Abstract
Autophagy is an intracellular mechanism of degradation of cytoplasmic molecules and organelles in autophagosomes, which is necessary for maintaining cellular homeostasis and normal functioning of neurons, both at rest and under the action of extreme factors. Despite this, the role of autophagy in the mechanisms of adaptive and pathological reactions of the brain is still poorly understood and is an important problem for research. The study of autophagy processes in neurons under conditions of damaging factors is of fundamental importance and, at the same time, can be useful in terms of the development of specific drugs aimed at the links of the autophagic cascade. Since one of the most common injurious factors is hypoxia, the aim of this work was to assess the activity of the autophagy process in the hippocampus of rats after exposure to severe hypoxia. The immunohistochemical method was used. It was revealed that severe hypobaric hypoxia (180-mm Hg, 3 h) increases the autophagy activity in neurons of the hippocampal fields. This is manifested by a decrease in the content of the LC3 marker 1 day after exposure. At the same time, 3 days after hypoxia, the LC3 level is restored to control values. Thus, the data that are obtained in the model in vivo indicate that the autophagic degradation is intensified in the hippocampus of rats in response to the action of hypobaric hypoxia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Autophagy is an intracellular regulated mechanism of degradation of proteins with disrupted structure and damaged organelles in autophagosomes (Klionsky and Emr, 2000; Levine and Klionsky, 2004; Mizushima et al., 2004; Komatsu et al., 2006). Autophagy mechanisms control the quality of intracellular components and are aimed at maintaining cellular homeostasis necessary for differentiation, development, and normal functioning of cells (Kuma et al., 2004). The autophagic degradation of damaged cell organelles and proteins becomes especially important under various extreme factors, including oxidative stress, due to the timely utilization of damaged components (Kiffin et al., 2004).
Hypoxia is one of the most widespread injurious exposures and a leading component of the pathogenesis of many diseases, including coronary heart disease, cerebral stroke, and many neurological disorders. In experimental animal models, it has been shown that hypoxia caused by a decrease in atmospheric pressure (severe hypobaric hypoxia (SHH), 180-mm Hg for 3 h) causes structural damage to neurons in the most vulnerable areas of the brain (neocortex, hippocampus) (Rybnikova et al., 2004). One of the most important features of the response of brain neurons to hypoxia could be mediated by the change in autophagic activity.
Neurons are highly differentiated cells with extended processes, and therefore their functioning largely depends on the mechanisms of autophagy (Hara et al., 2006; Komatsu et al., 2006). It has been shown that activation of autophagy is observed after cerebral ischemia (Zhu et al., 2005; Rami et al., 2008), as well as in neurons exposed to excitotoxic stress (Wang et al., 2008). Further detailed study of the role of autophagy in the mechanisms of the adverse effect of hypoxia on brain neurons is a pressing problem, the study of which is not only of fundamental, but also practical, importance, since it creates the basis for the development of specific drugs that have a targeted effect on individual links of the autophagy cascade.
The aim of this work was to assess the activity of autophagy in the structures of the rat brain (hippocampus) after exposure to SHH.
Autophagy is a dynamic process that depends on the rate of formation of autophagosomes and the rate of their degradation and is characterized by autophagic flux that implies the speed of autophagosome turnover in the cell. To assess the activity of autophagy, we used chloroquine, a nonselective inhibitor of late stage autophagy. Its action is associated with a change in the pH of lysosomes. As a result the completion of the autophagy process is blocked and the accumulation of autophagosomes in the cell occurs (Solomon and Lee, 2009). The number of autophagosomes and the activity of autophagy are proportional to the content of specific protein markers of autophagy and can be detected by immunohistochemistry. One of the widely used markers of autophagy is LC3, which is specifically localized in autophagic structures throughout the entire process from autophagosome formation to degradation in lysosomes (Mizushima et al, 2010). We used antibodies to the marker LC3 (microtubule-associated proteins 1A and 1B, MAP1LC3), and estimated the intensity of autophagy in accordance with the change in the LC3 levels in the cells of the hippocampus in response to SHH.
MATERIALS AND METHODS
The work was performed on adult male Wistar rats (200–220 g) obtained from the Center for Collective Use of the Biocollection Institute of Physiology, Russian Academy of Sciences.
