateral middle of the abdomen, using the pelvis as reference and the moving arm along the lateral middle of the femur. This test was performed once at each time-point evaluated.
Muscle soreness
Muscle soreness was evaluated with a 100-mm visual analog scale. Participants were asked to mark their level of muscle soreness in a scale where 0 mm represented “no pain” and 100 mm represented “extreme pain” after performing two full range of motion movement of knee flexion/extension in the standing position [19].
Voluntary peak torque and optimum angle
Isometric and concentric knee flexion peak torques were measured on the isokinetic dynamometer (Cybex Norm; Ronkonkoma, NY, USA). The optimum angle (i.e., peak torque angle) was analyzed from the dynamic maximal concentric knee flexion contraction. The participants were adjusted and stabilized to the dynamometer with the same description given in the eccentric exercise protocol. After a warm-up of ten submaximal (~ 50–60% of maximal voluntary contraction) concentric isokinetic contractions at 60°·s−1, the participants performed two maximal attempts of 3-s knee flexion isometric contractions at 40° of knee flexion (0° = full knee extension), with a 2-min recovery between each attempt, and the highest peak torque value was used for analysis. Participants were instructed to perform each trail as hard and fast as possible [20], and attempts with an initial countermovement were discarded and an extra trail was performed. The dynamometer force signal was sampled at 2000 Hz (Miotec suite, Brazil) and stored in an external computer for RTD analysis. The maximal isokinetic concentric peak torque and the optimum angle were assessed from five consecutive maximal concentric isokinetic contractions of the knee flexor muscles at 60°·s−1. The highest peak torque values and the joint angle at which the maximum peak torque occurred were used for analysis. Participants received verbal encouragement to perform maximal strength in both tests, and visual feedback was provided on a screen.
Rate of torque development
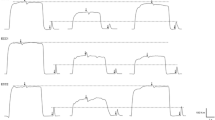
The RTD of the knee flexors was evaluated during the maximal isometric knee flexion as previously described and was analyzed at three time intervals: 0–50 ms (RTD0–50), 0–100 ms (RTD0–100), and 100–200 ms (RTD100–200). To determine the peak RTD (RTDpeak), a moving 20-ms window throughout the torque-time curve was used, and the highest RTD at time (from 0 to 200 ms) was defined as the RTDpeak [21]. To analyze the RTD, the torque curves were exported to an excel spreadsheet, and contraction onset was defined as the point at which the force curve exceeded average baseline force by more than three standard deviations as suggested previously [22]. RTD was manually calculated as the average slope of the initial phase of the force–time curve (Δtorque/Δtime) following the onset of the muscular contraction.
Statistical analysis
Normality of data was tested using the Shapiro–Wilk test, from which all variables resulted normally distributed. Mauchly’s test was used to verify sphericity; when sphericity was violated, a Greenhouse–Geiser correction was used. To compare the changes in the indirect markers of muscle damage a one-way analysis of variance (ANOVA), with a Bonferroni post hoc test to examine differences between time points was used. Significance level was set at α < 0.05. Effect sizes were calculated using Cohen's d. All analyses were performed with the software SPSS 17.0 (IBM, Somers, NY, USA), and results are reported as mean (standard deviation).
Results
The baseline values of all dependent variables are shown in Table 1. Total power output produced during the eccentric exercise protocol by knee flexors during all repetitions was 1238.7 ± 225.3 J.
Muscle thickness and echo intensity
Figure 1 shows an example of the images acquired for the biceps femoris (1) and semitendinosus (2) measured before (a) and after (b) eccentric exercise. As shown in Fig. 2a, biceps femoris muscle thickness increased at 0 h (5 ± 4%; p = 0.025; effect size: 0.37), 24 h (7 ± 4%; p = 0.003; effect size: 0.54), 48 h (10 ± 5%; p = 0.005; effect size: 0.71) and 72 h (13 ± 8%; p = 0.019; effect size: 0.83) after eccentric exercise. However, no changes were observed for the echo intensity assessments at any point (p = 0.177) in the biceps femoris muscle (Fig. 1b). As shown in Fig. 2a, semitendinosus muscle thickness increased at 0 h (9 ± 8%; p = 0.048; effect size: 0.48), 24 h (9 ± 8%; p = 0.048; effect size: 0.63), 48 h (18 ± 10%; p = 0.007; effect size: 0.48) and 72 h (24 ± 13% p = 0.003; effect size: 0.21) after exercise. The echo intensity of semitendinosus muscle increased only at 72 h (35 ± 26%; p = 0.026; effect size: 1.44) after exercise (Fig. 2b).
