Abstract
This study compared the antioxidant properties of oregano essential oil (OEO) and butylated hydroxytoluene (BHT) alone and when combined. The principal components in OEO were gamma terpinene (25.1 g/100 g), terpinen-4-ol (16.7 g/100 g), and carvacrol (16.2 g/100 g). OEO showed 60% DPPH inhibition and 10 mg/g total phenolic compounds. The antioxidant capacity of OEO (0.02, 0.10, and 0.20 g/100 g) and BHT (0.01 and 0.02 g/100 g) and their combinations were tested in sunflower oil oven-heated at 60 °C, by measuring the chemical (peroxide value, p-anisidine value, and conjugated dienes) and volatile (hexanal, 2-heptanal, and 2,4-decadienal) indicators over 14 days. The combined samples (oregano essential oil and BHT) showed the greatest protection against lipid oxidation. On day 14, the peroxide value of the control (without added antioxidants), OEO (0.02 g/100 g), BHT (0.01 g/100 g), and OEO + BHT (0.02 + 0.01 g/100 g) treatments decreased in the order of 136.36, 102.68, 83.24, and 41.37 meqO2/kg, respectively, for example. In the consumer sensory test, samples containing OEO at 0.02 and 0.10 g/100 g attained greater acceptance scores (7.3 and 6.7, respectively, on a 9-point hedonic scale) as compared with the control (6.1). Discriminative duo–trio testing presented significant differences between all OEO-containing samples relative to the control. The synergistic antioxidant activity between OEO (termination-enhancing antioxidant) and BHT (chain-breaking antioxidant) demonstrates an alternative approach to impede lipid oxidation in foods, by decreasing the use of synthetic compounds in the food industry.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lipid oxidation is one of the principal causes of deterioration of high-lipid food, during storage and processing (Wang et al. 2017). These oxidation reactions affect the chemical, sensory, and nutritional qualities of the food (Aladedunye and Przybylski 2013). Moreover, the ingestion of compounds produced from lipid oxidation has been associated with degenerative diseases, such as cancer, Alzheimer’s, and Parkinson’s (Guillen and Goicoechea 2008). A simple strategy to reduce lipid oxidation is the addition of antioxidants.
Synthetic antioxidants are commonly used to prevent lipid oxidation in foods by the food industry. The most important among these antioxidants are butylated hydroxyanisole (BHA), tert-butylhydroquinone (TBHQ), and butylated hydroxytoluene (BHT), which are incorporated into all kinds of food products, despite concerns about their safety, including strong evidence of their possible toxicity and cancer-causing potential in humans (Shearn et al. 2011).
Growing consumer awareness about these adverse health effects associated with the consumption of synthetic compounds has forced the food industry to either lower the amount of synthetic substances or replace them with natural alternatives (Boskou and Elmadfa 2011). Essential oils (EOs) are volatile, complex mixtures of natural compounds characterized by a strong odor and derive from the secondary metabolism of aromatic plants (Pan et al. 2007; Guimaraes et al. 2010; Tohidi et al. 2017).
Various plant EOs possess antioxidant activity. The incorporation of EOs as antioxidants in food products is well-documented (Dima and Dima 2015), showing benefits in a variety of foods, such as chicken meat (Chouliara et al. 2007), peanut products (Olmedo et al. 2012a, b), cream cheese (Olmedo et al. 2013), olive oil (Asensio et al. 2013), sunflower oil (Olmedo et al. 2015), and emulsion-like mayonnaise (Gorji et al. 2016).
Oregano (Origanum vulgare L.) is the most popular aromatic plant in Argentina, and the main areas of cultivation are in the central and southwest regions (Dambolena et al. 2010). The plant has a safe history of use as a culinary ingredient, and its extractives have generally recognized as safe (GRAS) status in the USA. The EO of oregano has proven antioxidant properties (Castilho et al. 2012), primarily linked to its phenolic compounds and terpenes (Asensio et al. 2012). The concentration of oregano essential oil (OEO) necessary to achieve similar antioxidant protection to that of BHT, however, imparts an intense odor in foods, which can make the products unacceptable to consumers.
BHT and EOs exert different antioxidant mechanisms. BHT is effective as a chain-breaking antioxidant that functions mainly as a primary antioxidant, reacting with peroxyl radicals and thereby interfering with the propagation of lipid peroxidation reactions, to inhibit the autoxidation of lipids. Unlike BHT, EOs are termed “termination-enhancing antioxidants”. When mixed with an oxidizable material, like unsaturated lipids, the EO components and lipid substrate are co-oxidized. Thereby, the antioxidant activity of the EO acts to enhance the termination reactions in autoxidation (Amorati et al. 2013). In this context, a combination of oregano essential oil (OEO) and BHT could allow diminishing the final contents of each one while retaining or, moreover, improving the antioxidant efficacy of the individual antioxidants, by capitalizing on both the chain-breaking antioxidation and termination-enhancing antioxidant mechanisms. The aim of this study was to evaluate the antioxidant effect of chain breaking antioxidant (BHT) combined with termination-enhancing antioxidant (OEO) in sunflower oil as lipid model and its impact on sensory properties.
