Abstract
Purpose
The current study was carried out to assess the effects of different levels of intermittent hypoxia (IH) on autophagy in hippocampal neurons, and explore the extent, frequency and duration of IH for researching on autophagy in hippocampal neurons.
Methods
Hippocampal neurons were exposed to different levels of IH. To analyze the oxygen level of neuronal exposure environment, we detected the oxygen concentration in the chamber by O2 analyzer, and monitored the oxygen partial pressure (PO2), carbon dioxide partial pressure (PCO2), and pH in the culture media by blood gas analyzer. After 4-, 8-, and 12-h IH, the morphology and quantity of neurons, as well as the expression of light chain 3 (LC3)-II positive dots were observed by immunofluorescence. The expression of apoptosis marker protein cleaved caspase-3 and autophagy marker protein LC3 were examined by western blotting.
Results
The oxygen level in the chamber and the neuronal culture media both reached to the values set previously in three models. The level of cleaved caspase-3 and LC3 had no significant changes in IH-1 group. The morphology and quantity had no significant changes, while the levels of cleaved caspase-3 and LC3 were both increased in IH-2 group. The quantity of neurons was reduced significantly, and the chromatin condensed and nuclei fragmented in IH-3 group.
Conclusions
The effects of varying degrees of IH on autophagy in hippocampal neurons are different. The IH model, hypoxia phase (1.5% O2, 5% CO2, and balance N2) for 5 min and reoxygenation phase (21% O2, 5% CO2, and balance N2) for 10 min, may be the best condition for researching on autophagy in hippocampal neurons.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intermittent hypoxia (IH), which resulted from recurring episodes of upper airway obstruction, the typical characteristic, and the most important pathophysiologic pathway of obstructive sleep apnea-hypopnea syndrome (OSAHS), is believed to be a potential major factor causing neuronal injury which may play the key role in the development and progression of cognitive impairments [1, 2]. It is worth noting that a wide range of neurocognitive impairments have been identified in OSAHS patients, and the greater hazard of cognitive dysfunctions is associated with the severity of IH, which is demonstrated by the higher percentage of patients experiencing poor cognition in the moderate-to-severe OSAHS patients than the primary snoring and mild OSAHS patients based on the subjective symptom assess [3]. Moreover, the injury induced by severe IH was more serious than those induced by moderate IH and mild IH in vivo and vitro experiments [4]. In a word, all these above suggest that the neuronal injury caused by IH is different and that is proportional to the severity of IH.
Autophagy is a phenomenon that widely presents in eukaryotic cells, and it can provide the required starting materials for cells to the synthesis of new proteins and update of organelles to maintain cellular homeostasis [5]. Many studies have confirmed that autophagy is increased in numerous hypoxic-excitotoxic conditions including cerebral hypoxia-ischemia [6], ischemia-reperfusion [7], and oxygen and glucose deprivation (OGD)-reperfusion (RP) [8]. And the increased level of autophagy is connected with the severity of hypoxia-related conditions. IH, a special form of hypoxia, also causes increased expression of autophagy in pancreatic cancer cells, human neutrophils, and animal tissue [9,10,11]. However, in hippocampal neurons which are more susceptible to IH, the effects of varying degrees of IH on autophagy are still unclear.
In many studies about OSAHS, IH was used as the sole exposure factors to simulating the disturbances of homeostasis of OSAHS patient [12, 13]. The extent, frequency, and duration of IH are various in different studies which focus on different research purposes. The oxygen concentration of hypoxic phase is usually maintained at 5% or more in IH animal model [12, 14, 15], and is held at 0.1∼5% in IH cell model [16, 17]. Furthermore, the duration of hypoxia and reoxygenation is also diverse, which ranges between tens of seconds and tens of minutes. Therefore, in present study, we have chosen 1.5% O2 and 21% O2 for simulating different frequencies and durations of IH, and explored the effects of IH on autophagy in hippocampal neurons, which will be meaningful for further understanding the mechanisms of hippocampal neuron injury induced by IH.
