Abstract
Introduction
Mayonnaise is an oil in water emulsion (O/W) consisting 70–80% oil. Lipid oxidation is a major cause of quality deterioration in mayonnaise. The effectiveness of antioxidants in a hetrophasic systems is highly dependent on their polarity and partitioning properties.
Objectives
The aim of the present study was to determine the effect of a hydrophilic [green tea extract (GTE)] and a lipophilic [tocopherol mixture (TOC)] and BHA on lipid oxidation in mayonnaise during 60 days of storage at 38 °C and to examine the interactions between GTE and TOC, to determine possible synergistic or antagonistic effects in antioxidant activity.
Methods
The oxidative stability was studied by measuring hydroperoxides, volatile organic compounds (VOCs) and colour of mayonnaise during storage. Comprehensive analysis of VOCs was done by static headspace extraction and separation by two-dimensional gas chromatography time of flight mass spectrometry. Sensory analysis was also carried out to study the effect of storage time and antioxidant type on sensory properties of mayonnaise and to investigate the predictive ability of volatile compounds for sensory terms.
Results and conclusion
Addition of GTE (500 ppm) and TOC (500 ppm) increased the formation of hydroperoxides and certain VOCs. The combination of GTE with TOC improved the antioxidant efficacy compared to the individual extracts. However, sensory evaluation demonstrated that GTE promoted the development of unpleasant fishy and rancid aroma. The volatile compound methional, was significantly and positively correlated with fishy and rancid flavour. Regarding colour analysis, GTE showed the highest increase in darkening and browning during storage.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Mayonnaise is an oil in water (O/W) emulsion consisting 70–80% oil. Lipid oxidation is a major cause of quality deterioration in mayonnaise. The most common strategy to retard lipid oxidation is the use of antioxidants (Coupland and McClements 1996). Antioxidants are divided into two categories based on their origin: synthetic and natural. Due to health concerns about synthetic antioxidants, there has been an increase in the use of natural antioxidants, which are efficient and safe in food systems. A number of studies investigated the potential of natural antioxidants in mayonnaise see e.g. review by Ghorbani Gorji et al. (2016).
Among naturally occurring antioxidants, the antioxidative efficacy of tocopherols (TOCs) has been examined extensively in food systems (Jacobsen et al. 2000; Jayasinghe et al. 2013; Karahadian and Lindsay 1989; Let et al. 2007; Panya et al. 2012; Rizner et al. 2000). TOCs can control autoxidation by acting as radical scavengers. However, the pro-oxidant effect of high concentrations of TOCs have been reported by promoting formation of hydroperoxides (Frankel et al. 1994; Karahadian and Lindsay 1989).
Green tea extract (GTE) is a water-soluble, polyphenolic compound. Catechins are the predominant group of polyphenols in GTE, and considered to act as antioxidants by scavenging radicals (Huang and Frankel 1997). GTEs have shown good antioxidative activity in oil (Chen et al. 2016; Chen and Chan 1996; Frankel et al. 1997; Wanasundara and Shahidi 1998; Yin et al. 2012). However, pro-oxidative behaviour was seen in corn O/W emulsion (Frankel et al. 1997; Huang and Frankel 1997).
The O/W emulsion is a system, containing oil droplets dispersed in an aqueous phase. It can consist of three regions: the continuous phase, the interfacial phase and the interior of a droplet. The effectiveness of antioxidants in such systems is highly dependent on their polarity and partitioning properties. The hydrophilic antioxidants predominantly are partitioned in the aqueous phase and the lipophilic antioxidants are mainly partitioned in the oil phase (Coupland and McClements 1996; Jacobsen et al. 1999c). In O/W emulsion systems, as the “polar paradox” theory states, non-polar antioxidants are more effective than their polar analogues (Frankel et al. 1994). Studies on combining water-soluble antioxidants, such as ascorbic acid and GTE, with TOC showed the regeneration of TOC by reducing the tocopheroxyl radical in the O/W emulsion (Buettner 1993; Yin et al. 2012). The potential activity of antioxidants is also dependent on the nature of the food system. In order to predict the antioxidant activity, the antioxidants should be tested in the product. To the best of our knowledge, no studies have evaluated the interactions of GTE and TOC in a mayonnaise-like emulsion (80% sunflower oil).
Frankel et al. (1994) showed that determination of antioxidant activity is affected by the method used to evaluate lipid oxidation. For this reason, in order to be able to understand whether GTE and TOC act as antioxidants or pro-oxidants, the progress of oxidation was monitored by measuring the formation of primary (hydroperoxides) and secondary oxidation products [volatile organic compounds (VOCs)]. Colour and sensory properties were also monitored.
The aim of the present study is to evaluate the anti- or pro-oxidative properties of hydrophilic and lipophilic antioxidants in a mayonnaise-like emulsion over the course of 60 days at 38 °C, using measurement of colour, hydroperoxide concentration and volatile oxidation compounds, as well as descriptive sensory analysis. We therefore chose a TOC mixture that is oil-soluble, and GTE, which is a water-soluble antioxidant. An additional objective was to examine the interactions between GTE and TOC, to determine possible synergistic or antagonistic effects in antioxidant activity.