Absolute changes in biceps femoris (1) and semitendinosus (2) muscle thickness (a) and echo intensity (b), immediately after (0 h) and 2–3 days after the eccentric exercise; *Significantly different (p < 0.05) from before eccentric exercise value (p < 0.05) for biceps femoris and semitendinosus muscle thickness, semitendinosus echo intensity
Range of motion and muscle soreness
The ROM decreased at 48 h (−17 ± 11%; p = 0.020; effect size: 1.68) and 72 h (−18 ± 13%; p = 0.039; effect size: 1.47) after eccentric exercise. Muscle soreness increased at 24 h (27.40 ± 13.72%; p = 0.001), 48 h (62.10 ± 14.73%; p < 0.001), and 72 h (59.70 ± 19.93%; p < 0.001) after eccentric exercise.
Isometric and concentric peak torque
Isometric peak torque decreased −30 ± 14% at 0 h (p = 0.010; effect size: 1.67), −37 ± 14% at 24 h (p = 0.003; effect size: 1.95), −47 ± 12% at 48 h (p < 0.001; effect size: 2.61), and −45 ± 20% at 72 h (p = 0.004; effect size: 2.26) after eccentric exercise compared to baseline (Fig. 3a). Concentric isokinetic peak torque decreased −29 ± 12% at 0 h (p = 0.001; effect size: 1.51), −32 ± 11% at 24 h (p < 0.001; effect size: 1.60), −44 ± 21% at 48 h (p = 0.001; effect size: 1.88), and −38 ± 28% at 72 h (p = 0.025; effect size: 1.54) after eccentric exercise compared to baseline (Fig. 3b). The optimum angle was not significantly changed after eccentric exercise at any time-point (p = 0.054).
Rate of torque development
As shown in Fig. 4, RTD0–50 was reduced only at 24 h (−47 ± 24%; p = 0.034; effect size: 1.67) after the eccentric exercise. The RTD0–100 was reduced at 24 h (−51 ± 21%; p = 0.011; effect size: 1.93), 48 h (−50 ± 23%; p = 0.015; effect size: 1.81), and 72 h (−49 ± 23%; p = 0.027; effect size: 1.83) after eccentric exercise. Similarly, RTD100–200 was reduced 24 h (−49 ± 24%; p = 0.003; effect size: 1.95), 48 h (−62 ± 13%; p < 0.001; effect size: 2.90), and at 72 h (−69 ± 16%; p < 0.001; effect size: 3.58) after eccentric exercise. Significant decreases were also observed in RTDpeak at 0 h (−37 ± 39%; p = 0.059; effect size: 1.81), 24 h (−50 ± 28%; p = 0.004; effect size: 2.32), 48 h (−63 ± 12%; p < 0.001; effect size: 2.96), and 72 h (−71 ± 14%; p < 001; effect size: 3.37).
Discussion
The aim of the present study was to evaluate the response of the RTD among other indirect markers of muscle damage to an EIMD protocol in the hamstrings. The main findings of the present study were that a significant reduction in the RTD was accompanied by changes in other more typical indirect markers of EIMD the knee flexors. The RTDpeak had similar time course to maximal isometric and concentric peak torque after eccentric exercise showing a decrease from immediately after (0 h) until 72 h after exercise. However, the RTD0–50 RTD0–100 and RTD100–200 showed no alterations at immediately after (0 h) the eccentric exercise protocol, but decreased 24 h after eccentric protocol, which may indicate that RTD is influenced by muscle fatigue immediately after exercise.
The decreases in peak isometric torque (30–45%) and peak concentric isokinetic torque (29–44%) strength after eccentric exercise are in line with similar protocols for the hamstring. For instance, previous studies [9, 23, 24] have reported similar reduction in the magnitude of isometric (25–39%) and dynamic (17–33%) strength of the knee flexors on the days after similar eccentric exercise protocols. During eccentric contractions, sarcomeres are overstretched, leading to sarcomere disruption, membrane damage, and interference with E–C coupling, which can interfere in the maximal force capacity [25]. We found that muscle soreness increased after exercise and peaked at 48 h after eccentric exercise. The increase of muscle soreness following eccentric exercise occurs as a sequence of events that produce substances that sensitize type III and IV nerve ending within 24–48 h [26]. It has been extensively reported in others muscle groups that the magnitude of soreness does not usually have the same time course of force loss [18] or is not accompanied by reduction in muscle activation [27]. Similar time course of recovery of soreness and the force loss in the hamstrings have also been reported [9, 24]. Thus, our eccentric exercise protocol induced similar magnitude of muscle damage as shown by similar changes in muscle strength and soreness induced.