Materials and methods
Essential oil extraction
Oregano (O. vulgare L. spp. vulgare) leaves from three different crop blocks were used as triplicates for distillation extraction. Ten plants of oregano were harvested from each block in autumn (May 2017), from the experimental station of the Faculty of Agricultural Sciences, National University of Córdoba, Córdoba, Argentina (google map position: GXCV + 8P). The leaves from 30 harvested plants were air-dried at room temperature for 1 week, to preserve the chemical components. The EO was extracted by steam distillation of oregano leaves (50 g aliquot) for 1 h at 100 °C, using a Clevenger-type apparatus, as described elsewhere (Olmedo et al. 2014). The steam-distilled EO (yield: 2,3 g of OEO/100 g dry leaves) was transferred to a glass vial and stored with sodium sulfate at 18 °C in darkness until its use.
Essential oil composition
Before determining its chemical components, the OEO was left to reach room temperature (2 h at 25 °C ± 5 °C). An aliquot of 1 µL was then injected in the gas chromatography–mass spectrometry (GC–MS) apparatus equipped with an ion-trap mass detector (Clarus 600 GC–MS, Perkin Elmer, Inc., Shelton, CT, USA) and a non-polar Elite-5MS capillary column (methylpolysiloxane, 5% phenyl, 30 m × 0.25 mm i.d., 0.25 µm coating thickness) with the injector at 250 °C, and the carrier gas (helium) at a flow rate of 0.9 mL/min. Chromatographic separation was achieved by adopting the same conditions as described by Olmedo et al. (2014): initial temperature at 40 °C for 3 min, then ramped to 100 °C at 10 °C/min, followed by a second ramp to 245 °C at 15 °C/min and held at this temperature for 2 min. Mass spectra were obtained in full-scan mode over the range 35 to 450 m/z, by electron impact at 70 eV. The chemical compounds were identified by comparison of their retention indices relative to a homologous n-alkane series and matching their mass spectra with those of reference compounds compiled by Adams (1995) and in the NIST (National Institute of Standards and Technology, Gaithersburg, MD, USA) database. In addition, some standard components (Sigma, St Louis, MO, USA) were co-injected to help in identifying the chemical constituents (Table 1: GC–MS–Co: Co-injected), based on their retention times and retention indices (Olmedo et al. 2015).
Indirect method for determination of antioxidant capacity in oregano essential oil
The 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging activity (DPPH-FRSA) and total phenolic content (TPC) were used to ascertain the indirect antioxidant capacity. For the TPC, the Folin–Ciocalteu reagent was combined with 10 µL of OEO before measuring the spectrophotometric absorbance at 760 nm (HP 8452A, Hewlett Packard, Palo Alto, CA, USA). The TPC concentration (mg/g) was calculated from a calibration curve using gallic acid standard (Sigma) (Olmedo et al. 2014).
The DPPH (Aldrich, Milwaukee, WI, USA) assay was conducted as described previously (Choi et al. 2000). The reduction in DPPH radicals was monitored spectrophotometrically at 517 nm for 30 min, and the FRSA (% DPPH inhibition) was calculated as follows: [(Ac − Aa)/Ac] × 100%, where Ac and Aa are the absorbance of the control and EO, respectively (Olmedo et al. 2015).
Preparation of samples for accelerated oxidation test of the lipidic model
Different proportions of OEO and BHT were combined and tested alone for their antioxidant efficacy in the stabilization of refined sunflower oil (Natura, Aceitera General Deheza, General Deheza, Argentina). Samples were prepared in glass test tubes (7 g: 5 g for peroxide value (PV), 0.1250 g for p-anisidine value (AV) and 0.0100 g for conjugated dienes (CD) value), as detailed in an earlier study (Olmedo et al. 2014). Treatments O1, O2, and O3 contained refined sunflower oil (7 g) and 0.02, 0.10, and 0.20 g/100 g OEO, respectively. BHT was used as a reference compound at two different concentrations of 0.01 (BHT1) and 0.02 g/100 g (BHT2) in sunflower oil (7 g). Control samples consisted of sunflower oil without added antioxidants. The OEO + BHT sunflower oil systems included O1B1 (0.02 g/100 g OEO and 0.01 g/100 g BHT), O1B2 (0.02 g/100 g OEO and 0.02 g/100 g BHT), O2B1 (0.10 g/100 g OEO and 0.01 g/100 g BHT), O2B2 (0.10 g/100 g OEO and 0.02 g/100 g BHT), O3B1 (0.20 g/100 g OEO and 0.01 g/100 g BHT), and O3B2 (0.20 g/100 g OEO and 0.02 g/100 g BHT). All samples were used to determine the lipid oxidation indicators, volatile compounds, and for sensory evaluation.