2 Materials and methods
2.1 Materials
Sunflower oil with and without butylated hydroxyanisole (BHA) added (200 mg/kg oil) was used. BHA is a synthetic antioxidant commonly used in the food industry. The characterisation of the oil used in the experiments was measured using the following techniques: acidity was determined by titration, hydroperoxide concentration was measured according to FOX2 assay (Nourooz-Zadeh et al. 1995), fatty acid (%) was measured as previously described by Concepcion et al. (2018), and different analogues of tocopherols were analysed by HPLC as previously described by (Zou et al. 2017). The characterisation of the oil is presented in Table S1 (see Supplementary material). The green tea extract (GUARDIANTM Green Tea Extract 20S) and tocopherol mixture (GUARDIANTM Toco 70) were donated by Danisco Australia (DuPont Nutrition & Health, Australia). Green tea extract was composed of about 20% of catechins (wt%) and salt (NaCl). It contains gallic acid (1.90 mg/g), thearubigin (0.24 mg/g), gallocatechin (6.61 mg/g), theo-phylline (0.14 mg/g), epigallocatechin (25.83 mg/g), caffeine (18.62 mg/g), epicatechin (0.75 mg/g), epigallocatechingallate (124.01 mg/g), and epicatechingallate (20.09 mg/g) (Soncu and Kolsarici 2017). The mixture of tocopherol was oil-soluble, comprising 70% natural tocopherol concentrate. The concentrations of the different analogues were analysed by HPLC are α-tocopherol, 15.4% w/w, β and γ-tocopherol, 59.1% w/w, and δ-tocopherol 25.5% w/w.
Ammonium ferrous sulfate, cumene hydroperoxide, butylated hydroxy toluene (BHT), xylenol orange [o-cresolsulfonphthalein-3,3-bis (methyliminoacetic acid sodium salt)], sulfuric acid, methanol and propan-1-ol were from Sigma Aldrich. External standards for identification of volatile compounds are listed in Table S2 (see Supplementary material).
2.2 Production of mayonnaise and storage experiment
An O/W emulsion modelled on mayonnaise was produced in 1.5 kg batches using a Kogan 1000 W Professional Food Processor & Blender. The mayonnaise recipe contained (all amounts are stated in wt%) sunflower oil (80%), egg (11%), white vinegar (3%), sugar (2%) and water (4%). Five different formulations of mayonnaise were produced to assess the effect of antioxidant type. Table S3 (see Supplementary material) shows antioxidant concentration, sample code and in vitro antioxidant activity of antioxidants. The doses of TOC and GTE were decided based on the supplier’s recommendation. Potential antioxidants were added in either the oil phase (BHA and TOC) or in the water phase (GTE) before mayonnaise production. TOC and GTE were made in a ratio of 1:1 and named GTT, in order to study the interactions between GTE and TOC, to determine possible synergistic or antagonistic effects in antioxidant activity. The GTE was mixed with the aqueous phase and TOC was mixed with the oil. A control (CON) sample was prepared without adding any antioxidants. Each formulation was made in three batches. pH o samples were 4.19–4.21 (Table S4, see Supplementary material). Samples were aseptically transferred into sterile specimen jars (75 ml) with caps and filled to the top, closed tightly (without headspace), and stored at 38 °C (elevated temperature) in dark for 60 days.
The VOCs and hydroperoxides were determined at day 1, 15, 30, 45, and 60 of storage, whereas sensory and colour measurements were performed at day 1, 30 and 60 of storage. Samples were kept at − 80 °C until the hydroperoxide and VOCs analysis while sensory and colour analyses were made directly after sampling. All determinations were made as triplicate.
2.3 Determination of in vitro antioxidant capacity
The assay for ABTS [2,2-azinobis-(3-ethylbenzothiazoline-6-sulphonic acid)] free radical scavenging capacity was conducted according to the method described by Danh et al. (2012) with some modifications. Briefly, the ABTS· radical cation stock solution was prepared by mixing 7 mM ABTS solution with 2.45 mM potassium persulphate (final concentration) and the mixture was left in the dark at room temperature for 12–16 h. Before performing the assay, the stock solution was diluted with methanol (HPLC grade) to an absorbance of 1.0, measured by a spectrophotometer at the wavelength of 734 nm. The antioxidants were diluted in order to acquire a readable range absorbance measurement. Thirty µl of extracts (three replicates per extract) were added to 1.5 ml of diluted ABTS· solution and incubated for 15 min in the dark. The absorbance was measured at 734 nm against blank sample of methanol. Gallic acid was used as the reference standard. The results of radical scavenging activity of the natural antioxidants tested were expressed as gallic acid equivalent (mmol GA/mg).
2.4 Colour measurements
Colour of mayonnaises was measured during storage in order to identify whether the addition of different potential antioxidants influenced the colour of samples since a different colour could decrease sensory appeal. Colour parameters were measured using the Minolta Chroma Meter CR-4 (Minolta Camera Co., Osaka, Japan) at designated time intervals to estimate mayonnaise colour in CIE space: lightness, (l*); redness, (a*); yellowness, (b*) (International Commission on Illumination, 2008).
2.5 Lipid separation from mayonnaise
The oil phase from the mayonnaise was separated according to the method (Jacobsen et al. 1999b) and kept at − 80 °C until analysis (hydroperoxide concentration).
2.6 Determination of hydroperoxides concentration
Lipid hydroperoxides of the oil phase separated from mayonnaise were measured according to FOX2 assay (Nourooz-Zadeh et al. 1995).