Interestingly, the eccentric exercise protocol decreased the knee flexors RTD for 72 h after exercise, similar to previous studies evaluating the decrease in rapid force production capacity following eccentric exercise in other muscle groups [6, 7]. Furthermore, similar to Jenkins et al. [7], the RTD0–50 was significantly reduced only at 24 h after exercise and was quickly recovered, which differed from the changes in peak isometric strength and the late phases of RTD (RTD0–100 and RTD100–200) that were significantly reduced from 24 to 72 h after exercise. In addition, the RTDpeak was significantly reduced at all time points after exercise, included the immediately post-exercise (0 h). The reduction in muscle function immediately after the maximal eccentric exercise protocol has been mainly explained by a mix between central and peripheral muscle fatigue resulting from a failure in the excitation–contraction coupling process and structural muscle damage [28, 29]. It might be speculated that the RTD0–50 had recovered faster than the late RTD due to the lesser impact of the eccentric exercise in the neuromuscular properties and/or sarcolemma excitability, suggestion supported by previous studies that also observed faster recovery of muscle activation after an eccentric protocol [6, 27]. In contrast, the late phase of the RTD (i.e., later than 100 ms RTD) and RTDpeak are thought to be related to muscle contractile properties, including release, diffusion, binding of Ca2+, and the rate of cross-bridge binding to thin filaments [30]. Thus, it is possible that the late phase of RTD is more influenced by muscle damage [31]. In this sense, the present results suggest that the late phase of the RTD in the knee flexors is a more specific indirect marker of EIMD compared to the early phase intervals, in accordance with Penailillo et al. [6], which also reported that the RTD100–200 is a better indirect marker of muscle damage for the knee extensors.
Interestingly, we showed that the magnitude of loss was greater for RTD100–200 (34–69%) and for RTDpeak (37–71%) compared to peak isometric (30–47%) and maximal isokinetic concentric torque (29–44%). Similarly, Crameri et al. [32] have shown a decrease of 25–45% on RTD0–100 at 4, 12, and 192 h after exercise in the knee extensor muscles, while the decrease in peak isometric torque was about 8–25%. The authors suggested that the greater decrease in RTD than peak isometric torque after eccentric exercise might have been associated with reduced stiffness of serial and/or lateral cellular force transmission, possibly due to changes in cytoskeletal integrity [32]. It is possible that our eccentric exercise protocol also caused disruption of the extracellular matrix and muscle fiber cytoskeletal as to decreased RTD to a greater extent than MICV. Furthermore, eccentric exercise induces impairments on force generation capacity [33] and greater damage of type II muscle fibers, which are critical muscular determinants of early phase RTD [34, 35]. Thus, it would be expected greater reduction in the early phase of the RTD after eccentric exercise. However, this suggestion should be interpreted with caution considering that the knee flexors are more affected by muscle damage following eccentric contractions than knee extensors [9]. In addition, the maximal strength measurements performed in the present investigation were not able to differentiate muscle damage and fatigue immediately (0 h) after exercise. Therefore, we suggest that the RTD0–100 and RTD100–200 are more specific indirect markers of muscle damage compared to isometric and concentric peak torque, RTD0–50 and RTDpeak, which confirms previous observations for the knee extensors [6].
The increases in muscle thickness and echo intensity assessed by ultrasound after eccentric exercise may result from muscle inflammation and edema induced by eccentric exercise [36]. Previous studies reported significant changes in the muscle thickness at all time points after eccentric exercise in the biceps femoris [9, 24], similar to the observed in the current study. However, Chen et al. [19] observed an increase in the biceps femoris echo intensity 48 h after exercise, while we found no alteration in the biceps femoris and an increase in the semitendinosus echo intensity was found only 72 h after exercise, similar to Brusco et al. (2018) who also observed increase in semitendinosus 72 h after exercise and reported no alteration in the biceps femoris. Differences between the eccentric exercise protocol performed in the present study (6 sets of 10 eccentric contractions at 60°/s) and previous (5 sets of six eccentric contractions at 90º/s) [9] and the method to analyze the echo intensity may explain the different results. Chen et al. [19] used a region of interest of 2 cm × 2 cm = 4 cm2, while in the present study, the maximal muscle area (i.e., all muscle tissue excluding fascia) was used. Thus, considering that the size of the region of interest may affect the values of the echo intensity of muscles [37], the different methods of analyses might explain the differences between studies. Furthermore, similar to other investigations using tomography imaging, we also observed different magnitudes of muscle damage between the hamstrings muscles [38]. Greater changes in semitendinosus compared to biceps femoris were found following eccentric exercise. It is possible that the isokinetic protocol overloaded the semitendinosus more than biceps femoris as the semitendinosus has shown a prominent role in producing and controlling the torques during single joint knee flexion exercises [38].
