Abstract
Background
Implementation of mandibular advancement splint (MAS) therapy as first-line treatment for obstructive sleep apnoea (OSA) is hindered by inter-individual variability of treatment outcomes and lack of robust patient selection methods. Optimal continuous positive airway pressure (CPAP) requirement provides an estimate of airway collapsibility severity, and high CPAP requirements predict MAS therapy failure in retrospective studies. Thus, understanding the effects of mandibular advancement on optimal CPAP requirements may enhance optimisation of patient selection for MAS therapy.
Objective
This study aims to determine dose-dependent effects of mandibular advancement on optimal CPAP requirements in OSA.
Methods
Prior to MAS therapy initiation, participants with OSA (apnoea-hypopnea index (AHI) > 10 events/h) underwent a research polysomnogram in which a remotely controlled mandibular positioner (RCMP) was used to determine dose–response effects of varying mandibular advancement positions (0% ‘habitual bite’ and 25, 50, 75 and 100% of maximum mandibular advancement, in random order) on optimal CPAP requirements. A separate polysomnography determined treatment outcome. Data are presented as mean ± SD or median (1st–3rd quartiles).
Results
Seventeen participants (age = 47 ± 9 years, body mass index = 26 kg/m2 (23–27), apnoea-hypopnea index = 18 events/h (14–44) and minimal oxygen saturation = 84 ± 7%) were studied. Optimal CPAP requirements were reduced with mandibular advancement in a dose-dependent manner (8.9 ± 2.4 vs. 7.9 ± 2.8, 6.4 ± 1.8, 5.7 ± 1.9 and 4.9 ± 1.8 cmH2O; respectively, p < 0.0001). Compared with non-responders, responders to MAS therapy had lower AHI, lower arousal index and greater MinSaO2 at baseline. Optimal CPAP requirements at 0% mandibular advancement (or other positions) were not different between groups.
Conclusions
Increasing mandibular advancement lowers optimal CPAP requirements in a dose-dependent manner. This supports prior work indicating a beneficial effect of MAS on upper airway collapsibility.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Obstructive sleep apnoea (OSA) is a debilitating health condition which puts a significant burden on patients and health systems worldwide [1,2,3,4,5]. First-line therapy, continuous positive airway pressure (CPAP), is highly efficacious in reducing OSA. However, it is not well-tolerated by many patients [6,7,8]. Mandibular advancement splints (MAS), the second-line therapy for OSA [9], tend to be less efficacious than CPAP but are associated with better adherence. This likely explains similar health outcomes of MAS compared with CPAP [10,11,12]. Nonetheless, there remain a significant proportion of people with OSA who experience suboptimal treatment efficacy with MAS therapy despite regular usage [13,14,15,16,17,18,19,20,21]. Thus, accurate patient selection is essential to optimise MAS therapy outcomes and avoid unnecessary costs.
A link between MAS therapy outcome and the pathophysiological causes of OSA (including poor airway anatomy and muscle function, high arousability from sleep and high sensitivity of respiratory control system [22, 23]) has been established [24]. Indeed, we recently found that mandibular advancement improves airway collapsibility during sleep in a dose-dependent manner [25]. This aligns with previous work suggesting that MAS therapy predominantly targets pharyngeal anatomy to improve OSA [26,27,28]. However, measurement of the pathophysiological causes of OSA requires invasive instrumentation and intensive staff training, which are not accessible for most clinical sleep laboratories.
The optimal CPAP requirement (the level of nasal pressure provided by CPAP at which all forms of obstructive respiratory events are rectified) has been found to reflect the severity of passive airway collapsibility in OSA patients [29]. Additionally, optimal CPAP requirements above 10.5 cmH2O in Japanese and 12–13 cmH2O in Caucasians have been reported to predict treatment failure with MAS [27, 30, 31]. Although these studies were retrospective, they highlight the potential to use CPAP requirement as a patient selection tool for MAS therapy in OSA. This has clinical appeal as the majority of people diagnosed with OSA receive a trial of CPAP in the first instance.
Thus, we aimed to prospectively determine the acute effects of varying mandibular advancement positions on optimal CPAP requirements during sleep in OSA patients before commencing MAS therapy. We also aimed to determine whether optimal CPAP requirement without mandibular advancement is a predictor of MAS therapy outcome in a prospective cohort. The findings of this study may provide a basis to assist in clinical selection of OSA patients who are likely responders to MAS therapy and, thereafter, facilitate first-line prescription of this treatment modality according to a personalised treatment approach [23, 24].
