Abstract
Purpose
To better understand neuromuscular characteristics of eccentric exercise-induced muscle damage, this study compared between concentric (CONC) and eccentric (ECC) exercises of knee extensor muscles, and the first (ECC1) and second bouts of the eccentric exercise (ECC2) for central and peripheral parameters associated with neuromuscular fatigue.
Methods
Twelve young men performed three exercise bouts separated by at least 1 week between CONC and ECC1, and 2 weeks between ECC1 and ECC2. In each exercise, maximal voluntary concentric or eccentric contractions of the knee extensors were performed until a reduction in maximal voluntary isometric contraction (MVC) torque of at least 40% MVC was achieved immediately post-exercise. MVC torque, central (voluntary activation and normalised electromyographic activity), and peripheral neuromuscular indices (evoked torque and M-wave amplitude), and muscle soreness were assessed before (PRE), immediately after (POST), 1 h (1H), and 1–4 days after exercise (D1, D2, D3, and D4).
Results
MVC torque decreased at only POST for CONC (− 52.8%), but remained below the baseline at POST (− 48.6%), 1H (− 34.1%), and D1–D4 (− 34.1 to − 18.2%) after ECC1, and at POST (− 45.2%), 1H (− 24.4%) and D1 (− 13.4%) after ECC2 (p < 0.05). Voluntary activation decreased immediately after ECC1 (− 21.6%) and ECC2 (− 21.1%), but not after CONC. Electrically evoked torques decreased similarly at POST and 1H for the three conditions, but remained below the baseline at D1 only post-ECC1.
Conclusion
These results showed that both central and peripheral factors contributed to the MVC decrease after ECC1 and ECC2, but the decrease was mainly due to peripheral factors after CONC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neuromuscular fatigue is defined as a decrease in capacity of a muscle to produce a level of strength or power (Bigland-Ritchie et al. 1986), and is classically categorised as central and peripheral fatigue, since fatigue is the resultant of a complex interaction between the two (Gandevia 2001). Central fatigue is attributable to a decrease in the capacity to fully activate a muscle during an exercise, whereas peripheral (or muscle) fatigue corresponds to the perturbation of the system involved from the muscle fibre excitation to the formation of cross bridges between actin and myosin (Gandevia 2001).
It appears that neuromuscular fatigue profiles are different between concentric and eccentric contractions. For example, Pasquet et al. (2000) reported a greater decrease in ankle dorsiflexor torque immediately after concentric (− 32%) than after eccentric exercise (− 24%) consisting of 5 sets of 30 maximal voluntary contractions at a constant angular velocity (50°/s) through a 30° range of motion. Similarly, Kay et al. (2000) showed that knee extensor strength decreased immediately after repeated maximal concentric contractions (− 42.3%) but not after maximal eccentric contractions (+ 8.6%). It seems likely that peripheral and central factors contribute differently to the post-exercise decreases in maximal voluntary contraction (MVC) torque between concentric and eccentric contractions.
Kay et al. (2000) reported a significant decrease in knee extensors MVC torque after a 100-s concentric endurance trial without any decrease in integrated electromyographic (EMG) signal, indicating that neural drive to muscle was not impaired. However, no previous studies have compared between concentric and eccentric contractions by matching the level of fatigue immediately post-exercise. Two studies (Prasartwuth et al. 2005, 2006) investigated the contribution of central and peripheral parameters in the decrease of maximal voluntary force after eccentric exercise for elbow flexor muscles. They indicated that modulations occurring both upstream (decrease in voluntary activation) and downstream (decrease in electrically evoked resting twitch amplitude) of the neuromuscular junction were associated with the MVC decreases after eccentric exercise (Prasartwuth et al. 2005, 2006).
To the best of our knowledge, only Dundon et al. (2008) have investigated peripheral and central parameters after concentric versus eccentric exercise of the elbow flexors inducing the same level of fatigue. In this study, central parameters were investigated only through surface EMG signals. Because of some limitations regarding the surface EMG data interpretation (Farina et al. 2014), modulations within the central nervous system after eccentric versus concentric exercise are not clear; thus, further investigation with more specific neuromuscular markers is necessary.
It is well documented that eccentric contractions induce greater muscle damage than concentric contractions (Lavender and Nosaka 2006). It is thought that mechanical stress is distributed to a smaller number of motor units during eccentric contractions than in concentric contractions, which leads to a perturbation in sarcomeric appearance and intracellular calcium homeostasis (Morgan 1990). A reduction in MVC strength in the days following an exercise is the best marker of muscle damage (Warren et al. 1999), and the strength loss lasts longer following eccentric exercise when compared with concentric or isometric exercise (Hamlin and Quigley 2001). However, it is not possible to determine whether muscle damage is induced from an MVC decrement immediately after exercise, since it also reflects neuromuscular fatigue.
Chen and Nosaka (2006) showed that after the second eccentric exercise bout, MVC returned close to the baseline by 1 day following the exercise, and this adaptation is referred to as the repeated bout effect. Similar results have been found in other studies in which elbow flexor eccentric exercise was performed (Janecki et al. 2014; Goodall et al. 2017). Although muscle damage is attenuated after the second bout of eccentric exercise (Hyldahl et al. 2017), the magnitude of changes in muscle damage markers such as MVC torque 1-day post-exercise is still greater when compared with a concentric exercise (Vissing et al. 2008). It is important to determine whether an MVC decrement is due to muscle damage or fatigue soon after exercise, since different strategies are required to recover muscles from damage versus fatigue. It may be that central and peripheral modulations are different between the first and second eccentric exercise bouts, and this can provide some clue for an early detection of muscle damage. However, this has never been assessed for knee extensor muscles, which play an important functional role in daily activities.