Direct method to determine antioxidant activity: Accelerated oxidation test
Chemical oxidation. The samples prepared for accelerated oxidation testing were stored in an oven (unsealed) with air convection at 60 °C, according to Olmedo et al. (2015). The experiment was carried out in triplicate, and the samples were removed at 0, 2, 4, 7, 9, 11, and 14 days. Peroxide value (AOAC 1980), p-anisidine value (IUPAC 1987), and conjugated dienes (COI 2001) were evaluated as the chemical indicators of lipid oxidation.
Volatile compounds. Volatile oxidation compounds from the different treatments were determined by GC–MS during an accelerated oxidation test. Ten grams of the treated sunflower oil prepared in a similar way than the treatments for accelerated oxidation (described in the previous section) was placed in a glass flask (capacity 50 mL), sealed, and stored in an oven with air convection at 60 °C. Samples were analyzed at 0, 7, and 14 days. Volatile compounds were captured using a polydimethylsiloxane/divinylbenzene (PDMS/DVB) solid phase microextraction (SPME) fiber (Supelco, Sigma) (Adams et al. 2011). The SPME fiber was injected into the headspace through the rubber stopper in the glass flask and then heated at 70 °C for 20 min. After absorbing the volatile compounds, the SPME fiber was injected into the GC–MS where it remained for 1 min. The GC–MS was run under the same conditions as those used to determine the EO composition, as described above and detailed in Olmedo et al. (2014). For volatile oxidation compound quantification (µg/g; Olmedo et al. 2015), calibration curves for hexanal, 2-heptanal, and 2,4-decadienal (E,Z) standards (Sigma) were constructed. These compounds represent the most important volatiles generated in sunflower oil, and their identification was made according to their standard retention times provided in the NIST-MS library (Feng et al. 2017a, b). Acetaldehyde (Sigma) was run as an internal standard in all samples.
Sensory evaluation
Discriminative evaluation. A duo–trio test was used to verify the sensory differences between the treatments at day 0. Ten semi-trained panelists (8 females and 2 males) participated in the evaluation. All panelists were presented with triplicates of the samples (0.5 mL oil on a 2 × 2 cm portion of bread) in three rounds, where the reference and sample combinations were modified in each round. In this test, the reference sample (labeled “R”) and then two 3-digited coded samples (duos), one of which matched the reference (Asensio et al. 2013), were presented to tasters, who were asked to identify which sample from the duos matched the reference sample. The duos selected for testing were control–O1, O1–O2, and O2–O3 (Samples were prepared according to accelerated oxidation test). Combined OEO + BHT samples were not tested because BHT did not affect the sensory characteristics on day 0.
Sensory test. A consumer analysis test was carried out using a 9-point hedonic scale (1 = dislike extremely; 9 = like extremely), according to Asensio et al. (2013). Non-trained consumers (n = 77) were invited to participate in the sensory analysis. The panelists were aged between 18 and 54 years. All samples were coded with three-digit numbers. The samples evaluated were the control, O1, O2, and O3, because BHT did not affect the sensory properties (Samples were prepared according to accelerated oxidation test).
Statistical analysis
The experiment was performed in triplicate. The statistical analysis was accomplished using Infostat software version 1.1 (Facultad de Ciencias Agropecuarias, Universidad Nacional de Córdoba, Córdoba, Argentina). Means, standard errors, and standard deviations were calculated. Analysis of variance (ANOVA) was used to detect significant differences between sampling days, and Fisher’s LSD test was undertaken to identify significant differences (α = 0.05) between means. Tables 2 and 3 provide the linear regression analysis results, including the regression coefficient analysis of residues/variables using the Shapiro–Wilks test for model verification. Principal components analysis (PCA) was used for correlation of the chemical and volatile oxidation indicators with the different treatments, using all data from all tests (Jonhson and Wichern 1998).