Methods
Participants
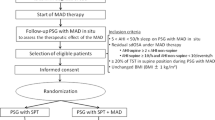
People with OSA (apnoea-hypopnea index (AHI) > 10 events/h), in whom MAS therapy was recommended (either due to patient preference or insufficient CPAP usage causing ineffective OSA treatment) by a sleep physician, were screened to ensure absence of contraindications to MAS therapy (e.g. gum diseases, insufficient number of teeth, severe daytime sleepiness and central sleep apnoea predominance). Twenty (76% males; 47% having previously trialled/used CPAP) eligible patients provided informed written consent to participate in the study. The Human Research Ethics Committee at North Sydney Local Health District provided ethical approval for the study (16/324).
Protocol
Prior to commencement of MAS therapy (SomnoDent Flex, SomnoMed Ltd., Australia), individual participants underwent a dental assessment to measure the maximal range of mandibular advancement (procedures outlined below) as previously described [32]. Participants also underwent a 1-week CPAP washout period before investigation if they were current CPAP users. A research polysomnography (instrumentation outlined below) was then performed to determine optimal CPAP requirements at five predetermined mandibular advancement positions (0% ‘habitual bite’ and 25, 50, 75 and 100% of the maximal voluntary mandibular advancement in randomised order). Optimal CPAP requirements were obtained in real time by a single researcher who was blinded to the randomisation order of the mandibular advancement positions. A second standard polysomnography [33] was performed approximately 6 weeks after initiation of MAS therapy to determine treatment outcome.
Measurement of mandibular motion range
During the dental assessment visit, two dental trays of a commercially available remotely controlled mandibular positioner (MATRx, Zephyr Sleep Technologies Inc., Calgary, AB, Canada) were filled with dental impression material and anchored onto the upper and lower dental arches of individual participants. The dental trays are coupled via a sliding bar in a manner that enables antero-posterior mandibular movement, provides a fixed vertical opening (~ 3 mm) and restricts lateral movement. A millimetre scale is printed on the sliding bar to enable quantification of the maximal voluntarily mandibular advancement (100% protrusion), which was measured relative to habitual bite (0 mm reference point; 0% protrusion). The remaining predetermined mandibular advancement positions were then calculated from these measurements to be entered in the software of the RCMP device during the research polysomnography with assistance from another overnight staff.
Instrumentation
In addition to standard polysomnography instrumentation (Grass Amplifier System, Natus Medical Inc., Pleasanton, CA, USA), participants were fitted with an RCMP device to enable precise mandibular positioning during sleep [32, 34,35,36,37]. Participants were also fitted with a non-vented nasal mask (GelMask; Philips Respironics, Murrysville, PA, USA) connected to a modified CPAP machine (PCRIT 3000, Philips Respironics, Murrysville, PA, USA) that can deliver up to + 20 cmH2O of CPAP and − 20 cmH2O of continuous negative airway pressure, a differential pressure sensor (DP15-16, Validyne Engineering, Northridge, CA, USA) to measure mask pressure, a pneumotachograph (3700A; Hans Rudolph Inc., Shawnee Mission, KS, USA) to measure airflow and a two-way valve (Series 1410; Hans Rudolph Inc., Shawnee Mission, KS, USA) to prevent CO2 rebreathing. We used an analogue-to-digital converter (Power1401, CED, Cambridge UK) to acquire data via Spike-2 software (version 7, CED, Cambridge, UK).
Measurement of optimal CPAP requirements
Before the lights were turned off, participants were asked to sleep supine throughout the night while the RCMP device was set to the first randomised mandibular position (with assistance from another overnight staff member) and CPAP was set to + 4 cmH2O. Air pressure was then incrementally titrated every 3–5 min until all forms of obstructive respiratory events were rectified (indicated by visual inspection of stable breathing in peak inspiratory flow signal without episodes of respiratory-related EEG-scored cortical arousals). To ensure optimisation of breathing, air pressure was increased by 2–3 cmH2O above the identified optimal CPAP level to detect any increase in peak inspiratory flow [38]. Next, mandibular advancement position was changed, CPAP level was reset to + 4 cmH2O and the titration procedure was repeated. If obstructive events were not detected while on + 4 cmH2O, the pressure was reduced to a lower level in an attempt to induce flow limitation before initiating incremental pressure titration again. Identification of optimal pressure requirements was repeated twice for each mandibular advancement position during the same overnight study to confirm reproducibility. Data analyses were repeated after sleep staging, and scoring was performed by an experienced technologist according to AASM criteria 2012 [33] to ensure intra-rater reliability.