Therefore, the objective of the present study was to compare the contribution of central and peripheral factors to the loss of MVC following concentric versus eccentric contractions of the knee extensors resulting in the same reduction in MVC immediately post-exercise, and to assess potential differences between central and peripheral adaptations associated with the repeated bout effect.
Methods
Participants
Twelve healthy young men (age: 22.6 ± 2.7 years, body mass: 78.2 ± 8.4 kg, height: 182.3 ± 9.6 cm) with no history of neuromuscular disorders participated in this study. They reported to perform mainly team sport activities (e.g., football) between 2 and 5 h per week. The participants did not perform any resistance training of the quadriceps muscle for at least 6 months prior to the commencement of the study and during the experimental period including the interval between the first and second eccentric exercise bouts. Since dietary strategies may influence the recovery process after muscle-damaging exercise [see Sousa et al. (2014) for a review], the participants were asked not to change their dietary intake pattern during the whole study. The sample-size was calculated using G*Power (version 3.1.9.2; Kiel University, Kiel, Germany), based on an expected “medium” effect size (f2 = 0.25) for a difference in MVC changes at 1 day post-exercise between concentric and eccentric conditions, with α level of 0.05, power (1 − β) of 0.8 and correlation among repeated measures of 0.85. Before testing, each participant was informed about the nature and the aim of this study, as well as risks and discomfort associated with electrical stimulation. The study was conducted in conformity with the Declaration of Helsinki and approved by the local ethics committee.
Experimental design
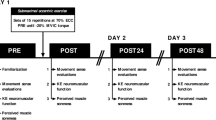
Each participant had three exercise sessions (about 90 min for each session) in which an exercise protocol consisting of concentric (CONC) contractions or two exercise protocols consisting of eccentric contractions (ECC1 and ECC2) of the knee extensors were performed. Each exercise session was performed at the same time of day. During each session, neuromuscular tests including voluntary and evoked contractions of the quadriceps femoris were performed before (PRE), immediately after (POST) and 1 h after (1H) as well as 1, 2, 3, and 4 days after the exercise (D1, D2, D3, and D4). The CONC was performed by one leg and the two ECCs (ECC1 and ECC2) were performed by the other leg. The choice of the leg and the order of the session were pseudo-randomised among the participants. Thus, seven participants performed the eccentric exercise with their dominant leg, whereas five participants performed the concentric exercise with their dominant leg, to control for any influence of leg dominancy on muscle fatigue, especially during the early recovery phase (Willems and Ponte 2013). There was a period of 1 or 2 weeks between concentric and eccentric sessions, while the two eccentric sessions were always separated by 2 weeks to ensure that the repeated bout effect (i.e., ECC1 versus ECC2) was assessed under the same experimental condition and was not influenced by the duration of the recovery phase.
Exercise protocol
All participants performed concentric or eccentric isokinetic maximal contractions of the knee extensors at an angular velocity of 60°·s− 1 for each condition, over a range of motion of 90° from 20° to 110° (ECC1 and ECC2) or from 110° to 20° (CONC), with 0° corresponding to the maximal leg extension. The leg was passively returned by the motor of the dynamometer (Biodex Shirley Corporation, New York, USA) to the initial position at 60°·s− 1, creating a ~ 1.5-s rest between contractions. The objective of each exercise session was to induce a decrease in isometric MVC torque from PRE to immediately post-exercise of at least 40%. The choice of this target was based on a study that showed ~ 40% decreases in MVC after concentric–eccentric contractions of the knee extensors (Behrens et al. 2012). At the end of the first 50 contractions, the participant performed one MVC. If the decrement was less than 40%, the participant continued the protocol with a series of 25 contractions, until reaching the target value. Once the target value was reached, the participant stopped the exercise and performed the neuromuscular tests as described in the following section.
The total number of contractions was recorded for each session, and the work quantity was calculated using the area under the torque–angle curve. The number of contractions was divided into five parts (every 20% of the total number), since the number of contraction performed in CONC, ECC1 and ECC2 was different between participants. The mean torque was calculated from the average of the five parts.
Muscle soreness
A 100-mm visual analogue scale (VAS) was used to assess muscle soreness (0 corresponding to “no pain” and 100 to “extremely painful”). The participants were asked to mark their subjective scale of soreness on the VAS after being palpated. For this purpose, all the areas of the superficial quadriceps muscles were checked (i.e., RF, VL, and VM) by applying the same pressure for 2 s. The palpation was always performed by the same investigator to ensure reproducible measurements.
Neuromuscular tests
Neuromuscular tests were performed on an isokinetic dynamometer (Biodex System 3 Pro®, Biodex Shirley Corporation, New York, USA). During the neuromuscular tests, the hip joint was set at 85° flexion (full hip extension = 0°), and the knee joint angle was set at 90° (full knee extension = 0°) with its axis being aligned with the axis of rotation of the dynamometer. Each participant was firmly strapped on the trunk and leg with belts to avoid any movements, and was asked to keep his arms crossed during all contractions. Mechanical data such as torque signals were recorded by a Delsys software (Delsys® Inc., Boston, MA, USA) and sampled at 5 kHz.
Maximal isometric voluntary contraction (MVC)
After a standardised warm-up protocol consisting of ten submaximal voluntary contractions at 30–80% of MVC, participants performed three MVCs at a knee joint angle of 90° (0° = full knee extension) on the dynamometer, interspaced by 1 min of rest. The first two maximal isometric contractions were performed without electrical stimulations, wherein the participant had to maintain the contraction for 4–5 s, and electrical stimulation was imposed during the third one (see next section). The participants were asked to generate their maximal torque as fast and forcefully as possible without any pre-activation (Aagaard et al. 2002). During these contractions, a visual feedback of the instantaneous torque was provided to the participants via a computer screen. The highest peak torque recorded from the two first MVC performed without electrical stimulation superimposition was used for further analysis.