Methods and materials
Hippocampal neuron culture
Primary hippocampal neurons were obtained from Sprague-Dawley rat pups (<24 h), which were purchased from Chinese Academy of Military Science (Beijing, China), and cultured according to the procedure described Pont-Lezica et al. [18]. Briefly, the brain was dissected and hippocampus was removed. The meninges and blood vessels were carefully striped and the hippocampus was minced into 1 mm3 fragments. Then, the hippocampal pieces were dissociated by 0.25% trypsin (Sigma, USA) and 100 ng/ml DNase (Roche, Sweden) at 37 °C for 30 min. The isolated cells were resuspended by DMEM medium (Hyclone, USA) with 10% FBS (Gibco, USA) and seeded into 6-well plates or 22 * 22 mm2 glass coverslips in 35-mm Petri dishes pre-coated with 10 μg/ml poly-d-lysine (Sigma, USA) at a density of 5 * 105 per well. After 4 h, neurons were cultured in the neurobasal-A medium with 2% B27, 1% glutamine, and HEPES (Gibco, USA) for 7 days at 37 °C in 5% CO2 and 95% O2 atmosphere.
Exposure of hippocampal neurons to IH
The culture medium was changed to fresh medium for routine culture before the cells were exposed to intermittent hypoxia. Hippocampal neurons grown in culture plates were placed in Plexiglas exposure chamber and were exposed to IH or normoxia conditions. We developed a gas delivery system that permits the exposure of cell cultures to hypoxia/reoxygenation cycles, simulating the pattern of hypoxic episodes seen in recurrent apnea. Visual C++ computer language was used in coding a control program (Breath-Simulating system 1.0) which regulated the delivery system as previously described [19]. Briefly, a personal computer drove a single chip through serial port communicating protocol, and with this system, solid relay was adjusted to modulate a gas resource system for controlling whether the gas mixture was given into the chamber or not. The gas mixture (Tai-ya Gas Limited Company, Tianjin, China) in this study consisted of O2 in particular concentration, 5% CO2, and balanced N2. The hypoxia gas mixture (1.5% O2, 5% CO2, and balanced N2, hypoxia phase) or normoxia gas mixture (21% O2, 5% CO2, and balanced N2, reoxygenation phase) was flushed alternatively into the customized small IH housing chamber to provide a designated IH or normoxia environment. The O2 concentration in the chamber was rapidly decreased to about 1.5% with hypoxia gas mixture during hypoxia phase. The O2 concentration was increased to a maximum of 21% by rapidly flushing the chamber with normoxia gas mixture during reoxygenation phase. In order to analyze the effect of varying degrees of IH on autophagy, cells were maintained in the cycle of 1.5% O2 for 2 min and 21% O2 for 5 min (IH-1 model), cycle of 1.5% O2 for 5 min and 21% O2 for 10 min (IH-2 model), cycle of 1.5% O2 for 10 min and 21% O2 for 20 min (IH-3 model), and sustained normoxia gas mixture (NC), respectively. The chamber was equipped with a humidifier, thermostat, and molecular sieve to maintain an inner temperature of 37 °C, humidity of 45%, and a relative germfree circumstance. Change of oxygen concentrations in the chamber was monitored by O2 analyzer. O2 levels in the culture media, 1 mm above the cell layer, were sampled and monitored by oxygen electrode (Lazar, USA) and blood gas analyzer (AVL OMNI, Swiss), with six samples per gas environment.
Immunofluorescence staining for microtubule-associated protein 2 (MAP-2), LC3, and DAPI
The primary hippocampal neurons, grown on coverslips in 3.5-cm dishes, were observed and took photograph by optical microscope after treated with IH or normoxia. The cells were washed with phosphate-buffered saline (PBS) and fixed in 4% paraformaldehyde for 15 min. Then, they were permeabilized with 0.1% Triton X-100 for 10 min and blocked with BSA buffer for 30 min at room temperature. Subsequently, cells were incubated with the anti-MAP-2 antibody (1:100, Boster Biological Technology, China) and anti-light chain 3B (LC3B) antibody (1:50, Cell Signaling Technology, USA) at 4 °C overnight. After being rinsed with PBS, the cells were incubated for 1 h at room temperature with an appropriate secondary antibody. Finally, 4,6-diamidino-2-phenylindole (DAPI) (Beyotime, C1002) was used as a counterstain. Images were taken under OLYMPUS microscope (cellSens system) and OLYMPUS confocal microscope (FV 1200). The total number of neurons was counted in individual views. LC3-II dots were counted in individual hippocampal neurons and the average of dots in at least 30 cells was presented in the figures.