SHH Model and Experimental Setup
SHH was created in a hypobaric chamber of a flowthrough type by maintaining an atmospheric pressure at 180-mm Hg for 3 h. The experiment involved the following groups of animals: (1) the control; (2) the chloroquine group, animals that were injected with chloroquine (an inhibitor of the completion of the autophagy process); (3) the SHH group, animals that were exposed to SHH; and (4) an SHH + chloroquine groups, animals that were exposed to SHH and injected with chloroquine immediately after the end of SHH. Rats of groups 1 and 3 were injected intraperitoneally with 200 μL of 0.9% NaCl, and groups 2 and 4 were injected with 200 μL of chloroquine solution prepared in 0.9% NaCl at a dose of 3 mg per 1 kg of animal weight. All groups consisted of five to six animals. The animals were anesthetized using an intraperitoneal injection of a two-component solution (60 mg of zoletil and 10 mg of 2% xylazine per 1 kg of body weight), transcardial perfusion was performed. After perfusion the brain was removed; the area containing the parietal neocortex and hippocampus was isolated and fixed for 1 day in 4% paraformaldehyde (0.01M phosphate buffer, pH 7.4).
Histological Tissue Processing
The fixed tissue samples were washed in running water for 2 h. The samples were dehydrated in alcohol of ascending concentration according to the following scheme: isopropanol 50% (1 h), isopropanol 70% (1 h), isopropanol 80% (overnight), isopropanol 100% (1 h), and isopropanol 100% (5 h). After that, the samples were put in paraffin (58°C) for 3 h (3 paraffin changes, 1 h each) and embedded into paraffin blocks. A series of paraffin frontal sections of the brain with a thickness of 4 μm at a level –2.8 mm from the bregma was cut and mounted on glass slides.
Immunohistochemical Staining
The slices were dewaxed in xylene (two changes, 5 min each) and rehydrated in descending concentration of ethanol, after which they were rinsed in phosphate buffered saline (PBS). Antigen unmasking was performed in citrate buffer (0.01 M, pH 6.0) at 100°C under pressure for 1 min. The samples were left to cool in the citrate buffer for 30 min, and were washed 2 times in PBS for 5 min. The tissue samples were then outlined with a hydrophobic marker and a blocking serum was applied to them; the slices were left for incubation in a humid chamber at room temperature for 30 min. Then, the excess blocking serum was carefully removed and the primary antibodies to LC3 were applied to the slides, the samples were left to incubate overnight in a humid chamber at 4°C. The samples were washed in PBS twice for 5 min. Then, the samples were incubated with secondary biotinylated antibodies and the avidin–biotin complex according to the manufacturer’s recommendations. Between incubations, the preparations were washed in PBS twice for 5 min. The reaction was visualized using a diaminobenzidine imaging kit. The samples were dehydrated and embedded in a mounting medium.
Data Processing
The samples were analyzed using an Olympus CX31 light microscope (Olympus, Japan), a ProgRes CT1 digital camera (Jenoptic, Germany), and a personal computer. The quantitative analysis of samples was performed using the ImageJ software (https://imagej.nih.gov/ij/) using the SDA plugin (Nurzynska et al., 2017). The integral brightness of the LC3 immunoreactive substance and its staining area were calculated; the obtained values were recalculated and expressed in conventional units of optical density. Thus, the calculated optical density values reflected the content of the LC3 protein in the cells. The results were statistically processed using the Microsoft Excel 2010 and STATISTICA 7.0 data analysis packages using the Mann–Whitney test. The differences between the samples were considered statistically significant when R < 0.05. The data in the figures are presented as mean and errors of the mean.
Reagents
We used primary antibodies to LC3 (Sigma Aldrich, United States); blocking serum, secondary biotinylated antibodies, and avidin–biotin complex in the VECTASTAIN ABC-Peroxidase Kit (Vector Laboratories PK-4001, Inc, United States); the DAB peroxidase substrate kit (Vector Laboratories, Inc, United States); the Bio-Mount mounting medium (Bio-Optica, Italy); chloroquine diphosphate (Sigma Aldrich, United States); paraformaldehyde (LenReaktiv, Russia); xylavette 2% (Alpha-Vet Veterinary Ltd, Hungary); and zoletil (Valdepharm, France).