Definition of MAS therapy outcome
Participants who achieved a residual AHI ≤ 5 events/h of sleep on MAS therapy were classified as responders compared with those whose treatment AHI was > 5 events/h (non-responders). A more stringent cutoff (5 events/h of sleep) of treatment success was used in this study to conform with clinical criteria for OSA diagnosis and CPAP outcomes.
Statistical analyses
SPSS software pack (V20, IBM Corp., NY, USA) was used to conduct statistical analyses. Shapiro-Wilk test was performed to assess normality distribution. Repeated measures analyses of variance (RM ANOVAs) or Kruskal-Wallis tests (as appropriate) were performed to examine differences in optimal CPAP requirements across mandibular advancement positions. Tukey’s (or Dunn’s for non-parametric variables) post hoc tests were used to assess multiple comparisons. Two-tailed paired t tests were used to compare anthropometric and polysomnographic parameters at baseline vs. MAS therapy. Unpaired t tests (or Mann-Whitney U test for non-parametric variables) were performed to examine potential differences in optimal CPAP requirement at 0% mandibular advancement between responders to MAS therapy vs. non-responders. Data were expressed as mean ± SD or median (25th–75th quartiles) according to normality distribution. A linear regression model was also built to assess the utility of optimal CPAP requirement at 0% mandibular advancement to predict the percent reduction in AHI with MAS compared with baseline.
Results
Participant characteristics at baseline diagnostic polysomnography
We obtained sufficient data for analyses during all mandibular advancement positions from 17 out of 20 participants. One patient had severe bruxism during the polysomnography study that prevented the RCMP device from working properly, and two patients were unable to sleep with the RCMP device. Table 1 summarises baseline patient characteristics and MAS settings for the 17 participants who completed the study protocol. Although dropouts were older compared with participants (65 ± 11 vs. 47 ± 9 years, p = 0.04), there was no significant difference between participants and dropouts in body mass index (BMI, p = 0.06) or OSA severity (AHI p = 0.62; minimal oxygen saturation (MinSaO2) p = 0.14; 4% oxygen desaturation index (ODI4%) p = 0.18; arousal index p = 0.12). There was also no difference in age, BMI or OSA severity between previous CPAP users and those who were CPAP naive.
Optimal CPAP requirements across five mandibular advancement positions
Measurements of optimal CPAP requirements were obtained at least twice at each mandibular advancement position with high reliability (overall intra-class correlation coefficient = 0.91). We observed a significant dose-dependent reduction in optimal CPAP requirements during NREM sleep across the five mandibular advancement positions (one-way RM ANOVA p < 0.0001, Fig. 1a), although to a different extent between individuals (Fig. 1b). Tukey’s multiple comparison testing showed stepwise reductions in the significance level (p value) with mandibular advancement compared with 0% position. The average reduction in optimal CPAP requirements was similar between each two consecutive mandibular advancement positions (1.0 ± 1.5 for 0 to 25% vs. 1.5 ± 2.6 for 25 to 50% vs. 0.7 ± 1.1 for 50 to 75% vs. 0.8 ± 1.3 for 75 to 100%, one-way RM ANOVA p = 0.62). The average reduction in optimal CPAP requirements from 0 to 100% advancement was 4.0 ± 2.7 cmH2O. We observed a significant correlation between the stepwise reduction in optimal CPAP requirements and the corresponding level of mandibular advancement in millimetres (r = − 0.588, p < 0.001; Fig. 2). However, no correlation was found between the total reduction in optimal CPAP requirements (i.e. the change from 0 to 100% advancement) and the maximum range of mandibular advancement in millimetres (r = 0.014, p = 0.888).
Optimal continuous positive airway pressure (CPAP) requirements (a) decreased across five mandibular advancement positions in a dose-dependent manner (8.9 ± 2.4 vs. 7.9 ± 2.8 vs. 6.4 ± 1.8 vs. 5.7 ± 1.9 vs. 4.9 ± 1.8 cmH2O; respectively, p < 0.0001), although to a different extent between individual participants (b). SEM, standard error of means; IQR, interquartile range; SD, standard deviation
Optimal CPAP requirements and response to MAS therapy
Table 1 summarises the changes in key clinical variables and polysomnographic indices with MAS therapy compared with baseline. In our sample, MAS therapy reduced total AHI by 72%, ODI4% by 82%, arousal index by 43%, maximal apnoea duration by 17%, maximal hypopnea duration by 42% and improved MinSaO2 by 5%. No difference in sleep latency or efficiency with MAS therapy was noted compared with baseline (unpaired t tests, p = 0.7 and 0.9, respectively).