Western blotting for cleaved caspase-3 and LC3 protein expression
Hippocampal neuron protein was extracted in accordance with the instructions of the protein extraction kit extracted (Promega, USA). Protein concentrations were measured using a BCA Assay Kit (Thermo, USA). Equal amounts of the protein (10 μg) were resolved by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto polyvinylidene difluoride (PVDF) membranes. The membranes were blocked with 5% non-fat milk containing Tris buffered saline and Tween 20 (TBS-T) for 2 h on a shaker at room temperature and then incubated with appropriate dilutions of the primary specific antibody: rabbit anti-LC3B antibody (1:1000, Cell Signaling Technology, USA); rabbit anti-cleaved caspase-3 antibody (1:1000, Cell Signaling Technology, USA); and rabbit anti-GAPDH antibody (1:1000, Cell Signaling Technology, USA) in TBS-T at 4 °C overnight. The membranes were washed with TBS-T on the shaker. The secondary antibody was horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (1:1000, Beyotime Biotechnology, China), incubation of which was carried out on the shaker at room temperature for 1 h. Chemiluminescence was imaged in a Molecular Imager Chemi Doc™ XRS + WB with Image Lab™ software (Bio-Rad, CA, USA), and band intensity was measured by ImageJ software.
Statistical analysis
SPSS 16.0 (SPSS Inc., USA) software package was used for statistical analysis. The data of blood gas analysis of culture media were obtained and were displayed as a descriptive analysis. The immunostaining was analyzed with the ImageJ software. Student’s t test was used for comparing with baseline values. Unless otherwise stated, values were reported as mean ± standard deviation (SD). The significance of variables between groups was tested by one-way ANOVA. P < 0.05 is considered statistically significant.
Results
Changes of oxygen concentration in the intermittent hypoxic cabin
We detected the level of oxygen concentration in the chamber. The results showed the process of hypoxia and normoxia alternately in IH models. As the Fig. 1 displayed, oxygen concentration decreased from 21 to 1.5%, and continued until 5 min. Then, it increased from 1.5 to 21%, and maintained 10 min. These results suggested that the level of oxygen in the cabin reached to the values (1.5 or 21%) set previously in three IH models.
PO2, PCO2, and pH values in the culture media of hippocampal neurons
We collected the culture medium samples from about 1 mm above the cell layer, and analyzed the PO2, PCO2, and pH values. The results are described as Table 1, which suggested that three IH models can provide hypoxia environment at the cellular level.
Effects of IH on morphology and quantity of hippocampal neurons
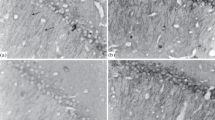
The morphology and quantity of hippocampal neurons were observed by optical microscope and fluorescence microscope after IH. The intact structure of neurons suffered severe damage, and the quantity of neurons was reduced significantly in IH-3 group compared with normoxia group (P < 0.05) (Fig. 2a, c), while neurons of IH-1 group and IH-2 group had no significant changes compared with normoxia group both in morphology and quantity (P > 0.05) (Fig. 2b, d).
The changes of morphology and quantity of hippocampal neurons after IH. a, b Hippocampal neurons were observed by optical microscope. Compared to normoxia group, the damage of neuronal integrity was more serious in IH-3 group. Scale bar: 50 μm. c, d Hippocampal neurons labeled MAP-2, DAPI, and merged. Compared to normoxia group, the number of neuron was decreased in the IH-3 group (***P < 0.001). Scale bar: 50 μm. e, f Fluorescent microscopy of neurons stained with DAPI. Chromatin condensation and nuclear fragmentation were observed in IH-3 group. Scale bar: 20 μm
The apoptosis induced by IH in hippocampal neurons was verified by examining nuclear morphological changes following staining of nuclei with DAPI. As indicated in Fig. 2, severe IH-treated neurons (Fig. 2e) exhibited typical late apoptotic morphological features, including condensed chromatin and fragmented nuclei. By contrast, the neurons exhibited homogenous in the normoxia group, IH-1 group, and IH-2 group (Fig. 2f).
Effects of IH on cleaved caspase-3 and LC3 expression in hippocampal neurons
We examined the expression of cleaved caspase-3 in IH-1 group and IH-2 group, and found that the level of cleaved caspase-3 was increased in these groups. The increased level of cleaved caspase-3 in IH-1 group had no statistical significance compared with normoxia group (P > 0.05) (Fig. 3a), while it was significant higher in the IH-2 group than those in normoxia group (P < 0.05) (Fig. 3b), especially at the time of 12 h of IH (P < 0.01). These results suggested that the IH model which induced hippocampal neuron injury was successfully established by episodic cycles of hypoxia phase (1.5% O2, 5% CO2, and balance N2) for 5 min and reoxygenation phase (21% O2, 5% CO2, and balance N2) for 10 min.