RESULTS AND DISCUSSION
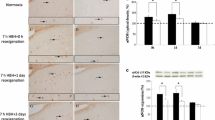
In the samples studied, the localization of LC3 was cytoplasmic. It is known that the isoforms of the LC3 protein are predominantly cytoplasmic (Koukourakis et al., 2015). In the investigated hippocampal fields (CA1, CA3, CA4) and in the dentate gyrus of the control group, the immunoreactivity to LC3 was concentrated both in the cell soma and in the cell processes. In particular, in field CA1 of the hippocampus, fragments of dendrites and in the field CA3 neuropil were intensely stained (Fig. 1a, black arrows). In rats injected with chloroquine, 1 day after injection, the level of immunoreactivity to LC3 in neurons of the studied hippocampal fields (CA1, CA3, CA4, dentate gyrus) was higher relative to the control, which was manifested by an increase in the optical density of the immunostaining (Figs. 1a, 1b, 2). After 3 days, the LC3 content did not differ from the control values in the CA1 and CA3 fields, but was higher than the control in the CA4 field (P = 0.005616) and dentate gyrus of hippocampus (R = 0.008352) (Fig. 2).
Histological specimen of the frontal section of the brain, illustrating immunoreactivity to LC3 in the CA1 field of the rat hippocampus: (a) control, (b) 1 day after chloroquine injection (3 mg/kg), (c) 1 day after severe hypobaric hypoxia (SHH, 180‑mm Hg, 3 h), and (d) 1 day after SHH and chloroquine injection at the end of SHH. The immunoperoxidase method with diaminobenzidine staining was used. Arrows in the photograph of the control specimen show neuronal processes that are immunoreactive to LC3.
Changes in LC3 immunoreactivity in (a) CA1, (b) CA3, (c) CA4, and (d) the dentate gyrus of the hippocampus after SHH (180-mm Hg, 3 h), as well as during the administration of chloroquine (3 mg/kg). Vertically—optical density of objects that are immunoreactive to LC3, arb. units. Data are presented as mean values and their errors. Differences between groups according to the Mann–Whitney test are significant when *P ≤ 0.05, **P ≤ 0.01, or ***P ≤ 0.001.
It has been shown in the model in vitro that a fairly high basal level of autophagy activity is maintained in neurons, though the number of autophagosomes is small due to their rapid degradation (Boland et al., 2008). Our results in vivo are consistent with this statement and indicate that the autophagy process is highly intensive in the cells of the rat hippocampus. Despite the relatively low level of immunoreactivity to LC3 in the hippocampal neurons of the control group of animals, 1 day after chloroquine administration, the level of LC3 increased significantly; accumulation of this protein occurred due to inhibition of its degradation in lysosomes.
A fairly large number of studies indicate that the basal level of autophagy is extremely important for the normal functioning of neurons (Boland et al., 2008; Tooze, Schiavo, 2008). In particular, it was shown that, in animals with gene knockout atg5 or atg7 (autophagosome assembly genes), the number of protein inclusions in neurons increases and neurodegeneration develops (Hara et al., 2006; Komatsu et al., 2006). Defects at various stages of the autophagy process are associated with the progression of Parkinson’s, Huntington’s, and Alzheimer’s diseases (see Ghavami et al., 2014). Therefore, the study of autophagy process, as one of the main mechanism for maintaining homeostasis in neurons, is important, especially under the action of various kinds of extreme factors, including hypoxia.
In the present study, we found that, 1 day after SHH, the level of immunoreactivity to LC3 was reduced relative to the control in the CA1 and CA4 fields and the dentate gyrus of the hippocampus (Figs. 1a, 1c, 2a, 2c, 2d). In the CA3 field, the SHH did not affect the level of immunoreactivity to LC3 (Fig. 2b). Since the level of immunoreactivity to LC3 in the control group is rather low, it is difficult to evaluate its decrease after SHH. Therefore, in the experiment we used an additional group of animals, which were injected with chloroquine after exposure to SHH. There was a decrease in the level of immunoreactivity to LC3 in the CA1 and CA3 fields and the dentate gyrus of the hippocampus of rats in the SHH + chloroquine group relative to the comparison group (chloroquine) 1 day after SHH (Figs. 1c, 1d, 2a, 2b, 2d).
The observed difference in the level of immunoreactivity to LC3 between the chloroquine and SHH + chloroquine groups in these areas of the hippocampus, we believe, is due to the fact that the intensification of autophagic degradation processes begins early after the onset of SHH exposure, when the animals are in the hypobaric chamber. In addition, the effect of the chloroquine did not start immediately, but some time after the injection, in accordance with the pharmacokinetics of the drug (Adelusi and Salako, 1982). Thus, by the time when chloroquine entered the cells and blocked lysosomal degradation, the intensity of autophagy was increased by SHH and, therefore, the rate of LC3 decay increased and its content decreased. In the CA4 field of the hippocampus, no significant changes in immunoreactivity to LC3 were revealed 1 day after SHH (Fig. 2c).