Result and discussion
Essential oil composition
The major compounds in OEO were γ-terpinene (25.1 g/100 g), terpinen-4-ol (16.7 g/100 g), and carvacrol (16.2 g/100 g), which, together, represented 58% of the total EO composition (22 compounds identified) (Table 1). To identify key compounds in OEO, the ratio between the principal components and other compounds must be above 2 (approximately). The ratios based on α-terpinene (8.5 g/100 g) were 2.9, 2.0, and 1.9 for γ-terpinene, terpinen-4-ol, and carvacrol (respectively). Identification of the key components in OEO is important, as these compounds possess antioxidant capacity, related to their electron-donating activity (Feng et al. 2017b). Asensio et al. (2012) studied four different varieties of Argentinean OEOs: Compacto, Cordobes, Criollo, and Mendocino, and found that terpineol and its derivatives (terpinen-4-ol and terpinen-4-acetate) were present in all EO samples, in addition to carvacrol/thymol. These molecules were abundant in all samples, in amounts between 16 and 24 g/100 g for terpineol and 13–30 g/100 g for thymol/carvacrol. The OEO varieties that were most similar to those tested in the current study were Criollo (16.45 g/100 g terpinen-4-ol and 30.19 g/100 g carvacrol) and Cordobes (20.09 g/100 g terpinen-4-ol and 24.54 g/100 g carvacrol). In the current study, the oregano essential oil was extracted from material collected in Cordoba (Argentina) corresponding to Origanum vulgare L. spp. Vulgare that was planted in the same lot and grown up under the same climatic conditions.
Antioxidant activity determination by indirect methods
Many EOs from aromatic culinary or medicinal plants show antioxidant properties. Various molecules in EOs present electron-donating activity and cause a decrease in radical activity. OEO was found to have a high TPC of 10.0 mg/g of gallic acid equivalent per gram of essential oil (Table 1), suggesting the presence of molecules with electron-donating activity. Yan et al. (2016) evaluated 42 oregano (O. vulgare) accessions from the Genebank in Gatersleben (Germany) and detected TPC values of 112.57 mg gallic acid equivalents/g dry weight. This high TPC is because Yan et al. (2016) analyzed an extract from oregano leaves (80% aqueous methanol) instead of the EO.
OEO contains only a small portion of the total amount of phenolic compounds in oregano leaves. However, the Folin–Ciocalteu reagent not only determines phenols with electron donor capacity but all molecules capable of reducing the Folin–Ciocalteu reagent. OEO demonstrated a high FRSA of 60.0% DPPH inhibition (Table 1). In a previous study, we reported 84% DPPH inhibition for OEO (Olmedo et al. 2014). These values indicate that OEO contains molecules with electron donor activity.
There is a direct relationship between the FRSA and TPC, as the TPC calculated by the Folin–Ciocalteu reaction relies on electron-transfer between the molecule and the Folin–Ciocalteu, and the FRSA measures the inhibition of DPPH by molecules that donate electrons (Lester et al. 2012). This association was verified by Olmedo et al. (2014) in an experiment with OEO and its fractions obtained by short path molecular distillation, reporting that the fraction with the highest FRSA-DPPH also had the greatest TPC. In this way, the correlation between the FRSA-DPPH and TPC, represents an approach for determining antioxidant activity, because the fraction with a lower content of phenolic molecules (lower FRSA and TPC) provided the best antioxidant protection in sunflower oil (Olmedo et al. 2014). While the indirect antioxidant measurements of EO constitute the majority of testing assays used, Amorati et al. (2013) indicated that the main limitation to these types of tests is that they do not involve substrate autoxidation. Indirect methods, like the DPPH assay, report a “radical trapping power” or “reducing power” rather than actual antioxidant activity. It means the EO could reduce the radical (DPPH), but does not necessarily indicate that the EO is going to stop the oxidative chain reaction. Foti (2015) explained that the relative concentration of antioxidant required to lower the initial DPPH concentration by 50% (EC50) in the DPPH test is not a kinetic parameter, and its correlation with the antioxidant properties of EOs is not justified with this assay. In addition, Sharma and Bhat (2009) mentioned the wide variability in DPPH results between different laboratories because of the lack of standard measurement conditions. For these reasons, it is important to evaluate the antioxidant capacity in a lipid oxidation model, such as sunflower oil (Roginsky and Lissi 2005).
Chemical oxidation indicators from the accelerated oxidation test
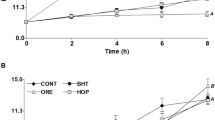
Chemical oxidation indicators. Chemical indicators for all samples increased throughout the 14-day storage at 60 °C. The variation in the chemical oxidation indicators, namely, PV (Fig. 1a), AV (Fig. 1b), and CD contents (Fig. 1c), revealed the control had the greatest PV among the samples. It was also observed that samples BHT1 and BHT2 had lower values compared with samples O1, O2, and O3. Although BHT was effective at protecting against lipid oxidation, the maximal protection was observed in samples containing OEO + BHT (Table 2), which were significantly different from the control, BHT, and OEO samples. Differences were also noted among the various combinations tested. For instance, at the end of storage (day 14), samples O2B1 and O3B1 exhibited a significantly higher PV compared with the other combinations.