The expression level of cleaved caspase-3 and LC3 in hippocampal neurons with western blotting. a, b The expression level of cleaved caspase-3 in hippocampal neurons. Compared to normoxia group, the level of cleaved caspase-3 was not statistically significant in IH-1 group and that was significantly higher at 8 and 12 h in IH-2 group (*P < 0.05, **P < 0.01). c, d The expression level of LC3 in hippocampal neurons. Compared to normoxia group, the level of LC3 was not statistic significant in IH-1 group and that was significantly higher at 8 and 12 h in IH-2 group (*P < 0.05, **P < 0.01)
As described above, the IH-2 model can not only provide hypoxia environment but also cause the hippocampal neuron injury. Thus, we examined the expression of LC3 in IH-2 group, and found that it was significantly increased, especially at the time of 12 h of IH (P < 0.01) (Fig. 3d). To further explore the effect of IH on autophagy in hippocampal neurons, we also examined the expression level of LC3 in IH-1 group, and found that its increase had no statistic significant (P > 0.05) (Fig. 3c). At the same time, compared with the normoxia group, the number of LC3-II positive dots was not statistic significant in IH-1 group (Fig. 4d) and that was significant higher at 12 h in IH-2 group (P < 0.01) (Fig. 4b).
Discussion
In the present study, we discussed the effects of varying degrees of IH on autophagy in hippocampal neurons, and found that the effects of varying degrees of IH on autophagy in hippocampal neurons are different. The IH model, hypoxia phase (1.5% O2, 5% CO2, and balance N2) for 5 min and reoxygenation phase (21% O2, 5% CO2 and balance N2) for 10 min, may be the best condition for researching on autophagy in hippocampal neurons.
Firstly, we successfully established IH model in hippocampal neurons by detecting the oxygen concentration in the chamber and PO2, PCO2, and pH in the culture media of hippocampal neurons. It is widely used that the hypoxia gas mixture and normoxia gas mixture were flushed alternatively into the customized small IH housing chamber to provide a designated hypoxia or normoxia environment. In previous studies, the cycle of hypoxia gas mixture (1.5% O2, 5% CO2, and balanced N2) and normoxia gas mixture (21% or 20% O2, 5% CO2, and balanced N2) has been used in many cells, such as pheochromocytoma PC12 cell [20] and the human lung microvascular endothelial cells [16]. The PO2 in the culture media of cells can more accurately detect the O2 concentration. He et al. [4] also monitored the O2 levels in the culture media by oxygen electrode and blood gas analyzer, and found that the PO2 was associated with the pattern of IH closely.
Secondly, we observed the morphology and quantity of hippocampal neurons before and after IH treatment. In this study, the morphology and quantity of neurons had no significant change in IH-1 group and IH-2 group, while the number of hippocampal neurons was decreased and the intact structure of neurons was rarely visible in the IH-3 group which suffered from more serious hypoxia. Then, we further investigated whether or not the apoptosis and necrosis are related to the reduction in the number of neurons. DAPI staining, a method for detecting apoptosis through observing the changes of nuclear morphology, is now widespread used [21,22,23]. Chromatin condensation and nuclear fragmentation are the typical morphological features in the late apoptosis [24]. In IH-3 group of our study, chromatin condensation and nuclear fragmentation were also observed. Thus, we thought that the apoptosis-induced by IH may play an important role in the reduction of neurons, but not exclude necrosis that will require large studies.
Due to severe injury and reduction of the neurons in IH-3 group, we thought that the model of IH-3 was not suitable for further study. To investigate whether IH model can induce cell injury, we examined the expression level of apoptosis marker protein cleaved caspase-3, and found that the increased level of cleaved caspase-3 in IH-1 group was not significant and that had significant statistic significance in IH-2 group, especially at the time of 12 h of IH. These results suggested that the IH-1 model was not neurotoxic, and the frequency and duration of IH-2 model could cause neuronal injury. In one study that focused on the effects of varying degrees of IH, the injury was proportional to the severity of IH, which was demonstrated through the level of systemic inflammatory markers describing as severe IH > moderate IH > mild IH [4]. The pathophysiological characteristics of IH and cerebral ischemia are similar, and the severity of cerebral ischemia is also proportional to the severity of hypoxia, which showed that the short hypoxic duration produced smaller average infarct size and more variable brain damage than the longer hypoxic duration [25]. These may be associated with the level of oxygen that affects mitochondrial function and structure [26].