The observed decrease in the level of LC3 in neurons of the hippocampus 1 day after SHH both in the SHH group and in the SHH + chloroquine group is possibly associated with rapid protein degradation upon activation of autophagy. The rate of protein breakdown increases and is not compensated by its synthesis de novo at this time. This statement is supported by the fact that the amount of LC3 is inversely proportional to the activity of autophagy; i.e. a decrease in the LC3 level is a sign of an intensification of the autophagy process at a later timepoint after the onset of autophagy activation (Mizushima et al., 2010).
In our experiments three days after SHH, the level of immunoreactivity to LC3 in the SHH group did not differ from that in the control group, while, in the SHH + chloroquine group, it did not differ from that in the chloroquine group (Fig. 2). It is possible that, 3 days after SHH, the intensity of the autophagy process decreases, or perhaps the balance between the level of degradation and protein synthesis is restored.
Thus, the analysis of the data obtained allows us to conclude that the process of autophagic degradation is enhanced by the action of SHH in the neurons of the rat hippocampus at an early stage (1 day) and is stabilized 3 days after exposure. It is possible that such dynamics of autophagy activity in response to SHH characterizes the adaptive mechanisms activated in neurons and is aimed at utilizing damaged organelles, proteins, and other toxic products. This view is supported by a number of studies. In particular, it has been shown that autophagy is one of the important mechanisms of regulation of the activity of the transcription factor HIF-1 (due to its selective degradation), one of the main players of the cellular response to hypoxia (Hubbi et al., 2013). Caspase-3 can undergo autophagic degradation (Dohi et al., 2012). In addition, it has been shown that activation of autophagy results in the detachment of the Bcl-2 antiapoptotic factor from the Ambra1 protein, as a result Bcl-2 becomes functionally active (Toozel, Codogno, 2011).
Autophagy has an important role in the antioxidative defense of the body (Giordano et al., 2013). Nevertheless, in some cases, activation of autophagy is associated with cell death, especially in acute conditions such as ischemia (Adhami et al., 2006; Koike et al., 2008; Rami et al., 2008; Puyal and Clarke, 2009). The question of whether an increase in autophagic activity in response to extreme factors is a mechanism of cell death or part of an adaptive strategy remains controversial (Zubova, 2019). This may largely depend on the parameters of the experimental model used. It is known that, under hypoxic (ischemic) exposure, the main mechanisms of cell death are necrosis, apoptosis, or a mixed type of death (Liu et al., 2004; Rybnikova et al., 2006). A number of studies have shown that the mechanisms of apoptosis and autophagy are interrelated and, when apoptosis is blocked, the cell death is mediated by activation of autophagy (Shimizu et al., 2004; Yu et al., 2004; Boya et al., 2005; Yousefi et al., 2006).
Thus, given the great importance of autophagy in the functioning of neurons, elucidation of its role in compensatory or proapoptotic mechanisms induced by SHH in neurons is an important fundamental problem and will become the subject of further research. At the same time, the development of drugs specific to the components of the autophagic cascade, substantiated by research in this area, may serve an effective strategy for the prevention or correction of diseases which have hypoxia as the main factor of pathogenesis.
REFERENCES
Adelusi, S.A. and Salako, L.A., Kinetics of the distribution and elimination of chloroquine in the rat, Gen. Pharmacol., 1982, vol. 13, p. 433.
Adhami, F., Liao, G., Morozov, Y.M., Schloemer, A., Schmithorst, V.J., Lorenz, J.N., Dunn, R.S., Vorhees, C.V., Wills-Karp, M., Degen, J.L., Davis, R.J., Mizushima, N., Rakic, P., Dardzinski, B.J., Holland, S.K., et al., Cerebral ischemia-hypoxia induces intravascular coagulation and autophagy, Am. J. Pathol., 2006, vol. 169, p. 566.
Boland, B., Kumar, A., Lee, S., Platt, F.M., Wegiel, J., Yu, W.H., and Nixon, R.A., Autophagy induction and autophagosome clearance in neurons: relationship to autophagic pathology in Alzheimer’s disease, J. Neurosci., 2008, vol. 28, p. 6926.
Boya, P., Gonzalez-Polo, R.A., Casares, N., Perfettini, J.L., Dessen, P., Larochette, N., Metivier, D., Meley, D., Souquere, S., Yoshimori, T., Pierron, G., Codogno, P., and Kroemer, G., Inhibition of macroautophagy triggers apoptosis, Mol. Cell Biol., 2005, vol. 25, p. 1025.