2.7 Analysis of volatile compounds
Comprehensive analysis of volatile compounds in mayonnaise was done by static headspace extraction and separation by two-dimensional gas chromatography time of flight mass spectrometry (GC × GC/TOF–MS; Leco, Australia). Briefly, headspace (1.5 ml) was collected using a 2.5 ml headspace syringe, after 10 min agitation of the sample at 80 °C. At each time point, mayonnaise (1 g) was weighed immediately into 20 ml GC headspace vials, covered with a silicon cap (Restek, Germany) and then placed at − 80 °C until analysis. On the day of analysis samples were equilibrated overnight at room temperature. The instrument parameters and data pre-processing were set as previously described by Daygon et al. (2016). The full details of the metabolite profiling including GC × GC program settings, MS conditions and chromatogram alignment parameters are given in Table S5 and Supplementary Information S1. Data pre-processing, baseline correction and identification of putative compounds were done using the software LECO ChromaTOF 4.50 with library match searching (Nist 11 v2.0). Volatile compounds were identified by combination of mass spectral library matching using NIST 11 v 2.0 library and an in-house library created by running authentic reference standards of common lipid oxidation products (Supplementary Table S2). Volatile compounds were putatively identified using mass spectra only when standards were not available. In this case the compounds that had ≥ 80% similarity with NIST library were taken into consideration.
Five volatiles were selected for quantification through calibration curves. The five volatiles were identified as: pentanal, hexanal, heptanal, octanal and nonanal. These aldehydes have previously been identified in the headspace of both sunflower oil and mayonnaise and have been shown to correlate with lipid oxidation in sunflower oil (Guillén et al. 2005). The antioxidant efficacy was calculated as the inhibition percentage according the following equation:
VOCsample,CON and VOCsample,antioxidant are samples without antioxidant and samples with antioxidant added, respectively. The antioxidant has a prooxidative effect if the inhibition % is negative (−) and an antioxidative effect if it is positive (+).
2.8 Descriptive sensory evaluation
The descriptive sensory evaluation test was carried out according to the method described by Tikapunya et al. (2018). The panel consisted of 12 members (3 males, 9 females, aged 37–66 years with a mean age of 48) who were recruited from a pool previously screened for ability to discriminate basic odours and tastes, according to standard ISO 3972 (ISO, 1991, 1992, 1993) procedures. The training and discussions sessions were conducted over 8 sessions (2 h each) in a meeting room, using a subset of samples selected to cover the total range of day/treatment variation. A total of 14 descriptors, definitions and sensory reference standards (7 aroma and 7 flavour) were developed (Table S6, see Supplementary material). Prior to each evaluation, the panellists were presented with the 13 sensory reference standards and definitions to review prior to assessment (Table S6, Supplementary material). The intensities of descriptive terms were quantified by use of line scale scoring on an unstructured line (10 cm), which was anchored from ‘‘none’’ and to ‘‘high’’. Scoring was computerised using the FIZZ acquisition software (Bio-System, Couternon, France).
2.9 Statistical analysis
Data from hydroperoxides, colour, sensory and volatile analysis were analysed by a one-way analysis of variance, and individual samples were compared on a 0.05 level of significance and Fisher’s test using XLSTAT (Add in soft 1995-204, CA, USA). All references to significant differences between samples or between sampling times are based on this statistical analysis of data. Multivariate data analysis was done using software Simca V14 (Umetrics).
The sensory results from FIZZ software were exported into Microsoft excel and were analysed by XLSTAT (Add in soft 1995-204, CA, USA). Factors and interactions effects were analysed using Mixed Model Analysis of Variance (three way and two-way ANOVA) applied to the raw data set (15 sample × 12 panellists × 3 replicates) for each attribute to determine significant differences (p < 0.05 and p < 0.01).
2.9.1 Principal component analysis (PCA)
The results from sensory analysis were subjected to principal component analysis (PCA). The PCA models were built on the average of the measured data, scaled to unit variance and full cross-validation was done.
2.9.2 Partial least square regression (PLS)
In the PLS analysis, the design variables used as X-variables were X = type of antioxidants (CON, BHA, GTE and TOC) and replicates [R1, R2, R3, AV (mean values)], and a single quantitative variable for ‘days’ of storage (D; with levels 1, 15, 30, 45 and 60). Analytical data from volatile oxidation products (peak areas) were used as Y-variables. The data was scaled to unit variance and full cross-validation was done. The model parameters such as R2 and Q2 is presented in Table S7 (see Supplementary material).
The sensory and chemical predictive ability of volatile compounds for sensory attributes was tested by PLS analysis with sensory terms as X-matrix and volatile compounds as the Y-matrix. The data was scaled to unit variance and full cross-validation was done. The model parameters such as R2 and Q2 is presented in Table S8 (see Supplementary material).
The regression coefficients were automatically calculated by the Simca V14 software relating to the calculations of the PLS model, so it was possible to assess whether regression coefficients for the different design variables were positive or negative for each of the measured variables. The + or − sign associated with the regression coefficient indicated either positive or negative correlation between the two variables. To gain information about significance indications for the relationship in the X- and Y-, regression coefficients were analysed by jack-knifing (p < 0.1) (Nissen et al. 2004) using Simca V14 software.