On day 14, the PVs of O3B2 (39.19 meqO2/kg), O1B1 (41.37 meqO2/kg), BHT1 (83.24 meqO2/kg), O1 (102.68 meqO2/kg), and the control (136.36 meqO2/kg) were all different to each other. The sum of the difference between control–BHT1 (53.12 meqO2/kg reduction) and control–O1 (33.68 meqO2/kg reduction) was 86.80 meqO2/kg, and the difference between control–O1B1 was 94.99 meqO2/kg, indicating a synergistic effect because the combination (O1B1) decreased the PV more than the sum of BHT1 and O1. Also, there was only a minor difference in the PVs between the combination with the greatest antioxidant concentrations (control–O3B2, 97.24 meqO2/kg) and O1B1 (control–O1B1, 94.99 meqO2/kg). The synergic effect was not observed in all the combinations. The ANOVA results for the slope as a function of storage showed that O1B1 (2.81), O1B2 (2.77), O2B2 (2.71), and O3B2 (2.67) had lower slope gradients than the control (9.87), and presented significant differences compared with all other treatments (R2 ≥ 0.70; p ≤ 0.05) (Table 2).
The AV showed similar behavior to the PV. The control had the highest AV, and BHT1 and BHT2 were significantly different from O1, O2, and O3, but samples containing only BHT or OEO were statistically identical. Samples incorporated with OEO + BHT had significantly lower AVs than the other samples (control, BHT only, and OEO only), presenting lower slope values in the regression equations (Table 2). The lowest slope gradients were exhibited equally by O3B2 (0.21) and O2B2 (0.21) (p > 0.05).
The CD values (Fig. 1c) displayed the same tendency as the PV and AV. The control samples had the greatest CD values. A significant difference was found between BHT1 and BHT2 and the samples with OEO (O1, O2, and O3) also differed among themselves. Oils treated with OEO + BHT exhibited the lowest values and were statistically analogous to each other, but there were significant differences when compared with the control, BHT, and OEO samples. The increase in these chemical indicators during storage was consistent with the analysis of sunflower oil supplemented with different kinds of EOs, described by Olmedo et al. (2009, 2012a, b).
Phenolic compounds, for instance, tocopherol or BHT, react with peroxyl radicals by hydrogen atom transfer, and these molecules are known as chain-breaking antioxidants (Amorati et al. 2013). Components in EOs, such as terpenes, do not have chain-breaking antioxidant properties because when non-phenolic terpenoids react with peroxyl radicals, a reactive alkyl radical is formed, which, on reaction with oxygen, regenerates a peroxyl radical. When non-phenolic components from EOs co-exist with oxidizable components (lipids), both will co-oxidize. However, the overall decrease in the chemical oxidation indicators is due to the increase in the rate of the oxidative termination step caused by “termination-enhancing antioxidants”. Ngo et al. (2016) showed this kind of activity in α-terpinene and γ-terpinene, using the density functional theory. After calculating the C–H bond dissociation enthalpy, proton affinity, ionization energy, proton dissociation enthalpy, and electron-transfer enthalpy, it was concluded that these non-phenolic terpenes exerted antioxidant capacity via a termination-enhancing process. This antioxidant mechanism might also be relevant to food preservation (Baschieri et al. 2017). Here, the combination of BHT and OEO, which is thought to provide chain-breaking and termination-enhancing antioxidant properties, respectively, acted in the prevention and termination of the lipid oxidation process.
Volatile oxidation compounds. The volatile indicators increased in all samples, during storage at 60 °C for 14 days (Fig. 2). Sunflower oil has a fatty acid profile that is susceptible to oxidation, as it predominantly contains linoleic acid (C18:2, 63 g/100 g) and oleic acid (C18:1, 20 g/100 g). When fatty acids are oxidized, they produce different kinds of molecules that are generally recognized as “off-flavor” (Guillen and Goicoechea 2008). Pentanal, hexanal, heptanal, octanal, 2,4-decadienal (E,E), and 2,4-decadienal (E,Z), among other molecules related to the lipid oxidation process, are responsible for the rancid, oxidized and paint odors in food with high-lipid content. In the current study, hexanal (Fig. 2a), 2-heptenal (Fig. 2b), and 2,4-decadienal (E,Z) were detected (Fig. 2c). Extraction and determination of the constituents in a fatty matrix are complex, and the extraction protocol must be appropriate for isolating the compounds of interest to avoid interference by other compounds, besides lipids, present in the matrix (Yu et al. 2017, 2019). When SPME/GC–MS is utilized for the determination of volatile compounds, the interferences can be minimized using an appropriate GC–MS program. In this research, the three volatiles analyzed were the major oxidation volatiles generated by sunflower oil and no interference in their analysis was detected. The control exhibited the highest values for all volatile compounds during storage and was significantly different when compared with all the other treatments. For hexanal, all samples showed significantly different slope values, except between BHT2 and O3, and BHT1 and BHT2 (Table 3). Both O3B2 (0.21) and O3B1 (0.48) had lower slope gradients than the control (1.98). 2-Heptenal evolved similarly to hexanal but presented a higher concentration (approximately 88 µg/g in the control at 14 days). In all samples, relatively low contents (< 100 µg/g) of volatile compounds were observed. Olmedo et al. (2015) detected between 10 and 80 µg/g in sunflower oil that contained laurel, oregano, and rosemary EOs, as natural antioxidants. Under comparatively more extreme experimental conditions, Belitz et al. (2009) revealed 1 g of oil could produce up to 5100 µg/g of hexanal, 450 µg/g of 2-heptenal, and 250 µg/g of 2,4-decadienal (E,Z).