Dohi, E., Tanaka, S., Seki, T., Miyagi, T., Hide, I., Takahashi, T., Matsumoto, M., and Sakai, N., Hypoxic stress activates chaperone-mediated autophagy and modulates neuronal cell survival, Neurochem. Int., 2012, vol. 60, p. 431.
Ghavami, S., Shojaei, S., Yeganeh, B., Andee, S.R., Jangamreddy, J.R., Mehrpour, M., Christoffersson, J., Chaabane, W., Moghadam, A.R., Kashani, H.H., Hashemi, M., Owji, A.A., and Łos, M.J., Autophagy and apoptosis dysfunction in neurodegenerative disorders, Progress Neurob-iol., 2014, vol. 112, p. 24.
Giordano, S., Darley-Usmar, V., and Zhang, J., Autophagy as an essential cellular antioxidant pathway in neurodegenerative disease, Redox Biol., 2013, vol. 2, p. 82.
Hara, T., Nakamura, K., Matsui, M., Yamamoto, A., Nakahara, Y., Suzuki-Migishima, R., Yokoyama, M., Mishima, K., Saito, I., Okano, H., and Mizushima, N., Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice, Nature, 2006, vol. 441, p. 885.
Hubbi, M.E., Hu, H., Kshitiz, Ahmed, I., Levchenko, A., and Semenza, G.L., Chaperone-mediated autophagy targets hypoxia-inducible factor-1α (HIF-1α) for lysosomal degradation, J. Biol. Chem., 2013, vol. 288, p. 10703.
Kiffin, R., Christian, C., Knecht, E., and Cuervo, A.M., Activation of chaperone-mediated autophagy during oxidative stress, Mol. Biol. Cell., 2004, vol. 15, p. 4829.
Klionsky, D.J. and Emr, S.D., Autophagy as a regulated pathway of cellular degradation, Science, 2000, vol. 290, p. 1717.
Koike, M., Shibata, M., Tadakoshi, M., Gotoh, K., Komatsu, M., Waguri, S., Kawahara, N., Kuida, K., Nagata, S., Kominami, E., Tanaka, K., and Uchiyama, Y., Inhibition of autophagy prevents hippocampal pyramidal neuron death after hypoxic/ischemic injury, Am. J. Pathol., 2008, vol. 172, p. 454.
Komatsu, M., Waguri, S., Chiba, T., Murata, S., Iwata, J., Tanida, I., Ueno, T., Koike, M., Uchiyama, Y., Kominami, E., and Tanaka, K., Loss of autophagy in the central nervous system causes neurodegeneration in mice, Nature, 2006, vol. 441, p. 880.
Koukourakis, M.I., Kalamida, D., Giatromanolaki, A., Zois, C.E., Sivridis, E., Pouliliou, S., Mitrakas, A., Gatter, K.C., and Harris, A.L., Autophagosome proteins LC3A, LC3B and LC3C have distinct subcellular distribution kinetics and expression in cancer cell lines, PLoS One, 2015, vol. 10. e0137675. https://doi.org/10.1371/journal.pone.0137675
Kuma, A., Hatano, M., Matsui, M., Yamamoto, A., Nakaya, H., Yoshimori, T., Ohsumi, Y., Tokuhisa, T., and Mizushima, N., The role of autophagy during the early neonatal starvation period, Nature, 2004, vol. 432, p. 1032.
Levine, B. and Klionsky, D.J., Development by self-digestion: molecular mechanisms and biological functions of autophagy, Dev. Cell, 2004, vol. 6, p. 463.
Liu, C.L., Siesjö, B.K., and Hu, B.R., Pathogenesis of hippocampal neuronal death after hypoxia-ischemia changes during brain development, Neuroscience, 2004, vol. 127, p. 113.
Mizushima, N., Yamamoto, A., Matsui, M., Yoshimori, T., and Ohsumi, Y., In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker, Mol. Biol. Cell, 2004, vol. 15, p. 1101.
Mizushima, N., Yoshimori, T., and Levine, B., Methods in mammalian autophagy research, Cell, 2010, vol. 140, p. 313.
Nurzynska, K., Mikhalkin, A., and Piorkowski, A., CAS: cell annotation software—research on neuronal tissue has never been so transparent, Neuroinformatics, 2017, vol. 15, p. 365.