3 Results
3.1 Effect of storage time and antioxidant type on lipid oxidation products
3.1.1 Formation of hydroperoxides
Generally, until day 30, there was no difference among CON, TOC and GTE mayonnaises. After day 30 the hydroperoxides decreased in CON, remained constant in TOC and increased in GTE (Fig. 1a). The hydroperoxides concentration increased significantly faster and to a higher level in mayonnaises with GTE than BHA during 60 days of storage. BHA reduced the formation of hydroperoxides in mayonnaise during storage and showed antioxidative activity whereas, GTE was pro-oxidant, and accelerated the formation of hydroperoxides.
a The concentration of hydroperoxides (mM) in mayonnaises during storage of 60 days. Data points and error bars represent means ± standard errors (n = 3). CON no antioxidant, BHA butylated hydroxyanisole, TOC tocopherol, GTE green tea extract. b, c Partial least square regression analysis (PC1, PC2), X = design variables (days of storage: 1, 15, 30, 60; antioxidant type: CON (no antioxidant); BHA (butylated hydroxyanisole); TOC (tocopherol); GTE (green tea extract), replicates (R1, R2, R3, AV (mean values)) and Y = volatile compounds: b scores plot of mayonnaises stored for 1 day (green circle), 15 days (orange circle), 30 days (blue circle), 45 days (yellow circle) and 60 days (red circle), c correlation loadings plot of X = design variables (red circle) and Y = analytical variables (volatile compounds) (green circle). Ellipses (solid line) represent r2 = 50%, 75% and 100% explained variance. Group: A positively correlated with GTE. Group B positively correlated with the storage time (Color figure online)
3.1.2 Formation of volatile compounds
From the GC × GC/TOF–MS analysis, 77 compounds were used for multivariate analysis. The compounds were identified using authentic standards (Supplementary Table S9). The 77 compounds identified included four acids, 11 alcohols, one ester, 11 ketones, 20 aldehydes, five furans, 19 hydrocarbons, one nitrogen derivative, two sulfur derivatives and three miscellaneous compounds. The identified compounds are listed in Table S9 together with peak number and method of identification.
The dataset of volatile compounds was subjected to PLS analysis to investigate the effect of storage time and antioxidant on lipid oxidation in mayonnaise. In total, two principal components (PCs) were validated, accounting for 26% of the variance in X variables (design) and 71% in Y variables (volatile compounds) (Fig. 1b, c).
As illustrated in the scores plot (Fig. 1b), all fresh samples (day 1 storage, green circles) were located to the left in the diagram, and differences in the volatile profile could be seen even at the initial phase of storage. Samples moved toward more positive values on PC1 with increasing storage days. Thus, PC1 mainly seemed to explain the variation in the data caused by storage time. The samples are located at different places along PC2 according to presence of different types of antioxidant. GTE samples had positive PC2, BHA and CON samples had negative PC2 and TOC samples were always located between GTE and BHA.
In the correlation loadings plot (Fig. 1c), design variable day (D) is located to the far-right part of the plot. Thus, the day design variable had a high PC1 value while the design variable antioxidant types were located along PC2. Interpretation of the effect of design variable antioxidant type is difficult because most of the volatiles were located to the far-right side of PC1. However, the model could indicate that volatiles could be categorised in two groups: A consisting of 29 volatiles which are positively correlated with GTE and B consisting of 48 volatiles which are positively correlated with the storage time as indicated by ellipses (Group A and B) in Fig. 1c and as listed in Table 1 (Group A).The examination of the regression coefficients of the volatile compounds for the antioxidant type design variable showed that GTE and TOC increased the concentration of the volatile compounds in group A (Table 1), the regression coefficient for GTE and TOC was significantly positive (p < 0.1). However, most of the variables for the volatiles in group A had significant negative regression coefficients for the BHA design variable (Table 1). The raw data showed that (data not shown) fresh mayonnaise containing GTE had already high concentrations of volatile compounds in group A, which indicates that volatiles in group A were rapidly formed in mayonnaises with GTE. Therefore, addition of GTE increased formation of certain volatile compounds in group A. The volatile compounds in group A are; four alcohols, two acids, four alkanals, four ketones, 12 hydrocarbons, three lactones and one miscellaneous. Further information on the origin of the compounds in group A is presented in Table 1.
Some of Group A volatile compounds e.g. heptanal, octanal and nonanal were quantified and the inhibition percentages for BHA, GTE and TOC were measured (Table 2). BHA was the only one acting as antioxidant, while GTE and TOC showed pro-oxidative effects. These results confirm our finding in Table 1.
3.2 Impact of combination of TOC and GTE on formation of hydroperoxides and volatile compounds
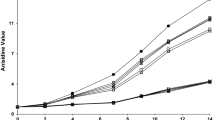
The combination of TOC and GTE antioxidants on the oxidative stability of mayonnaise was studied. In this study equal part blend of each antioxidant was investigated (250 mg/kg oil TOC + 250 mg/kg oil GTE). The improvement of antioxidant activity was seen in GTT compared to individual GTE (water-soluble antioxidant) and TOC (oil-soluble antioxidant). GTT showed antioxidative activity and reduced the formation of hydroperoxides Fig. S1 (see Supplementary material). GTT exhibited excellent antioxidative activity on the basis of pentanal and hexanal (Table 3) and its efficacy was higher than that of BHA until day 45 of storage.
3.3 Effect of storage time and antioxidant type on colour stability
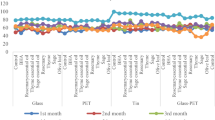
During storage of mayonnaise, l* (lightness) decreased, while a* (+ a* represents the red coordinate, − a is the green coordinate) increased (Fig. 2). Storage time caused a decrease in lightness and greenness of the samples (Fig. 2), which indicated that the samples darkened over time and became browner. The darkening and browning during storage was more pronounced in samples that had GTE (Fig. 2).