Voluntary activation, contractile properties, and torque–frequency relationship
Torque–frequency relationship, voluntary activation, and twitch-doublet properties were obtained using a constant-current electrical stimulator (Digitimer DS7A®. Digitimer Ltd., Hertfordshire, UK). We used the electrical stimulation of the femoral nerve to assess the voluntary activation and the twitch-doublet properties, and the stimulation of the quadriceps muscle to assess torque–frequency relationship (Doguet et al. 2016).
For the stimulation of the femoral nerve, the cathode (diameter = 1 cm; ADInstruments Pty Ltd.) was placed over the femoral nerve in the femoral triangle (3–5 cm below the inguinal ligament) and the anode (5 × 9 cm; Chattanooga Medical Supply Inc.) was placed on the gluteal fold. Rectangular pulses with 1-ms duration and 400 V maximal output voltage were applied. The best stimulation site was defined before the tests and then marked on the skin for the measurements on the following sessions. To determine the optimal intensity of stimulation, the intensity was increased by 5 mA until the amplitude of the twitch torque plateaued. Intensity of stimulation was ranged between 80 and 150 mA. The stimulating intensity was then increased by 50%, i.e., supramaximal intensity.
During the third MVC, three electrical nerve impulsions were delivered in the following order: (1) superimposed paired stimulation (i.e., doublet, frequency: 100 Hz) to the torque plateau to assess voluntary activation; (2) potentiated doublet 2 s after the MVC to assess contractile properties, and (3) potentiated single stimulation (twitch) 5 s after the MVC to obtain the maximal amplitude of the M wave. The superimposed doublet amplitude (DBsup) was measured to evaluate the level of voluntary activation (VA) using the following formula:
The potentiated doublet amplitude (DB) was also measured.
Electrical muscle stimulation was used to assess the torque–frequency relationship. For muscle stimulation, the cathode (8 × 13 cm, Stimex, Rouffach, France) was placed over the high insertion of quadriceps muscle and a large anode (8 × 13 cm, Stimex, Rouffach, France) was placed at the level of vastus lateralis (VL) muscle (distal 1/3) and vastus medialis (VM). Rectangular pulses with 1 s duration and 400 V maximal output voltage were randomly applied for each frequency of 5, 10, 15, 20, 30, 40, 50, 60, 70, and 100 Hz. The intensity of stimulation was set at the intensity necessary to evoke 30% MVC using a 1-s train stimulation at a frequency of 70 Hz. This intensity was retained and used for all the frequencies. Thus, ten frequencies of stimulations were delivered at rest to each participant, at the same intensity. The duration of stimulation was 1 s for each frequency, with 15 s of recovery between each stimulation. The electrically evoked torques of the ten different frequencies were assessed. The ratio 10 Hz/100 Hz was also calculated to assess the low-frequency fatigue.
Electromyography
After skin preparation (i.e., shaving, abrading, and cleaning with alcohol), pairs of circular electrodes (diameter = 1 cm, ADInstruments Pty Ltd.) with an inter-electrode distance (center to center) of 2 cm were put on the rectus femoris (RF) and the VL at 2/3 of the line from anterior superior iliac spine to the lateral side of the patella, according to SENIAM recommendation. The ground electrode (diameter = 1 cm, ADInstruments Pty Ltd.) was placed on the patella. All EMG signals were filtered with a band-pass filter 5 Hz–1 kHz, and were collected and stored with BIOPAC Student Lab Pro® software (BIOPAC System Inc., Goleta, USA). The placement of recording electrodes was marked on the skin for measurements on the following sessions.
M-wave peak-to-peak amplitude (MMAX) was calculated from EMG responses to single femoral nerve electrical stimulation on relaxed VL and RF muscles. For each repetition, a mean EMG root mean square (RMS) value was calculated on a time window corresponding to the time required to complete the range of motion (i.e., 1.5 s). RMS values of EMG activity were then normalised to MMAX (RMS/MMAX) for the five sets of the fatiguing protocol (see above) to evaluate the efficiency of the central drive throughout the concentric and eccentric fatiguing protocols.
Statistical analysis
Statistical tests were performed using a Statistica software (StatSoft Inc., Tulsa, Oklahoma, USA). Normality of the data was tested by a Shapiro–Wilk test. For mean torque and RMS/Mmax wave ratio (see data analysis), the number of contractions was divided into five sections (every 20% of the total number), since the number of contractions was different between CONC, ECC1, and ECC2 among participants. Two-way repeated measures analysis of variance (ANOVA) was used for mechanical and EMG data [condition (CONC, ECC1, ECC2) × time (PRE, POST, 1H, D1, D2, D3, D4)] and for the exercise measurements [condition (CONC, ECC1, or ECC2) × section (first, second, third, fourth, and fifth)]. For the torque–frequency relationship, a three-way repeated measures ANOVA was used [condition (concentric or eccentric) × time (PRE, POST, 1H, D1, D2, D3, D4) × stimulation frequencies (5, 10, 15, 20, 30, 40, 50, 60, 70, and 100 Hz)]. When the ANOVA showed a significant interaction effect, post-hoc analyses were performed using a Tukey’s HSD test. Partial eta-squared (pη2) was also reported as a measure of effect size, with 0.07 being considered as moderate and 0.14 as large effect (Racinais et al. 2013). Significance level was set at p < 0.05. All results are presented as mean values ± standard deviation (SD).