Eight (47%) out of seventeen participants were responders to MAS therapy (cutoff of residual AHI with MAS therapy ≤ 5 events/h of sleep). There was no difference between responders and non-responders in the maximum range of mandibular advancement (8.0 ± 2.4 vs. 8.3 ± 2.2 mm, respectively, unpaired t test p = 0.387) or therapeutic mandibular advancement (6.8 ± 2.1 vs. 7.6 ± 2.1 mm, respectively, unpaired t test p = 0.220). At baseline, responders, compared with non-responders, had significantly lower total AHI (15.0 (12.9–19.8) vs. 35.5 (16.8–46.4) events/h of sleep, Mann-Whitney U test p < 0.001), lower arousal index (30.4 ± 9.5 vs. 46.6 ± 16.3 events/h of sleep, unpaired t test p = 0.010), shorter longest apnoea duration (28.5 ± 13.4 vs. 42.2 ± 15.0 s, respectively, unpaired t test p = 0.030) and less overnight hypoxia (86 ± 4 vs. 81 ± 7%, unpaired t test p = 0.040). However, we found no difference between the two response groups in BMI (26.4 kg/m2 (22.8–26.7) vs. 26.3 kg/m2 (23.1–27.3), respectively, Mann-Whitney U test p = 0.281), age (49.4 ± 7.8 vs. 45.5 ± 10.3 years, respectively, unpaired t test p = 0.159), ODI4% (4.9 events/h (4.1–8.1) vs. 9.7 events/h (4.1–35.5), respectively, Mann-Whitney U test p = 0.129), longest hypopnea duration (45.0 s (35.5–85.1) vs. 68.5 s (57.0–100.0), respectively, Mann-Whitney U test p = 0.193) or Epworth Sleepiness Scale (7.5 ± 5.0 vs. 6.6 ± 3.4 s, respectively, unpaired t test p = 0.348).
The optimal CPAP requirement at 0% mandibular advancement was not significantly different between responders to MAS therapy vs. non-responders (8.7 ± 2.4 vs. 9.1 ± 2.6 cmH2O, unpaired t test p = 0.10; Fig. 3a). Optimal CPAP requirements for other mandibular advancement positions were also not different between the two response groups. The reduction in optimal CPAP requirements across the five mandibular advancement positions was similar between the two response groups (Fig. 3b). Univariable linear regression analyses showed two independent predictors of the percent reduction in AHI with MAS therapy: optimal CPAP requirement at 0% (95% CI = (3.6 to 1.5), Beta = 0.553, F = 5.7, p = 0.03) and maximum apnoea duration at diagnostic polysomnography (95% CI = (− 0.65 to 0.245), Beta = − 0.591, F = 7.0, p = 0.02).
Optimal continuous positive airway pressure (CPAP) requirement at 0% advancement (a) was not different (unpaired t test, p = 0.85) between responders to MAS therapy (residual AHI < 5 events/h of sleep) vs. non-responders. Reductions in optimal CPAP requirements across the five mandibular advancement positions (b) was also not different between the two response groups (two-way repeated measures analysis of variance, p = 0.907)
Discussion
This is the first study to identify a dose-dependent reduction in optimal CPAP requirements with increasing levels of mandibular advancement. This is in accordance with our previous findings of a dose-dependent reduction in airway collapsibility (measured by the critical closing pressure method) with mandibular advancement [25]. This also provides further evidence that the optimal CPAP requirement reflects airway collapsibility in OSA patients [29]. Indeed, the average reduction in optimal CPAP requirements with MAS therapy of ~ 4 cmH2O found in this study was similar to critical airway pressure changes with MAS in previous trials [25, 39,40,41]. This provides further support that optimal CPAP requirement may be a clinically useful method to estimate airway collapsibility in people with OSA.