Changes in a* and l* values during storage of 60 days. Error bars show standard errors. The data were compared using one-way analysis of variance followed by Fisher’s test. Data with different lowercase letters (for the same storage time), are significantly different (p < 0.05) and photos of mayonnaise samples during storage of 60 days. CON no antioxidant, BHA butylated hydroxyanisole, TOC tocopherol, GTE green tea extract, GTT tocopherol-green tea mixture
3.4 Effect of storage time and antioxidant type on sensory properties
Descriptive analysis was employed to quantify the intensity of the sensory attributes of mayonnaises stored for 60 days. A trained panel of 12 judges rated each mayonnaise in triplicate for the intensity of seven aroma and seven flavour attributes on a ten-point scale (0–10). The terms selected to describe lipid oxidation in mayonnaise in this study are somewhat similar to sensory attribute terms used in other studies for descriptive analysis of salad dressings based on an emulsion of sunflower oil (Sainsbury et al. 2016) and fish oil mayonnaise (Jacobsen et al. 2000; Jacobsen et al. 1999b; Jacobsen et al. 2001a; Timm-Heinrich et al. 2004). The sensory dataset with (n =15 samples × 12 panellists × 3 replicates) was analysed with a mixed model analysis of variance to explore the quality and robustness of the sensory data. The results of the model are shown in Table S10 (see Supplementary material). All sensory attributes were significantly different (p < 0.05) between the mayonnaises with the exception of the aroma attributes of plastic/chemical, eggy and flavour attribute of savoury, tangy, saltiness, and hotness which are marked with a star (*).
To explore groupings between the mayonnaises the sensory data were analysed by PCA which explained 63.9% of the variation of data set in the two first PCs (Fig. 3a). From the visual observations of the PCA bi-plot, PC1 separated the mayonnaises according to antioxidant type (samples having GTE have positive PC1) and PC2 separated the mayonnaises according to the day of storage. The sensory attributes of fishy flavour and aroma are located near fresh mayonnaises with GTE (GTE and GTT). Addition of GTE to the model mayonnaise caused immediate formation of distinct fishy aromas and flavours. Mayonnaises with GTE stored for 60 days were located in the same side of the plot as aroma intensity, rancid flavour and aroma.
a PCA bi-plot of sensory properties (aroma (A) and flavour (F) attributes, n = 3 replicates × 12 panellists) of samples stored for 1 day (green circle), 30 days (blue circle) and 60 days (red circle). (Asterisk) Sensory attributes not significantly different (p > 0.05) between the mayonnaises. CON no antioxidant, BHA butylated hydroxyanisole, TOC tocopherol, GTE green tea extract, GTT tocopherol-green tea mixture. b Correlation loadings plot of partial least square regression analysis (PC1, PC2), X = sensory attributes (aroma (A) and flavour (F)) (green circle) and Y = volatile compounds (red circle) (numbers represent peak number). Ellipses represent r2 = 50%, 75% and 100% explained variance (Color figure online)
3.5 Sensory and volatile compounds
The predictive ability of volatile compounds (GC × GC method) for sensory terms was further investigated by PLS analysis, with the X-matrix comprising sensory terms and volatile compounds as Y-matrix (Fig. 3b). Addition of GTE caused immediate formation of distinct fishy and rancid aroma and flavour. To identify the responsible volatile compounds for fishy and rancid flavour and aroma, and eggy flavour, the volatile compounds that had significant positive correlation with these terms were selected (Jack-knife uncertainty test) (Table 4). 3-methylsulfanylpropanal (methional) had the highest positive correlation with fishy, rancid and eggy aroma and flavour (Table 4; Fig. 3b) whereas vinegar term was negatively related.
4 Discussion
Lipid oxidation during storage of mayonnaise was demonstrated by the formation of lipid oxidation products (hydroperoxides and volatile compounds), change in sensory properties and colour stability.
Incorporation of GTE (500 mg kg oil), increased formation of hydroperoxides and certain volatile compounds. GTE used in this study is rich in polyphenolic compounds, containing 20% catechins and salt (NaCl). Jacobsen et al. (1999c) previously found that about 0% of catechin is in the oil phase of mayonnaise and 80% in the aqueous phase. Mayonnaise has a low pH and at this pH, the release of iron is caused because of the breakdown of the iron bridges between phosvitin, lipovitelin and low-density lipoprotein (Jacobsen et al. 1999a; Jacobsen et al. 2001a). GTE acting as a pro-oxidant could be due to reducing Fe3+ to Fe2+, which is more active as an oxidation catalyst than Fe3+ as shown in Fig. 4. A similar trend was previously reported for pro-oxidative activity of gallic acid on decomposition of hydroperoxides in fish-oil enriched mayonnaise (Jacobsen et al. 2001b). Zhou and Elias (2013) have previously reported that antioxidants such as epigallocatechin-3-gallate can act as antioxidants or pro-oxidants in O/W emulsions in that they can scavenge free radicals and reduce transition metals to their catalytically active state.
In other systems e.g. blubber oil, menhaden oil and corn O/W emulsion (Frankel et al. 1997; Huang and Frankel 1997) GTE exhibited pro-oxidative effect. Several reasons were reported for pro-oxidative effect of GTE, such as the catalytic effect of their chlorophylls (Wanasundara and Shahidi 1998), partitioning of hydrophilic tea catechins in the water phase of emulsion and becoming less protective (Frankel et al. 1997).