Results
Exercise
As shown in Table 1, no significant difference was observed for the total number of contractions between the three exercise sessions (p = 0.26, pη2 = 0.40). However, the total work necessary to reach the targeted level of fatigue was significantly greater for ECC1 (p = 0.004) and ECC2 (p = 0.008) when compared to CONC, without a significant difference between ECC1 and ECC2.
The mean torque was significantly reduced between the first 20% to the last 20% of the protocol during the three exercises (CONC − 48.2 ± 6.0%, ECC1 − 33.8 ± 8.0%, ECC2 − 25.9 ± 14.1%, p < 0.05, pη2 = 0.21) (Table 1). The average torque produced during contractions was greater for ECC1 and ECC2 than CONC (p < 0.05, pη2 = 0.77) for each 20% section. No significant changes (p > 0.05; pη2 = 0.10) in RMS/Mmax over contractions were evident for CONC, ECC1, and ECC2. However, the ratio was lower during ECC1 and ECC2 when compared to CONC (p < 0.05, pη2 = 0.52) (Table 1).
Muscle soreness
A significant condition × time interaction was found for muscle soreness upon palpation (p < 0.001). No significant increase in muscle soreness was found after CONC (p > 0.05, pη2 = 0.72), but muscle soreness significantly increased at D1–D4 from the baseline (p < 0.001), with a peak occurring at D2 after ECC1 (Fig. 2b). Muscle soreness also significantly increased up to D3 (p < 0.05) after ECC2, but the peak value was significantly lower after ECC2 than ECC1 (Fig. 2b).
Neuromuscular parameters
Isometric MVC torque
No significant difference (p > 0.05, pη2 = 0.23) in baseline MVC torque values was evident between CONC (292.0 ± 45.3 Nm), ECC1 (307.5 ± 43.4 Nm), and ECC2 (296.4 ± 49.6 Nm). A significant interaction effect (condition × time) was evident for the changes in this variable (p < 0.001). A significant decrease was observed at POST for CONC (− 52.8 ± 7.6%, p < 0.001), ECC1 (− 48.7 ± 7.7%, p < 0.001) and ECC2 (− 45.2 ± 6.9%, p < 0.001). As expected, no significant differences were reported in the magnitude of decrease in MVC at POST between sessions, indicating that the same level of fatigue was induced (Figs. 1, 2a). When compared to PRE, ECC1 resulted in a significantly greater decrease in MVC than CONC at 1H, D1, D2, D3, and D4, while ECC2 showed significant differences from CONC only at 1H and D1 (p < 0.001), indicating a quicker recovery than after ECC1 (Figs. 1, 2a). The magnitude of decrease in MVC recorded at 1H was significantly correlated with the magnitude of decrease in MVC recorded at D2 (r = 0.53, p < 0.01, Fig. 3).
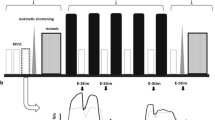
Representative traces of one participant recorded before (PRE), immediately after (POST) and 2 days after (D2) concentric (CONC) or eccentric exercises (ECC1 and ECC2 for the first and second bouts, respectively) for MVC force and potentiated doublets. Black arrows indicate the time of peripheral nerve stimulation. Dotted lines indicate the maximal value recorded at PRE for both MVC and DB
Changes in maximal voluntary isometric contraction (MVC) torque (a) and muscle soreness assessed by a visual analogic scale (VAS, b) before (PRE), immediately after (POST), 1 h after (1H) and 1, 2, 3, and 4 days (D1, D2, D3, and D4, respectively) after concentric (CONC), the first bout of eccentric (ECC1) and the second bout of eccentric exercise (ECC2). Mean ± SD values of 12 participants are shown. *Significantly different from PRE (p < 0.05). #Significantly different from CONC (p < 0.05)
Voluntary activation
No significant differences were found at baseline for VA among CONC, ECC1, and ECC2 (p > 0.05). There was a significant condition × time interaction (p = 0.001). VA decreased immediately after ECC1 (− 22.0 ± 13.7%) and ECC2 (− 21.4 ± 12.3%) (p < 0.001), thus highlighting the presence of central fatigue, but returned to pre-values at 1H (Fig. 4). Conversely, it seems that CONC did not induced central fatigue, since no significant reduction in VA was observed immediately after this session (− 3.8 ± 11.0%, p > 0.05).
Changes in maximal voluntary activation assessed by interpolated twitch technique of the knee extensors before (PRE), immediately after (POST), 1 h after (1H) and 1, 2, 3, and 4 days (D1, D2, D3, and D4, respectively) after concentric (CONC), the first bout of eccentric (ECC1) and the second bout of eccentric exercise (ECC2). Mean ± SD values of 12 participants are shown. *Significantly different from PRE (p < 0.05). #Significantly different from CONC (p < 0.05)
Peripheral parameters
A significant condition × time interaction effect was found for DB (p = 0.015, pη2 = 0.18). DB decreased (p < 0.05) at POST and 1H for CONC (− 35.9 ± 20.0 and − 21.3 ± 17.7%, respectively), thus highlighting the presence of peripheral fatigue. Even if a trend was observed for a change at POST for ECC1 (− 32.6 ± 23.7%, p = 0.10), DB was only significantly decreased at 1H for ECC1 and ECC2 (− 30.0 ± 15.7% and − 32.6 ± 17.2%, respectively). No significant differences were found at each timepoint between CONC and ECC1, and CONC and ECC2 (− > 0.05) (Table 2).
While a three-way ANOVA did not show a significant interaction effect for the torque–frequency relationship (condition × time × stimulation frequencies, p = 0.99), a significant condition × time interaction effect was found (p < 0.001). POST and 1H measurements were significantly decreased when compared with PRE for all the tested frequencies (p < 0.05), without significant (p > 0.05) differences between sessions (Fig. 5), thus highlighting the presence of peripheral fatigue after each session. When compared to PRE, ECC1 resulted in a greater decrease in evoked torques than CONC at D1 (p = 0.001), reflecting prolonged alterations in muscle properties following the first bout of eccentric exercise. Conversely, no significant difference between CONC and ECC2 was evident at the same timepoint (p > 0.05).