Finally, we detected the expression level of autophagy. The effects of varying degrees of hypoxia-condition on autophagy are different. Shi et al. [8] established the model of cerebral hypoxia and ischemia through oxygen and glucose deprivation (OGD) and reperfusion (RP), and they found that longer OGD injury activated higher autophagic activity as determined by the greater percentage of AVO-positive neurons to total neurons in the 6-h OGD group than that of 3-h OGD. Furthermore, they also showed that the LC3 levels were increased in a time-dependent fashion according to the RP time in the 6-h OGD group. In this study, we also found that the effects of varying degrees of IH on autophagy in hippocampal neurons are different, which indicated by these results that the increased level of LC3 in IH-1 group was not significant, while that had significant statistic significance in IH-2 group, especially at the time of 12 h of IH.
Conclusion
In conclusion, our results highlight the fact that the effects of varying degrees of IH on autophagy in hippocampal neurons are different. The model described as hypoxia phase for 2 min and reoxygenation phase for 5 min did not cause neuron damage and autophagy changes. Neuron injury and autophagy changes both occurred in the IH model described as hypoxia phase for 5 min and reoxygenation phase for 10 min, while more serious neuron injuries were caused by the IH model described as hypoxia phase for 10 min and reoxygenation phase for 20 min. Therefore, we think that the IH model, hypoxia phase (1.5% O2, 5% CO2, and balance N2) for 5 min and reoxygenation phase (21% O2, 5% CO2, and balance N2) for 10 min, may be the best condition for researching on autophagy in hippocampal neurons. Furthermore, autophagy may be involved in the injury induced by IH in hippocampal neurons. However, the specific mechanisms are still unclear and need further study.
References
Xie H, Yung WH (2012) Chronic intermittent hypoxia-induced deficits in synaptic plasticity and neurocognitive functions: a role for brain-derived neurotrophic factor. Acta Pharmacol Sin 33(1):5–10
Feng J, Wu Q, Zhang D, Chen BY (2012) Hippocampal impairments are associated with intermittent hypoxia of obstructive sleep apnea. Chin Med J 125(4):696–701
Chen R, Xiong KP, Lian YX, Huang JY, Zhao MY, Li JX, Liu CF (2011) Daytime sleepiness and its determining factors in Chinese obstructive sleep apnea patients. Sleep Breath 15(1):129–135
He Q, Yang QC, Zhou Q, Zhu H, Niu WY, Feng J, Wang Y, Cao J, Chen BY (2014) Effects of varying degrees of intermittent hypoxia on proinflammatory cytokines andadipokines in rats and 3T3-L1 adipocytes. PLoS One 9(1):e86326
Kroemer G, Mariño G, Levine B (2010) Autophagy and the integrated stress response. Mol Cell 40(2):280–293
Ginet V, Spiehlmann A, Rummel C, Rudinskiy N, Grishchuk Y, Luthi-Carter R, Clarke PG, Truttmann AC, Puyal J (2014) Involvement of autophagy in hypoxic-excitotoxic neuronal death. Autophagy 10(5):846–860
Zhou H, Zhu J, Yue S, Lu L, Busuttil RW, Kupiec-Weglinski JW, Wang X, Zhai Y (2016) The dichotomy of endoplasmic reticulum stress response in liver ischemia-reperfusion injury. Transplantation 100(2):365–372
Shi R, Weng J, Zhao L, Li XM, Gao TM, Kong J (2012) Excessive autophagy contributes to neuron death in cerebral ischemia. CNS Neurosci Ther 18(3):250–260
Zhu H, Wang D, Zhang L, Xie X, Wu Y, Liu Y, Shao G, Su Z (2014) Upregulation of autophagy by hypoxia-inducible factor-1α promotes EMT and metastatic ability of CD133+ pancreatic cancer stem-like cells during intermittent hypoxia. Oncol Rep 32(3):935–942
Dyugovskaya L, Berger S, Polyakov A, Lavie P, Lavie L (2016) Intermittent hypoxia affects the spontaneous differentiation in vitro of human neutrophils into long-lived giant phagocytes. Oxidative Med Cell Longev 2016:9636937
Maeda H, Nagai H, Takemura G, Shintani-Ishida K, Komatsu M, Ogura S, Aki T, Shirai M, Kuwahira I, Yoshida K (2013) Intermittent-hypoxia induced autophagy attenuates contractile dysfunction and myocardial injury in rat heart. Biochim Biophys Acta 1832(8):1159–1166