Incorporation of TOC (500 mg kg oil), seems to have slowed down the reactions that lead to the breakdown of the hydroperoxides once maximum hydroperoxides were reached. However increased formation of some of the volatile compounds. TOC is composed of 70% natural mixed TOCs and 30% vegetable oil. The pro-oxidative effect of TOC has been previously reported in several bulk oil and mayonnaise systems (Jacobsen et al. 2000, 2001a; Karahadian and Lindsay 1989; Rizner et al. 2000). TOCs act as antioxidants by donating hydrogen to lipid and/or to peroxide radicals. However, they can have pro-oxidant activity at high concentrations by promoting the formation of hydroperoxides (Frankel et al. 1994; Karahadian and Lindsay 1989). Initial oxidation of TOC results in tocopheroxyl radicals that can increase oxidation by abstracting hydrogen from lipid hydroperoxides and converting them to active peroxide radicals rather than inhibiting lipid oxidation (Let et al. 2007). The two factors that play the most important roles in determining anti-/pro-oxidant activity of phenolic compounds in lipid systems are pH and concentration (Zhou and Elias 2013). Low pH promotes pro-oxidant activity of polyphenols. To clarify the anti-/pro-oxidant activity of GTE and TOC more information is needed on the effect of other variables such as pH and concentration.
Combination of a hydrophilic antioxidant (GTE), with a hydrophobic antioxidant (TOCs), improved antioxidant activity compared to the individual antioxidants. GTT reduced formation of hydroperoxides, pentanal and hexanal. Antioxidants in a hetrophasic system such as mayonnaise, may partition into at least three phases. Studies showed that the differences in activity between TOC and GTE may be due to an interfacial phenomenon (Frankel et al. 1994, 1997). GTE partitions into the water phase and oil–water interfaces, because of its water solubility (Jacobsen et al. 1999c). Jacobsen et al. (1999c) reported that approximately 6% of the α-tocopherol present in mayonnaise was located at the interface whereas the remaining TOC was located in the oil phase. The improved protective effect of GTT could be due to reduction of the TOC radicals, resulting from initial oxidation of TOC, at the surface of lipid droplets, by the water-soluble reductant in GTE, in the pseudo-phase interfacial layer (Buettner 1993; Durand et al. 2015) or reduction of the lipophilic radicals (Durand et al. 2015) as shown in Fig. 4. Consistent with this, several studies have reported the regeneration of TOC by ascorbic acid, rosmarinic acid, caffeic acid and GTE (Durand et al. 2015; Panya et al. 2012; Sørensen et al. 2017; Yin et al. 2012).
Darkening and browning occurred during storage of mayonnaise. The decrease in lightness could be due to change in droplet size of emulsion. As the droplet size of the emulsion decreases, the colour would be lighter because of the light fractionation (Worrasinchai et al. 2006). The change in colour could also be due to oxidative decomposition of different ingredients, especially the oil. Addition of GTE caused a more pronounced change in colour during storage that could be due to oxidation of phenolic compounds in GTE during storage and forming brown colour.
Addition of green tea promoted fishy and rancid flavour and aroma in mayonnaise. The compound methional was significantly and positively correlated with fishy and rancid flavour and aroma whereas vinegar term was negatively related. This could be due to the ability of the high fishy and rancid aroma in mayonnaises to shield the natural vinegar aroma. Methional is a sulfur-containing compound (odour threshold value 0.2 ppb in water, a Strecker aldehyde of methionine) that has been found as one of the main odorants of GTE (Ho et al. 2015). In The Good Scents company database, the taste of methional at 0.01–5.00 ppm is described as egg and seafood nuances. Further investigations of, e.g. odour threshold of methional in oil, as well as exact concentration, are necessary to form an accurate conclusion.
5 Conclusion
The influence of hydrophilic and hydrophobic, and a mixture of hydrophilic/hydrophobic antioxidants was investigated by monitoring colour, hydroperoxides, volatile compounds and sensory properties of mayonnaise samples during storage for 60 days at 38 °C. Addition of GTE, a water-soluble antioxidant, caused an increase in hydroperoxide concentration, was a pro-oxidant on certain volatile compounds, and promoted fishy and rancid flavours and aromas. The results of the predictive PLS (volatile compounds vs sensory) revealed that methional is positively correlated with fishy, rancid and eggy aroma and flavour. The pro-oxidative effect of GTE could be due to partitioning of hydrophilic tea catechins in the water phase of the emulsion and becoming less protective and/or reducing transition metals to their catalytically active state. Further investigation is needed to confirm these hypotheses. GTE changed the colour of mayonnaise during storage. Evaluation of data shows that incorporation of GTE at 500 mg/kg (oil) concentration is not a suitable antioxidant for mayonnaise made with sunflower oil. TOC at 500 mg/kg (oil), an oil-soluble antioxidant, showed pro-oxidative effects on formation of some volatile compounds. Antioxidant efficacy of TOC mixtures could be influenced by the presence of a hydrophobic antioxidant (GTE). GTT appeared to inhibit the formation of hydroperoxides and certain volatile compounds to a greater extent, compared with individual extracts (TOC and GTE). More investigation needs to be done to understand the synergistic effect of TOC and GTE in terms of concentration, and type of lipid. The origin of volatile oxidation compounds that are promoted by the presence of GTE should be studied in future.
References
Buettner, G. R. (1993). The pecking order of free radicals and antioxidants: Lipid peroxidation, α-tocopherol, and ascorbate. Archives of Biochemistry and Biophysics, 300, 535–543.
Chen, B., Rao, J., Ding, Y., McClements, D. J., & Decker, E. A. (2016). Lipid oxidation in base algae oil and water-in-algae oil emulsion: Impact of natural antioxidants and emulsifiers. Food Research International, 85, 162–169.
Chen, Z., & Chan, P. (1996). Antioxidative activity of green tea catechins in canola oil. Chemistry and Physics of Lipids, 82, 163–172.