Torque–frequency relationship assessed by electrical muscle stimulation before (square), immediately after (diamond), 1 h after (circle) and 1 day after (triangle) concentric (CONC), the first bout of eccentric (ECC1) and the second bout of eccentric exercise (ECC2). Mean ± SD values of 12 participants are shown. *Significantly different from PRE (p < 0.05). #Significantly different from CONC (p < 0.05)
No significant condition × time interaction was found for 10 Hz/100 Hz ratio (p = 0.16), while there was a main time effect (p < 0.001), meaning that prolonged low-frequency force depression was not influenced by the type of exercise (i.e., concentric or eccentric). Indeed, a significant decrease of 10 Hz/100 Hz ratio at POST and 1H was evident for CONC, ECC1, and ECC2 (p < 0.05), as shown in Table 2. As displayed in Table 2, no modifications were observed for MMAX on the VL and the RF (p > 0.05, pη2 = 0.10).
Discussion
The present study compared changes in neuromuscular parameters after concentric versus eccentric exercise of the knee extensors resulting in the same level of decrease in MVC torque immediately post-exercise, i.e., decrease of at least 40% MVC. We hypothesised that the contribution of central and peripheral parameters in the MVC decrease would be different between concentric and eccentric exercises and that neuromuscular adaptations after ECC1 would decrease the changes in the parameters after ECC2. The present study confirmed the hypotheses and showed that the contribution of central and peripheral factors associated with the decreases in MVC torque was clearly different between CONC and ECC. When comparing between ECC1 and ECC2, the magnitude of muscle damage was lower after ECC2.
The mean torque produced in the exercise was significantly greater during ECC1 (234.7 ± 55.2 Nm) and ECC2 (225.6 ± 45.1 Nm) when compared with CONC (118.9 ± 23.1 Nm). Consequently, the work produced in the exercise was significantly greater for both ECC sessions than CONC, indicating that more work was produced during ECC to induce the same decrement of MVC torque. During eccentric contractions, the active muscles resist to a higher external torque than the torque developed by the active motor units (Enoka 1996). Once all motor units are recruited, the maximal torque is higher for eccentric than concentric contraction for the same angular velocity (Åstrand et al. 1994). This is mainly due to the lengthening of the elastic structures of the muscle–tendon complex, adding a torque to the one already produced by the activated muscle fibres (Guilhem et al. 2010). This also leads to a lower energy cost for eccentric contractions than in concentric contractions. Middleton and Montero (2004) reported a preservation of phosphagen (e.g., adenosine triphosphate, phosphocreatine) during eccentric contractions.
It seems likely that neuromuscular fatigue plays a major role in the decreases of MVC torque immediately and 1 h after eccentric exercise in addition to muscle damage. MVC torque decreased at POST and 1H for ECC1 and ECC2, but only at POST for CONC (Fig. 2). Behrens et al. (2012) reported 16% decrease of VA immediately after 100 maximal voluntary concentric-eccentric contractions of the knee extensors. Prasartwuth et al. (2005) showed 19% decrease in VA immediately after eccentric exercise of the elbow flexors that resulted in 40% decrease in MVC. A significant decrease in VA measured by electrical stimulation at the motor point (− 28%), and transcranial magnetic stimulation (− 19%) was reported immediately after 30 maximal eccentric contractions of the elbow flexors (Goodall et al. 2017). In the present study, VA significantly decreased approximately 20% at immediately after ECC1 and ECC2 only. The magnitude of decrease in VA at immediately after ECC appears comparable to that reported in the previous studies shown above. Babault et al. (2006) showed a decreased VA after three sets of 30 concentric leg extensions (1st − 11.2%, 2nd − 15.2% and 3rd − 27.0%). However, the present study did not find any decrease in VA after CONC. This discrepancy may be due to the methodological difference in the VA assessment. Babault et al. (2006) assessed the VA during the last dynamic concentric contraction, while in our study VA was assessed during maximal isometric contraction after concentric exercise, with a 5–10 s period of rest between the end of the exercise and the VA measurement. It has been documented that neuromuscular assessment must be performed as soon as possible after exercise termination to be a reliable estimate of neuromuscular fatigue (Froyd et al. 2013). However, Bigland-Ritchie et al. (1978) reported that central mechanisms of acute muscle fatigue recover in about 3 min, so changes in VA should have been expected after 5–10 s of the end of the concentric exercise. Considering these conflicting results, future studies should be conducted to give more insight on VA after repeated concentric contractions.
One might assume that muscle soreness associated with muscle damage after ECC was responsible for the decreased VA. Nociceptive III and IV afferents may induce modulations at spinal and/or supra-spinal levels that could impact the level of VA after ECC (Behrens et al. 2012). However, in the present study, the decreases in VA were not observed after 1-day post-ECC when muscle soreness developed. This suggests no effect of muscle soreness on the capacity for the central nervous system to voluntary activate the muscle. Modulations occurring at the spinal level may be considered as a plausible explanation for the decreased VA after ECC but not CONC. Indeed, it was reported H-reflex amplitude decreased during eccentric contractions while it was unchanged during concentric contractions in a non-fatigued state (Duclay and Martin 2005; Duclay et al. 2011). Thus, it seems possible that increased spinal modulations such as higher presynaptic inhibition exerted on Ia afferents terminals (Rudomin and Schmidt 1999), led to a decreased VA after ECC1 and ECC2. Lactate measurement could have provided more insight on our results, especially because lactate accumulation can significantly increase the response of group III and IV nociceptive afferents thus influencing the level of voluntary activation after the fatiguing concentric or eccentric exercise (Kennedy et al. 2014).