Multivariate principal components analysis (PCA)
In general, a set of variables is chosen to describe and analyze an antioxidant effect. A system involves different variables and samples, and it is difficult to determine which of these treatments showed the best properties. PCA captures the structure of the underlying data distribution, by providing a visual biplot containing all treatments and samples. In this study, a multivariate PCA was performed to determine which treatments presented the best antioxidant properties. Figure 3a shows all data during storage (14 days) and all variables, as a global result. Additionally, the score plot for samples at 0, 7, and 14 days for all treatments (Fig. 3b) and the loading plots of the variables for these days (Fig. 3c) are given, thereby allowing a detailed interpretation of the results (Zhang et al. 2018).
Principal component (PC) 1 (93.9%) and PC2 (5.2%) represented 99.1% of the total data variability in the system (Fig. 3a). All indicator variables and the location of the samples according to their relationship with these variables are projected along the PC1 axis. The samples located on the negative PCI axis (i.e., opposite side to the variables) showed the best antioxidant properties. The OEO + BHT treatments (negative PC1 axis) exerted superior antioxidant properties compared with those containing only BHT or OEO (positive PC1 axis). The samples were further divided into two groups: one included the OEO + BHT treatments, and the other, the BHT, OEO, and control treatments. For samples treated with OEO + BHT, there was no change in the oxidation indicators between day 7 (Fig. 3c) and day 14 (Fig. 3b), as evident from the grouping of the variables into chemical and volatile indicators of oxidation.
BHT can regenerate α-tocopherol radicals from oxidized α-tocopherol after they react with DPPH radicals. Marteau et al. (2014) demonstrated that the matrix in which the antioxidants reacted, influenced this synergistic relationship between BHT and α-tocopherol. Hamdo et al. (2014) studied the relationship between four different tocopherols (α, β, γ, and δ) and three synthetic antioxidants (BHT, BHA, and ascorbyl palmitate [AP]) when combined. The combination of AP and tocopherol displayed the greatest antioxidant synergism effect (16.4% for AP + β-tocopherol, and 21.7% for AP + δ-tocopherol) compared with the other combinations measured by the DPPH test (% inhibition). The synergism between BHT and the tocopherols was 5.10% (BHT + δ-tocopherol). 5.98% (BHT + α-tocopherol), and 6.29% (BHT + β-tocopherol and BHT + γ-tocopherol). These studies demonstrate the potential existence of a synergistic effect when natural and synthetic antioxidants are combined.
Sensory analysis
Sensory test analysis. Consumer acceptance scores of the control (6.1 ± 0.3b), O1 (7.3 ± 0.2d), O2 (6.7 ± 0.3c), and O3 (5.1 ± 0.3a), were all above 5, on the 9-point hedonic scale, with significant differences between all samples. Sample O3 had the lowest acceptance score (5.1) because the concentration of 0.20 g/100 g provided a strong oregano odor, whereas sample O1 (0.02 g/100 g) had the highest score. Asensio et al. (2013) noticed OEO tended to enhance the odor and flavor acceptance of olive oil. Of the four different varieties of OEO tested, three (Compacto, Mendocino, and Criollo) showed greater acceptance (5.7–6.1, on a 9-point hedonic scale) than the control. These scores were generally lower than those reported in the current study, and this may be explained by the use of different kinds of edible oil.
Discriminative analysis. A directional duo–trio test was used for the discriminative analysis. All panelists found differences between samples (α = 0.01). The duo combinations used in the test were control–O1, O1–O2, and O2–O3. Samples containing OEO had an odor that was recognizable by the panelists. Addition of OEO to the refined sunflower oil samples resulted in a different odor, which was perceived by the panel. Although the panel identified all samples with EO in the discriminative test, it did not mean a negative result in the acceptance test, especially for the samples with the least amount of OEO added to sunflower oil. Similarly to the current finding, in the discriminative directional duo–trio test documented by Asensio et al. (2013), the panelists detected the olive oil sample containing OEO.