Concepcion, J. C. T., Ouk, S., Riedel, A., Calingacion, M., Zhao, D., Ouk, M., et al. (2018). Quality evaluation, fatty acid analysis and untargeted profiling of volatiles in Cambodian rice. Food Chemistry, 240, 1014–1021.
Coupland, J. N., & McClements, D. J. (1996). Lipid oxidation in food emulsions. Trends in Food Science & Technology, 7, 83–91.
Danh, L. T., Triet, N. D. A., Zhao, J., Mammucari, R., & Foster, N. (2012). Antioxidant activity, yield and chemical composition of lavender essential oil extracted by supercritical CO2. The Journal of Supercritical Fluids, 70, 27–34.
Daygon, V. D., Prakash, S., Calingacion, M., Riedel, A., Ovenden, B., Snell, P., et al. (2016). Understanding the Jasmine phenotype of rice through metabolite profiling and sensory evaluation. Metabolomics, 12, 63.
Durand, E., Zhao, Y., Coupland, J. N., & Elias, R. J. (2015). Assessing interactions between lipophilic and hydrophilic antioxidants in food emulsions. Journal of Agricultural and Food Chemistry, 63, 10655–10661.
Frankel, E. N., Huang, S.-W., & Aeschbach, R. (1997). Antioxidant activity of green teas in different lipid systems. Journal of the American Oil Chemists’ Society, 74, 1309–1315.
Frankel, E. N., Huang, S.-W., Kanner, J., & German, J. B. (1994). Interfacial phenomena in the evaluation of antioxidants: bulk oils vs emulsions. Journal of Agricultural and Food Chemistry, 42, 1054–1059.
Ghorbani Gorji, S., Smyth, H. E., Sharma, M., & Fitzgerald, M. (2016). Lipid oxidation in mayonnaise and the role of natural antioxidants: A review. Trends in Food Science & Technology, 56, 88–102.
Guillén, M. D., Cabo, N., Ibargoitia, M. L., & Ruiz, A. (2005). Study of both sunflower oil and its headspace throughout the oxidation process. Occurrence in the headspace of toxic oxygenated aldehydes. Journal of Agricultural and Food Chemistry, 53, 1093–1101.
Ho, C.-T., Zheng, X., & Li, S. (2015). Tea aroma formation. Food Science and Human Wellness, 4, 9–27.
Huang, S.-W., & Frankel, E. N. (1997). Antioxidant activity of tea catechins in different lipid systems. Journal of Agricultural and Food Chemistry, 45, 3033–3038.
Jacobsen, C., Adler-Nissen, J., & Meyer, A. S. (1999a). Effect of ascorbic acid on iron release from the emulsifier interface and on the oxidative flavor deterioration in fish oil enriched mayonnaise. Journal of Agricultural and Food Chemistry, 47, 4917–4926.
Jacobsen, C., Hartvigsen, K., Lund, P., Adler-Nissen, J., Holmer, G., & Meyer, A. S. (2000). Oxidation in fish-oil-enriched mayonnaise 2. Assessment of the efficacy of different tocopherol antioxidant systems by discriminant partial least squares regression analysis. European Food Research and Technology, 210, 0242–0257.
Jacobsen, C., Hartvigsen, K., Lund, P., Meyer, A. S., Adler-Nissen, J., Holstborg, J., et al. (1999b). Oxidation in fish-oil-enriched mayonnaise 1. Assessment of propyl gallate as an antioxidant by discriminant partial least squares regression analysis. European Food Research and Technology, 210, 13–30.
Jacobsen, C., Hartvigsen, K., Lund, P., Thomsen, M. K., Skibsted, L. H., Hølmer, G., et al. (2001a). Oxidation in fish oil-enriched mayonnaise: 4. Effect of tocopherol concentration on oxidative deterioration. European Food Research and Technology, 212, 308–318.
Jacobsen, C., Hartvigsen, K., Thomsen, M. K., Hansen, L. F., Lund, P., Skibsted, L. H., et al. (2001b). Lipid oxidation in fish oil enriched mayonnaise: Calcium disodium ethylenediaminetetraacetate, but not gallic acid, strongly inhibited oxidative deterioration. Journal of Agricultural and Food Chemistry, 49, 1009–1019.
Jacobsen, C., Schwarz, K., Stöckmann, H., Meyer, A. S., & Adler-Nissen, J. (1999c). Partitioning of selected antioxidants in mayonnaise. Journal of Agricultural and Food Chemistry, 47, 3601–3610.
Jayasinghe, C., Gotoh, N., & Wada, S. (2013). Pro-oxidant/antioxidant behaviours of ascorbic acid, tocopherol, and plant extracts in n-3 highly unsaturated fatty acid rich oil-in-water emulsions. Food Chemistry, 141, 3077–3084.
Kalua, C., Allen, M., Bedgood, D., Jr., Bishop, A., Prenzler, P. D., & Robards, K. (2007). Olive oil volatile compounds, flavour development and quality: A critical review. Food Chemistry, 100, 273–286.
Karahadian, C., & Lindsay, R. C. (1989). Action of tocopherol-type compounds in directing reactions forming flavor compounds in autoxidizing fish oils. Journal of the American Oil Chemists’ Society, 66, 1302–1308.
Keszler, Á., Kriska, T., & Németh, A. (2000). Mechanism of volatile compound production during storage of sunflower oil. Journal of Agricultural and Food Chemistry, 48, 5981–5985.
Kochhar, S. (1996). Oxidative pathways to the formation of off-flavours. In Food taints and off-flavours. Springer.