One of the possible limitations of the present study is that we had no control group. However, this choice was made based on the assumption that the eccentric exercise performed was enough to induce exercise-induced muscle damage as shown in the previous studies [19, 24]. Therefore, no further individuals would need to be submitted to this intense maximal exercise protocol. In conclusion, the present results showed that while the RTDpeak responded similarly to the isometric and concentric peak torque and muscle soreness, the RTD0–50 RTD0–100 and RTD100–200 had different time course and were not changed immediately after exercise (0 h). Therefore, the RTD analyses may be implemented in future studies aiming to investigate early and late responses to eccentric exercise as an additional indirect marker of exercise-induced muscle damage. As a practical application, coaches and practitioners should expect that their ability to produce force rapidly will be negatively affected by muscle damage, and it is important to be considerate during training routine design.
References
Friden J, Lieber RL (2001) Eccentric exercise-induced injuries to contractile and cytoskeletal muscle fibre components. Acta Physiol Scand 171:321–326
Nosaka K, Chapman D, Newton M, Sacco P (2006) Is isometric strength loss immediately after eccentric exercise related to changes in indirect markers of muscle damage? Applied physiology, nutrition, and metabolism. Physiol Appl Nutr Metabol 31:313–9
Warren GL, Lowe DA, Armstrong RB (1999) Measurement tools used in the study of eccentric contraction-induced injury. Sports Med 27:43–59
Raastad T, Owe SG, Paulsen G et al (2010) Changes in calpain activity, muscle structure, and function after eccentric exercise. Med Sci Sports Exerc 42:86–95
Ingalls CP, Warren GL, Williams JH, Ward CW, Armstrong RB (1998) E-c coupling failure in mouse edl muscle after in vivo eccentric contractions. J Appl Physiol 85:58–67
Penailillo L, Blazevich A, Numazawa H, Nosaka K (2015) Rate of force development as a measure of muscle damage. Scand J Med Sci Sports 25:417–427
Jenkins ND, Housh TJ, Traylor DA et al (2014) The rate of torque development: a unique, non-invasive indicator of eccentric-induced muscle damage? Int J Sports Med 35:1190–1195
Andersen LL, Aagaard P (2006) Influence of maximal muscle strength and intrinsic muscle contractile properties on contractile rate of force development. Eur J Appl Physiol 96:46–52
Chen TC, Lin KY, Chen HL, Lin MJ, Nosaka K (2011) Comparison in eccentric exercise-induced muscle damage among four limb muscles. Eur J Appl Physiol 111:211–223
Sangnier S, Tourny-Chollet C (2007) Comparison of the decrease in strength between hamstrings and quadriceps during isokinetic fatigue testing in semiprofessional soccer players. Int J Sports Med 28:952–957
Macaluso F, Isaacs AW, Myburgh KH (2012) Preferential type ii muscle fiber damage from plyometric exercise. J Athl Train 47:414–420
Korhonen MT, Cristea A, Alen M et al (2006) Aging, muscle fiber type, and contractile function in sprint-trained athletes. J Appl Physiol 101:906–917
Hvid L, Aagaard P, Justesen L et al (2010) Effects of aging on muscle mechanical function and muscle fiber morphology during short-term immobilization and subsequent retraining. J Appl Physiol 109:1628–1634
Farup J, Sorensen H, Kjolhede T (2014) Similar changes in muscle fiber phenotype with differentiated consequences for rate of force development: endurance versus resistance training. Hum Mov Sci 34:109–119
McHugh MP, Connolly DA, Eston RG, Kremenic IJ, Nicholas SJ, Gleim GW (1999) The role of passive muscle stiffness in symptoms of exercise-induced muscle damage. Am J Sports Med 27:594–599
Ayala F, de Baranda PS, De Ste Croix M, Santonja F (2013) Comparison of active stretching technique in males with normal and limited hamstring flexibility. Phys Therapy Sport 14:98–104
Blackburn JT, Pamukoff DN (2014) Geometric and architectural contributions to hamstring musculotendinous stiffness. Clin Biomech 29:105–110
Radaelli R, Bottaro M, Wilhelm EN, Wagner DR, Pinto RS (2012) Time course of strength and echo intensity recovery after resistance exercise in women. J Strength Condition Res 26:2577–2584
Chen CH, Nosaka K, Chen HL, Lin MJ, Tseng KW, Chen TC (2011) Effects of flexibility training on eccentric exercise-induced muscle damage. Med Sci Sports Exerc 43:491–500