Conclusion
OEO + BHT exerted superior antioxidant properties in sunflower oil than the use of only OEO or BHT, as measured by lipid oxidation indicators and volatile compounds. The lowest concentrations of the combined antioxidants (O1B1) afforded the best protection against lipid oxidation in sunflower oil. The consumer acceptance of sunflower oil was greatest for O1, which contained the same concentration of OEO as sample O1B1. The use of OEO + BHT blends, as antioxidant mixtures, represents a novel way to preserve food with high-lipid content, without needing to markedly increase the concentration of OEO or BHT.
References
Adams RP (1995) Identification of essential oils by ion trap mass spectroscopy. Allured Press, Carol Stream
Adams A, Kruma Z, Verhé R, De Kimpe N, Kreicbergs V (2011) Volatile profiles of rapeseed oil flavored with basil, oregano and thyme as a function of flavoring conditions. J Ame Oil Chem Soc 88(2):201–212
Aladedunye F, Przybylski R (2013) Frying stability of high oleic sunflower oils as affected by composition of tocopherol isomers and linoleic acid content. Food Chem 141:2373–2378
Amorati R, Foti M, Valgimigli L (2013) Antioxidant activity of essential oils. J Agric Food Chem 61:10835–10847
AOAC (1980) Official methods of analysis of the AOAC. Horwitz W (ed) 13th edn. Washington, DC
Asensio CM, Nepote V, Grosso NR (2012) Sensory attribute preservation in extra virgin olive oil with addition of oregano essential oil as natural antioxidant. J Food Sci 77:294–301
Asensio CM, Nepote V, Grosso NR (2013) Consumers’ acceptance and quality stability of olive oil flavoured with essential oils of different oregano species. I J Food Sci Technol 48:2417–2428
Baschieri A, Ajvazi MD, Tonfack JLF, Valgimigli L, Amorati R (2017) Explaining the antioxidant activity of some common non-phenolic components of essential oils. Food Chem 232:656–663
Belitz HD, Grosch W, Schielberle P (2009) Food chemistry. Springer, Berlin
Boskou D, Elmadfa I (2011) Frying of food. CRC Press, Boca Raton
Castilho PC, Savluchinske-Feio S, Weinhold TS, Gouveia SC (2012) Evaluation of the antimicrobial and antioxidant activities of essential oils, extracts and their main components from oregano from Madeira Island, Portugal. Food Control 23(2):552–558
Choi HS, Song HS, Ukeda H, Sawamura M (2000) Radical scavenging activities of citrus essential oils and their compoents. Detection using 1,1 diphenyl-2-picrylhydrazyl. J Agric Food Chem 48:4156–4161
Chouliara E, Karatapanis A, Savvaidis IN, Kontominas MG (2007) Combined effect of oregano essential oil and modified atmosphere packaging on shelf-life extension of fresh chicken breast meat, stored at 4 °C. Food Microbiol 24:607–617
COI (2001) Método de análisis, prueba espectrofotométrica en el ultravioleta. Document COI/T, 20/Doc no 19/Rev. 1. International Olive Oil Council (IOOC), Madrid
Dambolena JS, Zunino MP, Lucini EI, Olmedo R, Banchio E, Bima P, Zygadlo JA (2010) Total phenolic content, radical scavenging properties and essential oil composition of origanum species from different populations. J Agric Food Chem 58:1115–1120
Dima C, Dima S (2015) Essential oils in foods: extraction, stabilization, and toxicity. Curr Opin Food Sci 5:29–35
Feng X, Ng VK, Mikš-Krajnik M, Yang H (2017a) Effects of fish gelatin and tea polyphenol coating on the spoilage and degradation of myofibril in fish fillet during cold storage. Food Bioprocess Technol 10:89–102
Feng X, Zhu Y, Liu Q, Lai S, Yang H (2017b) Effects of bromelain tenderization on myofibillar proteins, texture and flavor of fish balls prepared from golden pomfret. Food Bioprocess Technol 10(10):1918–1930
Foti MC (2015) Use and abuse of the DPPH Radical. J Agric Food Chem 63:8765–8776
Gorji SG, Smyth HE, Sharma M, Fitzgerald M (2016) Lipid oxidation in mayonnaise and the role of natural antioxidants: a review. Trends Food Sci Technol 56:88–102
Guillen MD, Goicoechea E (2008) Formation of oxygenated α, β-unsaturated aldehydes and other toxic compounds in sunflower oil oxidation at room temperature in closed receptacles. Food Chem 111:157–164
Guimaraes R, Sousa MJ, Ferrerira ICFR (2010) Contribution of essential oils and phenolics to the antioxidant properties of aromatic plants. Ind Crops Prod 32:152–156
Hamdo HH, Khayata W, Al-Assaf Z (2014) Synergistic effect of combined some natural and synthetic antioxidants to increase oxidative stability using DPPP test. Int J Chem Tech Res 6(4):2539–2545