The present study showed a significant reduction of electrically evoked torque after each exercise (Table 2). DB decreased at POST and 1H for CONC but only at 1H for ECC1 and ECC2, while torque–frequency relationship decreased similarly at POST and 1H for CONC, ECC1 and ECC2 (Fig. 5). An alteration of the neuromuscular propagation, a failure of the excitation–contraction coupling, a modification of substrates reserves or possible modifications of intracellular environment could likely explain these results (Feasson et al. 2006). Nevertheless, the perturbation of the neuromuscular propagation does not seem to be a valid explanation for the decrease in the electrically evoked torque, since no decreases in VL and RF MMAX were found immediately and 1 h after CONC, ECC1, and ECC2. An alteration of the calcium release via the sarcoplasmic reticulum could explain the evoked torque decrease (Westerblad and Allen 2002). Metabolite accumulation (e.g., inorganic phosphate, adenosine diphosphate or hydrogen) disturbs the calcium release and the excitation–contraction coupling (Babault et al. 2006). Although there was no difference in the magnitude of the decrease in 10 Hz/100 Hz ratio between CONC, ECC1, and ECC2 at POST and 1H, DB was significantly reduced at POST after CONC, but not after ECC1 and ECC2, even if a trend (p = 0.10) for a decrease was observed after ECC1. We cannot exclude that this study was underpowered to detect (1) significant changes in DB after eccentric exercises and (2) higher levels of prolonged low-frequency force depression (i.e., low-frequency fatigue) after eccentric exercise than after concentric exercise, as previously demonstrated (Iguchi and Shields 2010; Janecki et al. 2016; Newham et al. 1983). However, our DB-related results are in accordance with the finding of Pasquet et al. (2000) who showed a greater decrease in evoked torque immediately after concentric exercise than after eccentric exercise. It is possible that an alteration of the excitation–contraction coupling process is associated with a higher energy requirement during the concentric than in eccentric exercise (Pasquet et al. 2000).
In the present study, a prolonged decrease of MVC was observed up to 4 days after ECC1, while it was only decreased up to 1 h after CONC. These results support the findings of the previous study showing that muscle damage is peculiar to unaccustomed eccentric exercise (Coudreuse et al. 2004). The present study observed a strong correlation between the magnitude of decrease in MVC torque at 1 h after and that at 2 days after ECC, indicating that MVC decrement recorded at 1 h post-exercise can be a relevant marker of muscle damage (Fig. 3). This is particularly relevant for coaches and trainers to evaluate the impact of a training session or a match on the neuromuscular system. The greater mechanical stress applied on each muscle fibre during eccentric contractions may be a major cause of the muscle damage (Bigland-Ritchie et al. 1986). Although muscle damage is related to the exercise intensity and volume (Nosaka and Newton 2002), it should be noted that the total work was significantly greater for ECC than CONC. We cannot exclude the possibility that the greater damage shown after ECC1 compared to CONC was due to the difference in work quantity. Further research is needed to assess if muscle damage is still greater after eccentric than after concentric exercise of equal work quantity.
It is important to note that the prolonged decreases in MVC torque after ECC1 were mainly due to alterations of the peripheral factors, while central factors do not seem to be altered, since the VA level returned close to the baseline already at 1 day after ECC1. However, this result appears inconsistent with the studies reporting a prolonged decrease of VA up to one (Goodall et al. 2017; Prasartwuth et al. 2005) and 2 days (Racinais et al. 2008) after eccentric exercise of the plantar flexors or the elbow flexors, respectively. Racinais et al. (2008) suggested that a supra-spinal modulation of muscle activation was associated with the prolonged decreases in VA, since the H-reflex was not affected by the eccentric exercise. Albeit speculative, these discrepancies to our results may be due to the fact that the eccentric exercise performed on an isokinetic dynamometer in the current study was unusual for the participants, contrary to the study of Racinais et al. (2008) using daily movement (i.e., walking) on a treadmill.
The decrease in the torque–frequency relationship remained up to 1 day after ECC1, while the values returned close to the baseline after CONC (Fig. 5), which confirmed that the prolonged MVC decrease was mainly attributed to peripheral alterations. Dundon et al. (2008) explained that the damage to the muscle fibre membrane could alter the amplitude or frequency of the sarcolemma action potential, which may influence the summation of the muscle fibre action potentials (Coudreuse et al. 2004; Proske and Allen 2005) and the amplitude of the electrically evoked torque. However, it is unlikely that such alterations could have explained our results since no significant changes were reported in VL and RF M-wave amplitude (Table 2) as well as in VL M-wave duration (data not shown).
The adaptations induced by the initial eccentric exercise attenuate muscle damage after the second bout of the same eccentric exercise, which is known as the repeated bout effect (McHugh 2003; Chen and Nosaka 2006; Guilhem et al. 2010; Goodall et al. 2017). In the current study, MVC torque, muscle soreness, and force–frequency relationship were less impacted after ECC2 than ECC1, suggesting that some neuromuscular adaptations occurred after ECC1, leading to less muscle damage after ECC2. It is important to note that some indications of muscle damage were still present after ECC2, which were not observed after CONC. Mechanical adaptations such as cytoskeletal protein reorganisation responsible for aligning the sarcomere structure (i.e., titin and desmin) and/or remodelling of extra cellular matrix including connective tissue surrounding muscle fibres and fascicles, are associated with the repeated bout effect (Hyldahl et al. 2017), which may allow a better dissipation of the mechanical constraint (Lapier et al. 1995).