Although responders to MAS therapy had less-severe OSA compared with non-responders, we observed no difference between the two groups in the optimal CPAP requirement at 0% mandibular advancement or in the reduction of optimal CPAP requirements from 0 to 100% mandibular advancement. This may be attributed, at least in part, to the fact that the majority of participants in this study had lower than average age, BMI and baseline AHI compared with a typical OSA population. Although participants were recruited by three different sleep physicians, selection-bias possibly contributed to the lower BMI and mild AHI of our participants. Mild OSA has been shown to reflect a distinct phenotype that is more likely caused by non-anatomical mechanisms (e.g. poor pharyngeal muscle function, high loop gain and low arousal threshold [22, 42, 43]). These mechanisms are less likely to be corrected by MAS therapy. Our study design was intended to be clinically applicable, and hence we did not directly measure anatomical collapsibility or non-anatomical mechanisms due to the invasive nature of measurement techniques [22]. Therefore, we cannot elucidate how differences in the presence and extent of these mechanisms between individual patients contributed to optimal CPAP requirements. MAS therapy could have provided suboptimal efficacy in a proportion of our participants (with higher levels of non-anatomical disturbances) which in turn resulted in a homogenous levels of optimal CPAP requirement at 0% position as well as reduction in optimal CPAP requirements with mandibular advancement between the two response groups [23]. Additionally, our study design did not account for variations in craniofacial dimensions (e.g. via X-ray imaging) between responders and non-responders to MAS therapy. These variations may result in differences in the extent and characterisation of structural displacement of the upper airways, and thus, optimal CPAP requirements, in response to mandibular advancement. Nasal resistance also contributes to the development of OSA and has a role in determining response to MAS therapy [44, 45]. Concurrent analysis of anatomical, physiological and clinical factors may further enhance our knowledge of MAS therapy outcome in OSA. Noteworthy, optimal CPAP requirement at 0% mandibular advancement in the current study was in a lower range (~ 9 cmH2O) compared with previously reported cutoffs (12–13 cmH2O in predominantly Caucasian population) predicting MAS failure. This may have affected the study results by skewing MAS response rate (according to a response definition: AHI ≤ 5 events/h) which would influence the differences in baseline characteristics between MAS response groups. Nonetheless, the findings of this study do not strongly support a role for optimal CPAP in prediction of MAS therapy outcome in mild to moderate OSA. However, the optimal CPAP requirement at 0% was an independent predictor in the univariate analysis. Future replication of this study in patients with diverse OSA severities is required to provide a clearer understanding.
Methodological considerations
Randomisation of mandibular advancement conditions during measurement and blinded data acquisition and analysis of optimal CPAP requirements are major design strengths of the current study. However, several methodological aspects should be considered before drawing final inferences from the current findings. Although a larger sample size would improve the reliability and generatability of the study, our findings are consistent with those from previous trials examining the effects of mandibular advancement on the pathophysiological mechanisms causing OSA. Although we used specialised equipment to ensure accurate measurements, the technique implemented for titration of optimal CPAP requirements was based on visual inspection of airflow pressure and EEG signals to resemble measurements made in clinical sleep laboratories, and in accordance with clinical guidelines (AASM [33]). For the same reason, we chose not to instrument participants with an oesophageal pressure catheter (gold-standard indicator of airway collapsibility) as this may affect breathing and introduce bias in measurements of optimal CPAP requirements compared with clinical practice. Nonetheless, other trials from our laboratory (unpublished work) found that measurement of the optimal CPAP requirement based on stabilisation of epiglottic pressure swings is not different from those measured via visual inspection of airflow signals. We also focused our analysis on NREM sleep to facilitate comparison with previous work related to MAS therapy outcome and to enhance the study feasibility to obtain measurements from multiple conditions of mandibular advancement during a single polysomnography night. Nonetheless, responses to MAS therapy did not change when the definition of treatment success (i.e. ≤ 5 events/h) was based on NREM AHI. Thus, we speculate that the effects of mandibular advancement on optimal CPAP requirements would extend to REM sleep, although with higher absolute CPAP requirements compared with NREM sleep. Furthermore, it is possible that 0% mandibular advancement with RCMP device in situ, compared with no device, may stabilise the mandible and the airway in patients with less-severe OSA [46], yet, we did not include a no-device condition to the already challenging protocol to enhance the feasibility of the current study.
The maximal mandibular advancement range (and other predetermined positions) implemented in our study was calculated relative to habitual bite rather than maximal retrusion, and thus, was shorter (8.2 ± 2.3 mm) compared with previous trials (~ 11–13 mm [47]). Nonetheless, the average maximal advancement relative to maximal retrusion was also measured in the current study (12.2 ± 2.4 mm) and was comparable with previously reported ranges. We note that this method of mandibular advancement measurement was adopted here as we could not find clinical guidelines to recommend a certain method of measurement with inconsistent methodology reported across previous studies pertaining to MAS therapy outcome.
Although mandibular advancement range was variable between participants in this study (4–12 mm), there was no difference in mandibular advancement range between responders and non-responders to MAS therapy. Thus, this variability is not expected to influence optimal CPAP requirements at baseline or assist in predicting MAS therapy outcome. We also elected to standardise our methodology via implementing mandibular advancement conditions using a percentage format (e.g. 0%, 25%, 50% etc.), rather than absolute millimetres (e.g. 0, 2, 4, etc.) to account for structural (e.g. bone size) and functional (e.g. temporomandibular joint motion) differences between individual participants. Vertical opening of RCMP and MAS devices is another important element in determining optimal CPAP requirement and treatment outcome, respectively. Vertical opening causes posterior re-positioning of the mandible which restricts the maximal mandibular advancement and aggravates airway narrowing/collapsibility [14, 47, 48]. Thus, we attempted to keep the vertical opening of RCMP and MAS devices consistent across our participants to avoid effects on optimal CPAP requirements and MAS therapy outcome. Nonetheless, examining the effects of vertical opening on the dose–response relationship between mandibular advancement and optimal CPAP requirements would be an interesting aim for future studies.