Puyal, J. and Clarke, P.G.H., Targeting autophagy to prevent neonatal stroke damage, Autophagy, 2009, vol. 5, p. 1060.
Rami, A., Langhagen, A., and Steiger, S., Focal cerebral ischemia induces upregulation of beclin 1 and autophagy-like cell death, Neurobiology, 2008, vol. 29, p. 132.
Rybnikova, E.A., Khozhai, L.I., Tyul’kova, E.I., Glushchenko, T.S., Sitnik, N.A., Pelto-Huikko, M., Otellin, V.A., and Samoilov, M.O., Expression of early gene proteins, structural changes in brain neurons in hypobaric hypoxia and the correcting effects of preconditioning, Neurosci. Behav. Physiol., 2005, vol. 35, no. 4, p. 383.
Rybnikova, E., Sitnik, N., Gluschenko, T., Tjulkova, E., and Samoilov, M.O., The preconditioning modified neuronal expression of apoptosis-related proteins of Bcl-2 superfamily following severe hypobaric hypoxia in rats, Brain Res., 2006, vol. 1089, p. 195.
Shimizu, S., Kanaseki, T., Mizushima, N., Mizuta, T., Arakawa-Kobayashi, S., Thompson, C.B., and Tsujimoto, Y., Role of Bcl-2 family proteins in a nonapoptotic programmed cell death dependent on autophagy genes, Nat. Cell Biol., 2004, vol. 6, p. 1221.
Solomon, V.R. and Lee, H., Chloroquine and its analogs: a new promise of an old drug for effective and safe cancer therapies, Eur. J. Pharmacol., 2009, vol. 625, p. 220.
Tooze, S.A. and Schiavo, G., Liaisons dangereuses: autophagy, neuronal survival and neurodegeneration, Curr. Opin. Neurobiol., 2008, vol. 18, p. 504.
Toozel, S.A. and Codogno, P., Compartmentalized regulatio-n of autophagy regulators: fine-tuning AMBRA1 by Bcl-2, EMBO J., 2011, vol. V 30, p. 1185.
Wang, Y., Han, R., Liang, Z.Q., Wu, J.C., Zhang, X.D., Gu, Z.L., and Qin, Z.H., An autophagic mechanism is involved in apoptotic death of rat striatal neurons induced by the non-N-methyl-D-aspartate receptor agonist kainic acid, Autophagy, 2008, vol. 4, p. 214.
Yousefi, S., Perozzo, R., Schmid, I., Ziemiecki, A., Schaffner, T., Scapozza, L., Brunner, T., and Simon, H.U., Calpain-mediated cleavage of Atg5 switches autophagy to apoptosis, Nat. Cell Biol., 2006, vol. 8, p. 1124.
Yu, L., Alva, A., Su, H., Dutt, P., Freundt, E., Welsh, S., Baehrecke, E.H., and Lenardo, M.J., Regulation of an ATG7-beclin 1 program of autophagic cell death by caspase-8, Science, 2004, vol. 304, p. 1500.
Zhu, C., Wang, X., Xu, F., Bahr, B.A., Shibata, M., Uchiyama, Y., Hagberg, H., and Blomgren, K., The Influence of age on apoptotic and other mechanisms of cell death after cerebral hypoxia–ischemia, Cell Death Differ., 2005, vol. 12, p. 162.
Zubova, S.G., The diversity of autophagy and its controversial role in biological processes, Tsitologiia, 2019, vol. 6, no. 12, p. 941.
Funding
This work was financially supported by the Russian Foundation for Basic Research, project no. 19-04-01152.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest. The authors declare that they have no conflict of interest.
Statement on the welfare of animals. The experiments were carried out in accordance with the principles laid down in the directives of the European Council (2010/63/EU) on the use of animals for experimental research and the Declaration of Animal Rights of the World Medical Association of Helsinki. Experimental protocols were approved by the Commission for the Humane Treatment of Animals of the Pavlova Institute of Physiology, Russian Academy of Sciences.
Additional information
Abbreviations: SHH—severe hypobaric hypoxia.
Rights and permissions
About this article
Cite this article
Churilova, A.V., Zachepilo, T.G. & Zenko, M.Y. The Effect of Severe Hypobaric Hypoxia on the Content of the Autophagy Marker LC3 in the Hippocampus of Rats. Cell Tiss. Biol. 15, 174–180 (2021). https://doi.org/10.1134/S1990519X21020036
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990519X21020036