In conclusion, this study showed that the origins of decrease in MVC torque were different between concentric and eccentric exercises when the magnitude of MVC torque change was matched. The decrease of MVC after CONC was mainly attributed to peripheral mechanisms while the same decrease observed after ECC1 and ECC2 was attributed to both peripheral and central mechanisms. Moreover, the repeated bout effect appears to be associated mainly with peripheral adaptation mechanisms.
Abbreviations
- DB:
-
Potentiated doublet amplitude evoked at rest
- DBsup:
-
Superimposed doublet amplitude
- EMG:
-
Electromyography
- MMAX :
-
Maximal M wave
- MVC:
-
Maximal voluntary contraction
- pη 2 :
-
Partial eta-squared
- RF:
-
Rectus femoris
- RMS:
-
Root mean square
- VA:
-
Voluntary activation
- VAS:
-
Visual analogue scale
- VL:
-
Vastus lateralis
- VM:
-
Vastus medialis
References
Aagaard P, Simonsen EB, Andersen JL, Magnusson P, Dyhre-Poulsen P (2002) Increased rate of force development and neural drive of human skeletal muscle following resistance training. J Appl Physiol 93(4):1318–1326. https://doi.org/10.1152/japplphysiol.00283.2002
Åstrand P-O, Rodahl K, Lacour J-R, Duizabo D (1994) Précis de physiologie de l’exercice musculaire. Masson, Paris, p 420
Babault N, Desbrosses K, Fabre MS, Michaut A, Pousson M (2006) Neuromuscular fatigue development during maximal concentric and isometric knee extensions. J Appl Physiol 100(3):780–785. https://doi.org/10.1152/japplphysiol.00737.2005
Behrens M, Mau-Moeller A, Bruhn S (2012) Effect of exercise-induced muscle damage on neuromuscular function of the quadriceps muscle. Int J Sports Med 33(8):600–606. https://doi.org/10.1055/s-0032-1304642
Bigland-Ritchie B, Jones D, Hosking G, Edwards R (1978) Central and peripheral fatigue in sustained maximum voluntary contractions of human quadriceps muscle. Clin Sci Mol Med 54(6):609–614
Bigland-Ritchie BR, Dawson NJ, Johansson RS, Lippold OC (1986) Reflex origin for the slowing of motoneurone firing rates in fatigue of human voluntary contractions. J Physiol 379:451–459
Chen TC, Nosaka K (2006) Effects of number of eccentric muscle actions on first and second bouts of eccentric exercise of the elbow flexors. J Sci Med Sport 9(1):57–66
Coudreuse JM, Dupont P, Nicol C (2004) Delayed post effort muscle soreness. Ann Readapt Med Phys 47:290–298
Doguet V, Nosaka K, Plautard M, Gross R, Guilhem G, Guével A, Jubeau M (2016) Neuromuscular changes and damage after isoload versus isokinetic eccentric exercise. Med Sci Sports Exerc 48(12):2526–2535
Duclay J, Martin A (2005) Evoked H-reflex and V-wave responses during maximal isometric, concentric, and eccentric muscle contraction. J Neurophysiol 94(5):3555–3562. https://doi.org/10.1152/jn.00348.2005
Duclay J, Pasquet B, Martin A, Duchateau J (2011) Specific modulation of corticospinal and spinal excitabilities during maximal voluntary isometric, shortening and lengthening contractions in synergist muscles. J Physiol 589(Pt 11):2901–2916. https://doi.org/10.1113/jphysiol.2011.207472
Dundon JM, Cirillo J, Semmler JG (2008) Low-frequency fatigue and neuromuscular performance after exercise-induced damage to elbow flexor muscles. J Appl Physiol 105(4):1146–1155. https://doi.org/10.1152/japplphysiol.01339.2007
Enoka RM (1996) Eccentric contractions require unique activation strategies by the nervous system. J Appl Physiol 81(6):2339–2346
Farina D, Merletti R, Enoka RM (2014) The extraction of neural strategies from the surface EMG: an update. J Appl Physiol 117(11):1215–1230. https://doi.org/10.1152/japplphysiol.00162.2014
Feasson L, Camdessanche JP, El Mandhi L, Calmels P, Millet GY (2006) Fatigue and neuromuscular diseases. Ann Readapt Med Phys 49(6):289–300, 375–284. https://doi.org/10.1016/j.annrmp.2006.04.015
Froyd C, Millet GY, Noakes TD (2013) The development of peripheral fatigue and short-term recovery during self-paced high-intensity exercise. J Physiol 591(5):1339–1346. https://doi.org/10.1113/jphysiol.2012.245316
Gandevia SC (2001) Spinal and supraspinal factors in human muscle fatigue. Physiol Rev 81(4):1725–1789
Goodall S, Thomas K, Barwood M, Keane K, Gonzalez JT, St Clair Gibson A, Howatson G (2017) Neuromuscular changes and the rapid adaptation following a bout of damaging eccentric exercise. Acta Physiol (Oxf) 220(4):486:500. https://doi.org/10.1111/apha.12844
Guilhem G, Cornu C, Guevel A (2010) Neuromuscular and muscle-tendon system adaptations to isotonic and isokinetic eccentric exercise. Ann Phys Rehabil Med 53(5):319–341. https://doi.org/10.1016/j.rehab.2010.04.003
Hamlin MJ, Quigley BM (2001) Quadriceps concentric and eccentric exercise 1: changes in contractile and electrical activity following eccentric and concentric exercise. J Sci Med Sport 4(1):88–103
Hyldahl RD, Chen TC, Nosaka K (2017) Mechanisms and mediators of the skeletal muscle repeated bout effect. Exerc Sport Sci Rev 45(1):24–33. https://doi.org/10.1249/JES.0000000000000095
Iguchi M, Shields RK (2010) Quadriceps low-frequency fatigue and muscle pain are contraction-type-dependent. Muscle Nerve 42(2):230–238. https://doi.org/10.1002/mus.21679