Conclusions
Optimal CPAP requirements decrease with mandibular advancement in a dose-dependent manner. The reduction in optimal CPAP requirements correlates with the level of mandibular advancement measured in millimetres (where habitual bite is the ‘0-mm reference point’). The reduction in optimal CPAP requirements was not different between MAS therapy responders vs. non-responders. These findings support previous work showing beneficial effects of MAS on upper airway collapsibility and the potential for optimal CPAP requirement to be utilised as a marker for upper airway collapsibility. Further prospective validation of our results in a larger sample size with more diverse OSA severities is warranted.
References
Bixler E (2000) Association of hypertension and sleep-disordered breathing. Arch Intern Med 160(15):2289–2295
Nieto FJ, Young TB, Lind BK et al (2000) Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study: Sleep Heart Health Study. JAMA. 283(14):1829–1836
Peker Y, Hedner J, Norum J, Kraiczi H, Carlson J (2002) Increased incidence of cardiovascular disease in middle-aged men with obstructive sleep apnea. Am J Respir Crit Care Med 166(2):159–165
Punjabi NM, Ahmed MM, Polotsky VY, Beamer BA, O’Donnell CP (2003) Sleep-disordered breathing, glucose intolerance, and insulin resistance. Respir Physiol Neurobiol 136(2–3):167–178
Heinzer R, Vat S, Marques-Vidal P, Marti-Soler H, Andries D, Tobback N, Mooser V, Preisig M, Malhotra A, Waeber G, Vollenweider P, Tafti M, Haba-Rubio J (2015) Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med 3(4):310–318
Weaver TE, Grunstein RR (2008) Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc 5(2):173–178
Catcheside PG (2010) Predictors of continuous positive airway pressure adherence. F1000 Med Rep 2:70
Rotenberg BW, Murariu D, Pang KP (2016) Trends in CPAP adherence over twenty years of data collection: a flattened curve. J Otolaryngol Head Neck Surg 45(1):43
Ramar K, Dort LC, Katz SG et al (2015) Clinical practice guideline for the treatment of obstructive sleep apnea and snoring with oral appliance therapy: an update. J Clin Sleep Med 11(7):773–827
Aarab G, Lobbezoo F, Heymans MW, Hamburger HL, Naeije M (2011) Long-term follow-up of a randomized controlled trial of oral appliance therapy in obstructive sleep apnea. Respiration. 82(2):162–168
Phillips CL, Grunstein RR, Darendeliler MA, Mihailidou AS, Srinivasan VK, Yee BJ, Marks GB, Cistulli PA (2013) Health outcomes of continuous positive airway pressure versus oral appliance treatment for obstructive sleep apnea: a randomized controlled trial. Am J Respir Crit Care Med 187(8):879–887
Li W, Xiao L, Hu J (2013) The comparison of CPAP and oral appliances in treatment of patients with OSA: a systematic review and meta-analysis. Respir Care 58(7):1184–1195
Bloch KE, Iseli A, Zhang JN et al (2000) A randomized controlled crossover trial of two oral appliances for sleep apnea treatment. Am J Respir Crit Care Med 162(1):246–251
Pitsis AJ, Darendeliler MA, Gotsopoulos H, Petocz P, Cistulli PA (2002) Effect of vertical dimension on efficacy of oral appliance therapy in obstructive sleep apnea. Am J Respir Crit Care Med 166(6):860–864
Gotsopoulos H, Chen C, Qian J, Cistulli PA (2002) Oral appliance therapy improves symptoms in obstructive sleep apnea: a randomized controlled trial. Am J Respir Crit Care Med 166(5):743–748
Walker-Engstrom ML, Ringqvist I, Vestling O, Wilhelmsson B, Tegelberg A (2003) A prospective randomized study comparing two different degrees of mandibular advancement with a dental appliance in treatment of severe obstructive sleep apnea. Sleep Breath 7(3):119–130
Tegelberg A, Walker-Engstrom ML, Vestling O, Wilhelmsson B (2003) Two different degrees of mandibular advancement with a dental appliance in treatment of patients with mild to moderate obstructive sleep apnea. Acta Odontol Scand 61(6):356–362
Blanco J, Zamarron C, Abeleira Pazos MT, Lamela C, Suarez Quintanilla D (2005) Prospective evaluation of an oral appliance in the treatment of obstructive sleep apnea syndrome. Sleep Breath 9(1):20–25