Let, M. B., Jacobsen, C., & Meyer, A. S. (2007). Ascorbyl palmitate, γ-tocopherol, and EDTA affect lipid oxidation in fish oil enriched salad dressing differently. Journal of Agricultural and Food Chemistry, 55, 2369–2375.
Matheis, K., Granvogl, M., & Schieberle, P. (2016). Quantitation and enantiomeric ratios of aroma compounds formed by an Ehrlich degradation of l-isoleucine in fermented foods. Journal of Agricultural and Food Chemistry, 64, 646–652.
Nissen, L. R., Byrne, D. V., Bertelsen, G., & Skibsted, L. H. (2004). The antioxidative activity of plant extracts in cooked pork patties as evaluated by descriptive sensory profiling and chemical analysis. Meat Science, 68, 485–495.
Nourooz-Zadeh, J., Tajaddini-Sarmadi, J., Birlouez-Aragon, I., & Wolff, S. P. (1995). Measurement of hydroperoxides in edible oils using the ferrous oxidation in xylenol orange assay. Journal of Agricultural and Food Chemistry, 43, 17–21.
Panya, A., Kittipongpittaya, K., Laguerre, M., Bayrasy, C., Lecomte, J., Villeneuve, P., et al. (2012). Interactions between α-tocopherol and rosmarinic acid and its alkyl esters in emulsions: Synergistic, additive, or antagonistic effect? Journal of Agricultural and Food Chemistry, 60, 10320–10330.
Rizner, A., Hadolin, M., Knez, Z., & Bauman, D. (2000). Comparison of antioxidative and synergistic effects of rosemary extract with a-tocopherol, ascorbyl palmitate and citric acid in sunflower oil. Food Chemistry, 71, 229–233.
Sainsbury, J., Grypa, R., Ellingworth, J., Duodu, K. G., & De Kock, H. L. (2016). The effects of antioxidants and shelf life conditions on oxidation markers in a sunflower oil salad dressing emulsion (SOSDE). Food Chemistry, 213, 230–237.
Soncu, E. D., & Kolsarici, N. (2017). Microwave thawing and green tea extract efficiency for the formation of acrylamide throughout the production process of chicken burgers and chicken nuggets. Journal of the Science of Food and Agriculture, 97, 1790–1797.
Sørensen, A.D.M., Villeneuve, P., & Jacobsen, C. (2017). Alkyl caffeates as antioxidants in O/W emulsions: Impact of emulsifier type and endogenous tocopherols. European Journal of Lipid Science and Technology 119.
Tikapunya, T., Henry, R. J., & Smyth, H. (2018). Evaluating the sensory properties of unpolished Australian wild rice. Food Research International, 103, 406–414.
Timm-Heinrich, M., Xu, X., Nielsen, N. S., & Jacobsen, C. (2004). Oxidative stability of mayonnaise and milk drink produced with structured lipids based on fish oil and caprylic acid. European Food Research and Technology, 219, 32–41.
Wanasundara, U. N., & Shahidi, F. (1998). Antioxidant and pro-oxidant activity of green tea extracts in marine oils. Food Chemistry, 63, 335–342.
Worrasinchai, S., Suphantharika, M., Pinjai, S., & Jamnong, P. (2006). β-Glucan prepared from spent brewer’s yeast as a fat replacer in mayonnaise. Food Hydrocolloids, 20, 68–78.
Yin, J., Becker, E. M., Andersen, M. L., & Skibsted, L. H. (2012). Green tea extract as food antioxidant. Synergism and antagonism with α-tocopherol in vegetable oils and their colloidal systems. Food Chemistry, 135, 2195–2202.
Zhou, L., & Elias, R. J. (2013). Antioxidant and pro-oxidant activity of (−)-epigallocatechin-3-gallate in food emulsions: Influence of pH and phenolic concentration. Food Chemistry, 138, 1503–1509.
Zhou, L., Zhao, M., Bindler, F., & Marchioni, E. (2014). Comparison of the volatiles formed by oxidation of phosphatidylcholine to triglyceride in model systems. Journal of Agricultural and Food Chemistry, 62, 8295–8301.
Zou, X.-G., Chen, X.-L., Hu, J.-N., Wang, Y.-F., Gong, D.-M., Zhu, X.-M., et al. (2017). Comparisons of proximate compositions, fatty acids profile and micronutrients between fiber and oil flaxseeds (Linum usitatissimum L.). Journal of Food Composition and Analysis, 62, 168–176.
Acknowledgements
The authors would like to acknowledge Mary Sharma, Adam Mayne and Goodman Fielder Company for supplying the ingredients and production of samples. The authors thank the sensory evaluation panel of the Health and Food Sciences Precinct, Coopers Plains, Australia for their dedication.
Funding
This study was funded by the Industry Transformation Training Centre Australian Research Council Project, ARC: IC130100011 and The University of Queensland.
Author information
Authors and Affiliations
Contributions
MF and SGG designed the study. SGG performed all experiments, interpreted data and wrote the manuscript. MC, MF designed and participated in the interpretation of the GC × GC/TOF–MS test. HES designed the sensory test and participated in the data interpretation. The paper was edited by all. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that no conflict of interest exists.
Ethical approval
This study was conducted under the Human Ethics Approval # 2015001700. All procedures for conducting the sensory analysis were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ghorbani Gorji, S., Calingacion, M., Smyth, H.E. et al. Effect of natural antioxidants on lipid oxidation in mayonnaise compared with BHA, the industry standard. Metabolomics 15, 106 (2019). https://doi.org/10.1007/s11306-019-1568-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11306-019-1568-4