Janecki D, Jaskolska A, Marusiak J, Andrzejewska R, Jaskolski A (2014) Twitch mechanical properties after repeated eccentric exercise of the elbow flexors. Appl Physiol Nutr Metab 39(1):74–81. https://doi.org/10.1139/apnm-2013-0097
Janecki D, Jaskolska A, Marusiak J, Jaskolski A (2016) Low-frequency fatigue assessed as double to single twitch ratio after two bouts of eccentric exercise of the elbow flexors. J Sports Sci Med 15(4):697–703
Kay D, Gibson ASC, Mitchell MJ, Lambert MI, Noakes TD (2000) Different neuromuscular recruitment patterns during eccentric, concentric and isometric contractions. J Electromyogr Kinesiol 10(6):425–431
Kennedy DS, McNeil CJ, Gandevia SC, Taylor JL (2014) Fatigue-related firing of distal muscle nociceptors reduces voluntary activation of proximal muscles of the same limb. J Appl Physiol 116(4):385–394. https://doi.org/10.1152/japplphysiol.01166.2013
Lapier TK, Burton HW, Almon R, Cerny F (1995) Alterations in intramuscular connective tissue after limb casting affect contraction-induced muscle injury. J Appl Physiol 78(3):1065–1069
Lavender AP, Nosaka K (2006) Changes in fluctuation of isometric force following eccentric and concentric exercise of the elbow flexors. Eur J Appl Physiol 96(3):235–240
McHugh MP (2003) Recent advances in the understanding of the repeated bout effect: the protective effect against muscle damage from a single bout of eccentric exercise. Scand J Med Sci Sports 13(2):88–97
Middleton P, Montero C (2004) Eccentric muscular contraction: implications in treatment of athletes. Ann Readapt Med Phys 47(6):282–289
Morgan DL (1990) New insights into the behavior of muscle during active lengthening. Biophys J 57(2):209
Newham DJ, Mills KR, Quigley BM, Edwards RH (1983) Pain and fatigue after concentric and eccentric muscle contractions. Clin Sci (Lond) 64(1):55–62
Nosaka K, Newton M (2002) Difference in the magnitude of muscle damage between maximal and submaximal eccentric loading. J Strength Cond Res 16(2):202–208
Pasquet B, Carpentier A, Duchateau J, Hainaut K (2000) Muscle fatigue during concentric and eccentric contractions. Muscle Nerve 23(11):1727–1735
Prasartwuth O, Taylor JL, Gandevia SC (2005) Maximal force, voluntary activation and muscle soreness after eccentric damage to human elbow flexor muscles. J Physiol 567(1):337–348
Prasartwuth O, Allen T, Butler J, Gandevia S, Taylor J (2006) Length-dependent changes in voluntary activation, maximum voluntary torque and twitch responses after eccentric damage in humans. J Physiol 571(1):243–252
Proske U, Allen TJ (2005) Damage to skeletal muscle from eccentric exercise. Exerc Sport Sci Rev 33(2):98–104
Racinais S, Bringard A, Puchaux K, Noakes TD, Perrey S (2008) Modulation in voluntary neural drive in relation to muscle soreness. Eur J Appl Physiol 102(4):439–446
Racinais S, Maffiuletti NA, Girard O (2013) M-wave, H-and V-reflex recruitment curves during maximal voluntary contraction. J Clin Neurophysiol 30(4):415–421. https://doi.org/10.1097/WNP.0b013e31829ddcf1
Rudomin P, Schmidt RF (1999) Presynaptic inhibition in the vertebrate spinal cord revisited. Exp Brain Res 129(1):1–37
Sousa M, Teixeira VH, Soares J (2014) Dietary strategies to recover from exercise-induced muscle damage. Int J Food Sci Nutr 65(2):151–163. https://doi.org/10.3109/09637486.2013.849662
Vissing K, Overgaard K, Nedergaard A, Fredsted A, Schjerling P (2008) Effects of concentric and repeated eccentric exercise on muscle damage and calpain–calpastatin gene expression in human skeletal muscle. Eur J Appl Physiol 103(3):323–332. https://doi.org/10.1007/s00421-008-0709-7
Warren GL, Lowe DA, Armstrong RB (1999) Measurement tools used in the study of eccentric contraction-induced injury. Sports Med 27(1):43–59
Westerblad H, Allen DG (2002) Recent advances in the understanding of skeletal muscle fatigue. Curr Opin Rheumatol 14(6):648–652
Willems ME, Ponte JP (2013) Divergent muscle fatigue during unilateral isometric contractions of dominant and non-dominant quadriceps. J Sci Med Sport 16(3):240–244. https://doi.org/10.1016/j.jsams.2012.06.005
Acknowledgements
The authors sincerely thank Clément Billet for his essential help during all the testing sessions.
Author information
Authors and Affiliations
Contributions
RS was involved in the conception of the research, the acquisition, analysis, and interpretation of the data. RS was also involved in the revision of the manuscript. MJ and KN were involved in the conception of the research design, the analysis and interpretation of the results and the writing of the manuscript (revising the work). As the contributing author, MJ gave his final approval for submission.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Nicolas Place.
Rights and permissions
About this article
Cite this article
Souron, R., Nosaka, K. & Jubeau, M. Changes in central and peripheral neuromuscular fatigue indices after concentric versus eccentric contractions of the knee extensors. Eur J Appl Physiol 118, 805–816 (2018). https://doi.org/10.1007/s00421-018-3816-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-018-3816-0