Petri N, Svanholt P, Solow B, Wildschiodtz G, Winkel P (2008) Mandibular advancement appliance for obstructive sleep apnoea: results of a randomised placebo controlled trial using parallel group design. J Sleep Res 17(2):221–229
Tsuiki S, Ito E, Isono S, Ryan CF, Komada Y, Matsuura M, Inoue Y (2013) Oropharyngeal crowding and obesity as predictors of oral appliance treatment response to moderate obstructive sleep apnea. Chest. 144(2):558–563
Sutherland K, Takaya H, Qian J, Petocz P, Ng AT, Cistulli PA (2015) Oral appliance treatment response and polysomnographic phenotypes of obstructive sleep apnea. J Clin Sleep Med 11(8):861–868
Eckert DJ, White DP, Jordan AS, Malhotra A, Wellman A (2013) Defining phenotypic causes of obstructive sleep apnea: identification of novel therapeutic targets. Am J Respir Crit Care Med 188(8):996–1004
Eckert DJ (2018) Phenotypic approaches to obstructive sleep apnoea: new pathways for targeted therapy. Sleep Med Rev 37:45–59
Edwards BA, Andara C, Landry S, Sands SA, Joosten SA, Owens RL, White DP, Hamilton GS, Wellman A (2016) Upper airway collapsibility and loop gain predict the response to oral appliance therapy in patients with obstructive sleep apnea. Am J Respir Crit Care Med 194(11):1413–1422
Bamagoos AA, Cistulli PA, Sutherland K et al (2019) Dose-dependent effects of mandibular advancement on upper airway collapsibility and muscle function in obstructive sleep apnea. Sleep 42(6)
Ng AT, Qian J, Cistulli PA (2006) Oropharyngeal collapse predicts treatment response with oral appliance therapy in obstructive sleep apnea. Sleep. 29(5):666–671
Sutherland K, Phillips CL, Davies A, Srinivasan VK, Dalci O, Yee BJ, Darendeliler MA, Grunstein RR, Cistulli PA (2014) CPAP pressure for prediction of oral appliance treatment response in obstructive sleep apnea. J Clin Sleep Med 10(9):943–949
Chan AS, Sutherland K, Schwab RJ et al (2010) The effect of mandibular advancement on upper airway structure in obstructive sleep apnoea. Thorax. 65(8):726–732
Landry SA, Joosten SA, Eckert DJ et al (2017) Therapeutic CPAP level predicts upper airway collapsibility in patients with obstructive sleep apnea. Sleep 40(6)
Storesund A, Johansson A, Bjorvatn B, Lehmann S (2018) Oral appliance treatment outcome can be predicted by continuous positive airway pressure in moderate to severe obstructive sleep apnea. Sleep Breath 22(2):385–392
Tsuiki S, Kobayashi M, Namba K, Oka Y, Komada Y, Kagimura T, Inoue Y (2010) Optimal positive airway pressure predicts oral appliance response to sleep apnoea. Eur Respir J 35(5):1098–1105
Remmers J, Charkhandeh S, Grosse J, Topor Z, Brant R, Santosham P, Bruehlmann S (2013) Remotely controlled mandibular protrusion during sleep predicts therapeutic success with oral appliances in patients with obstructive sleep apnea. Sleep. 36(10):1517–1525
Berry RB, Budhiraja R, Gottlieb DJ et al (2012) Rules for scoring respiratory events in sleep: update of the 2007 American Academy of Sleep Medicine manual for the scoring of sleep and associated events. J Clin Sleep Med 8(5):597–619
Petelle B, Vincent G, Gagnadoux F, Rakotonanahary D, Meyer B, Fleury B (2002) One-night mandibular advancement titration for obstructive sleep apnea syndrome: a pilot study. Am J Respir Crit Care Med 165(8):1150–1153
Tsai WH, Vazquez JC, Oshima T, Dort L, Roycroft B, Lowe AA, Hajduk E, Remmers JE (2004) Remotely controlled mandibular positioner predicts efficacy of oral appliances in sleep apnea. Am J Respir Crit Care Med 170(4):366–370
Dort LC, Hadjuk E, Remmers JE (2006) Mandibular advancement and obstructive sleep apnoea: a method for determining effective mandibular protrusion. Eur Respir J 27(5):1003–1009
Sutherland K, Ngiam J, Cistulli PA (2017) Performance of remotely controlled mandibular protrusion sleep studies for prediction of oral appliance treatment response. J Clin Sleep Med 13(3):411–417
Kushida CA, Chediak A, Berry RB et al (2008) Clinical guidelines for the manual titration of positive airway pressure in patients with obstructive sleep apnea. J Clin Sleep Med 4(2):157–171