Xu XM, Yao D, Cai XD, Ding C, Lin QD, Wang LX, Huang XY (2015) Effect of chronic continual- and intermittent hypoxia-induced systemic inflammation on the cardiovascular system in rats. Sleep Breath 19(2):677–684
Angelo MF, Aguirre A, Avilés Reyes RX, Villarreal A, Lukin J, Melendez M, Vanasco V, Barker P, Alvarez S, Epstein A, Jerusalinsky D, Ramos AJ (2014) The proinflammatory RAGE/NF-κB pathway is involved in neuronal damage and reactive gliosis in a model of sleep apnea by intermittent hypoxia. PLoS One 9(9):e107901
Huang H, Jiang X, Dong Y, Zhang X, Ding N, Liu J, Hutchinson SZ, Lu G, Zhang X (2015) Adiponectin alleviates genioglossal mitochondrial dysfunction in rats exposed to intermittent hypoxia. PLoS One 9(10):e109284
Wu J, Li P, Wu X, Chen W (2015) Chronic intermittent hypoxia decreases pain sensitivity and increases the expression of HIF1α and opioid receptors in experimental rats. Sleep Breath 19(2):561–568
Makarenko VV, Usatyuk PV, Yuan G, Lee MM, Nanduri J, Natarajan V, Kumar GK, Prabhakar NR (2014) Intermittent hypoxia-induced endothelial barrier dysfunction requires ROS-dependent MAP kinase activation. Am J Physiol Cell Physiol 306(8):C745–C752
Chen TI, Chiu HW, Pan YC, Hsu ST, Lin JH, Yang KT (2015) Intermittent hypoxia-induced protein phosphatase 2A activation reduces PC12 cell proliferation and differentiation. J Biomed Sci 21:46
Pont-Lezica L, Colasse S, Bessis A (2013) Depletion of microglia from primary cellular cultures. Methods Mol Biol 1041:55–61
Magalang UJ, Cruff JP, Rajappan R, Hunter MG, Patel T, Marsh CB, Raman SV, Parinandi NL (2009) Intermittent hypoxia suppresses adiponectin secretion by adipocytes. Exp Clin Endocrinol Diabetes 117(3):129–134
Yuan G, Khan SA, Luo W, Nanduri J, Semenza GL, Prabhakar NR (2011) Hypoxia-inducible factor 1 mediates increased expression of NADPH oxidase-2 in response to intermittent hypoxia. J Cell Physiol 226(11):2925–2933
Rahbar Saadat Y, Saeidi N, Zununi Vahed S, Barzegari A, Barar J (2015) An update to DNA ladder assay for apoptosis detection. Bioimpacts 5(1):25–28
Pang L, Zhao X, Liu W, Deng J, Tan X, Qiu L (2015) Anticancer effect of ursodeoxycholic acid in human oral squamous carcinoma HSC-3 cells through the caspases. Nutrients 7(5):3200–3218
Choi H, Lee DG (2015) Lycopene induces apoptosis in Candida albicans through reactive oxygen species production and mitochondrial dysfunction. Biochimie 115:108–115
Lee SL, Chin TY, Lai CL, Wang WH (2015) Sedum mexicanum Britt. induces apoptosis of primary rat activated hepatic stellate cells. Evid Based Complement Alternat Med 2015:194373
Adhami F, Liao G, Morozov YM, Schloemer A, Schmithorst VJ, Lorenz JN, Dunn RS, Vorhees CV, Wills-Karp M, Degen JL, Davis RJ, Mizushima N, Rakic P, Dardzinski BJ, Holland SK, Sharp FR, Kuan CY (2006) Cerebral ischemia-hypoxia induces intravascular coagulation and autophagy. Am J Pathol 169(2):566–583
Tiede LM, Cook EA, Morsey B, Fox HS (2011) Oxygen matters: tissue culture oxygen levels affect mitochondrial function and structure as well as responses to HIV viroproteins. Cell Death Dis 2:e246
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by the National Natural Science Foundation of China (Grant Nos. 81670086 and 81370183), the National Clinical Key Subject Construction Project of NHFPC Fund, and the National Training Program of Innovation and Entrepreneurship for Undergraduates (Grant No. 201510062005).
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and institutional guidelines for the care and use of animals were followed. The Animal Ethics Committee of Tianjin Medical University approved this study.
Rights and permissions
About this article
Cite this article
Song, S., Tan, J., Miao, Y. et al. Effect of different levels of intermittent hypoxia on autophagy of hippocampal neurons. Sleep Breath 21, 791–798 (2017). https://doi.org/10.1007/s11325-017-1512-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-017-1512-7