Maffiuletti NA, Aagaard P, Blazevich AJ, Folland J, Tillin N, Duchateau J (2016) Rate of force development: physiological and methodological considerations. Eur J Appl Physiol 116:1091–1116
Haff GG, Ruben RP, Lider J, Twine C, Cormie P (2015) A comparison of methods for determining the rate of force development during isometric midthigh clean pulls. J Strength Condition Res 29:386–395
de Ruiter CJ, Kooistra RD, Paalman MI, de Haan A (2004) Initial phase of maximal voluntary and electrically stimulated knee extension torque development at different knee angles. J Appl Physiol 97:1693–1701
Penailillo L, Guzman N, Cangas J, Reyes A, Zbinden-Foncea H (2017) Metabolic demand and muscle damage induced by eccentric cycling of knee extensor and flexor muscles. Eur J Sport Sci 17:179–187
Brusco CM, Blazevich AJ, Radaelli R et al (2018) The effects of flexibility training on exercise-induced muscle damage in young men with limited hamstrings flexibility. Scand J Med Sci Sports 28:1671–1680
Proske U, Allen TJ (2005) Damage to skeletal muscle from eccentric exercise. Exerc Sport Sci Rev 33:98–104
Armstrong RB (1984) Mechanisms of exercise-induced delayed onset muscular soreness: a brief review. Med Sci Sports Exerc 16:529–538
Behrens M, Mau-Moeller A, Bruhn S (2012) Effect of exercise-induced muscle damage on neuromuscular function of the quadriceps muscle. Int J Sports Med 33:600–606
Ferri A, Narici M, Grassi B, Pousson M (2006) Neuromuscular recovery after a strength training session in elderly people. Eur J Appl Physiol 97:272–279
Raastad T, Hallen J (2000) Recovery of skeletal muscle contractility after high- and moderate-intensity strength exercise. Eur J Appl Physiol 82:206–214
Edman KA, Josephson RK (2007) Determinants of force rise time during isometric contraction of frog muscle fibres. J Physiol 580:1007–1019
Warren GL, Ingalls CP, Lowe DA, Armstrong RB (2002) What mechanisms contribute to the strength loss that occurs during and in the recovery from skeletal muscle injury? J Orthop Sports Phys Ther 32:58–64
Crameri RM, Aagaard P, Qvortrup K, Langberg H, Olesen J, Kjaer M (2007) Myofibre damage in human skeletal muscle: effects of electrical stimulation versus voluntary contraction. J Physiol 583:365–380
Raastad T, Risoy BA, Benestad HB, Fjeld JG, Hallen J (2003) Temporal relation between leukocyte accumulation in muscles and halted recovery 10–20 h after strength exercise. J Appl Physiol 95:2503–2509
Taekema DG, Westendorp RG, Frolich M, Gussekloo J (2007) High innate production capacity of tumor necrosis factor-alpha and decline of handgrip strength in old age. Mech Ageing Dev 128:517–521
Folland JP, Buckthorpe MW, Hannah R (2014) Human capacity for explosive force production: neural and contractile determinants. Scand J Med Sci Sports 24:894–906
Chen TC, Nosaka K, Sacco P (2007) Intensity of eccentric exercise, shift of optimum angle, and the magnitude of repeated-bout effect. J Appl Physiol 102:992–999
Caresio C, Molinari F, Emanuel G, Minetto MA (2015) Muscle echo intensity: reliability and conditioning factors. Clin Physiol Funct Imaging 35:393–403
Schuermans J, Van Tiggelen D, Danneels L, Witvrouw E (2014) Biceps femoris and semitendinosus–teammates or competitors? New insights into hamstring injury mechanisms in male football players: a muscle functional mri study. Br J Sports Med 48:1599–1606
Funding
PhD scholarship to Clarissa M Brusco was provided by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) –Brazil (88887.357700/2019-00).
Author information
Authors and Affiliations
Contributions
C.M.B., R.R., L.E.P., and R.S.P. participated in the conception and design of the study. C.M.B. and R.R. did the data acquisition. All authors participated in data analysis and interpretation and wrote the main manuscript. The manuscript was reviewed and approved by all authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
Ethics approval was given by the Ethics committee of the Universidade Federal do Rio Grande do Sul, approval number 965.097.
Informed consent
The participants were carefully informed of the purpose, procedures, and risks of study participation, and written informed consent was obtained from all participants before commence of the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Brusco, C.M., Radaelli, R., Neske, R. et al. Rate of torque development as an indirect marker of muscle damage in the knee flexors. Sport Sci Health 18, 75–83 (2022). https://doi.org/10.1007/s11332-021-00776-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11332-021-00776-1