Kato J, Isono S, Tanaka A, Watanabe T, Araki D, Tanzawa H, Nishino T (2000) Dose-dependent effects of mandibular advancement on pharyngeal mechanics and nocturnal oxygenation in patients with sleep-disordered breathing. Chest. 117(4):1065–1072
Ng AT, Gotsopoulos H, Qian J, Cistulli PA (2003) Effect of oral appliance therapy on upper airway collapsibility in obstructive sleep apnea. Am J Respir Crit Care Med 168(2):238–241
Marques M, Genta P, Sands SA et al (2017) Characterizing site and severity of upper airway collapse to guide patient selection for oral appliance therapy for obstructive sleep apnea [abstract]. Am J Respir Crit Care Med 195:A2584–A2584
Edwards BA, Eckert DJ, Jordan AS (2017) Obstructive sleep apnoea pathogenesis from mild to severe: is it all the same? Respirology. 22(1):33–42
Gray EL, McKenzie DK, Eckert DJ (2017) Obstructive sleep apnea without obesity is common and difficult to treat: evidence for a distinct pathophysiological phenotype. J Clin Sleep Med 13(1):81–88
Lai V, Tong BK, Tran C et al (2019) Combination therapy with mandibular dvancement and expiratory positive airway pressure valves reduces obstructive sleep apnea severity. Sleep
Zeng B, Ng AT, Qian J, Petocz P, Darendeliler MA, Cistulli PA (2008) Influence of nasal resistance on oral appliance treatment outcome in obstructive sleep apnea. Sleep. 31(4):543–547
Anitua E, Duran-Cantolla J, Almeida GZ, Alkhraisat MH (2017) Minimizing the mandibular advancement in an oral appliance for the treatment of obstructive sleep apnea. Sleep Med 34:226–231
Mayoral P, Lagravere MO, Miguez-Contreras M, Garcia M (2019) Antero-posterior mandibular position at different vertical levels for mandibular advancing device design. BMC Oral Health 19(1):85
Vroegop AV, Vanderveken OM, Van de Heyning PH, Braem MJ (2012) Effects of vertical opening on pharyngeal dimensions in patients with obstructive sleep apnoea. Sleep Med 13(3):314–316
Acknowledgements
The authors of this study are grateful for the support provided by members of the Sleep and Breathing Laboratory at NeuRA and the Centre for Sleep Health and Research at Royal North Shore Hospital including their roles in participants setup, data acquisition and data analysis. These individuals include: Carolin Tran, Andrea Ricciardiello, Alan Chiang, Benjamin Tong, Paras Acharya, Hanna Hensen, Mohammed Ahmadi and Aimee Lowth. The authors are also grateful to the study participants for taking the time to volunteer for the study.
Funding
This study was funded by the institutional research funds of PAC, DJE (NHMRC Senior Research Fellowship 1116942) and AAB (Scholarship for PhD studies from King Abdul-Aziz University). The study received equipment support from SomnoMed in the form of mandibular advancement splints.
Author information
Authors and Affiliations
Contributions
Study conception and design: PAC conceived the study and received design input from AAB, KS and DJE. Data collection: AAB, DJE and JN. Data analysis: AAB. Dental consultation and technical oversight: JN and AAB. Data interpretation, discussion, editing and critical revision of the manuscript: all authors.
Corresponding author
Ethics declarations
Conflict of interest
DJE held a Project Grant from the Commonwealth Government of Australia Cooperative Research Centres (industry partner: Oventus Medical) during the conduct of the study and received research grants from Bayer and Apnimed outside the submitted work. PAC has an appointment to an endowed academic Chair at the University of Sydney that was established from ResMed funding. He has received research support from ResMed, SomnoMed, Zephyr Sleep Technologies, and Bayer. He is a consultant to Zephyr Sleep Technologies and ResMed Narval. He has a pecuniary interest in SomnoMed related to a previous role in R&D (2004). AAB, KS and JN has no conflict of interest to disclose.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bamagoos, A.A., Eckert, D.J., Sutherland, K. et al. Dose-dependent effects of mandibular advancement on optimal positive airway pressure requirements in obstructive sleep apnoea. Sleep Breath 24, 961–969 (2020). https://doi.org/10.1007/s11325-019-01930-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-019-01930-3