IUPAC (1987) Method number 2.504. Determination of the p-anisidine value. In: Paquot C, Hautfenne A (eds) Standard methods for the analysis of oils, fats and derivatives, 7th edn. Blackwell Scientific Publications, Oxford
Jonhson RA, Wichern DW (1998) Applied multivariate statistical analysis. Pretince Hall, Englewook Cliffs
Lester GE, Lewers KS, Medina MB, Saftner RA (2012) Comparative analysis of strawberry total phenolics via Fast Blue BB vs. Folin-Ciocalteu: assay interference by ascorbic acid. J Food Compost Anal 27:102–107
Marteau C, Favier D, Nardello-Rataj V, Aubry JM (2014) Dramatic solven effect on the synergy between α-tocopherol and BHT antioxidants. Food Chem 160:190–195
Ngo TC, Dao DQ, Thong NM, Nam PC (2016) Insight into the antioxidant properties of non-phenolic terpenoids contained in essential oils extracted from the buds of Cleistocalyx operculatus: a DFT study. RSC Adv 6:30824–30834
Olmedo RH, Asensio C, Nepote V, Mestrallet MG, Grosso NR (2009) Chemical and sensory stability of fried-salted peanuts flavored with oregano essential oil and olive oil. J Sci Food Agric 89:2128–2136
Olmedo RH, Nepote V, Grosso NR (2012a) Aguaribay and Cedron essential oils as natural antioxidants in oil-roasted and salted peanuts. J Am Oil Chem Soc 89(12):2195–2205
Olmedo RH, Nepote V, Grosso NR (2012b) Sensory and chemical stability in coated peanuts with the addition of essential oils and synthetic antioxidants. Int J Fats Oils 63(1):5–13
Olmedo RH, Nepote V, Grosso NR (2013) Preservation of sensory and chemical properties in flavored cheese prepared with cream cheese base using oregano and rosemary essential oils. LWT-Food Sci Technol 53:409–417
Olmedo R, Nepote V, Grosso NR (2014) Antioxidant activity of fractions from oregano essential oils obtained by molecular distillation. Food Chem 156:212–219
Olmedo R, Asensio CM, Grosso NR (2015) Thermal stability and antioxidant activity of essential oil from aromatic plants farmed in Argentina. Ind Crops Prod 69:21–28
Pan Y, Zhu J, Wang H, Zhang X, Zhang Y, He C, Ji X, Li H (2007) Antioxidant activity of ethanolic extract of Cortex fraxini and use in peanut oil. Food Chem 103:913–918
Roginsky V, Lissi EA (2005) Review of methods to determine chain-breaking antioxidant activity in food. Food Chem 92:235–254
Sharma OP, Bhat TK (2009) DPPH antioxidant assay revisited. Food Chem 113:1202–1205
Shearn CT, Fritz KS, Thompson JA (2011) Protein damage from electrophiles and oxidans in lung of mice chronically exposed to the tumor promoter butylated hydroxytoluene. Chem-Biol Interact 192:278–286
Tohidi B, Rahimmalek M, Arzani A (2017) Essential oil composition, total phenolic, flavonoid contents and antioxidant activity of Thymus species collected from different regions of Iran. Food Chem 220:153–161
Wang S, Adhikari K, Hung YC (2017) Effects of short storage on consumer acceptability and volatile compound profile of roasted peanuts. Food Pack Shelf Life 13:27–34
Yan F, Azizi A, Janke S, Schwarz M, Zeller S, Honermeier B (2016) Antioxidant capacity variation in the oregano (Origanum vulgare L.) collection of the German National Genebank. Ind Crops Prod 92:19–25
Yu X, Ang HC, Yang H, Zheng C, Zhang Y (2017) Low temperature cleanup combined with magnetic nanoparticle extraction to determine pyrethroids residue in vegetables oils. Food Control 74:112–120
Yu X, Li Z, Zhao M, Lau SCS, Tan HR, Teh WJ, Yang H, Zheng C, Zhang Y (2019) Quantification of aflatoxin B1 in vegetable oils using low temperature clean-up followed by immune-magnetic solid phase extraction. Food Chem 275:390–396
Zhang L, Chen F, Lai S, Wang H, Yang H (2018) Impact of soybean protein isolate-chitosan edible coating on the softening of apricot fruit during storage. LWT-Food Sci Technol 96:604–611
Acknowledgements
This work was supported by Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) and Secretaria de Ciencia y Tecnología de la Universidad Nacional de Córdoba (SECYT-UNC).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Olmedo, R., Ribotta, P. & Grosso, N.R. Decrease of chemical and volatile oxidation indicators using oregano essential oil combined with BHT in sunflower oil under accelerated storage conditions. J Food Sci Technol 56, 2522–2535 (2019). https://doi.org/10.1007/s13197-019-03731-8
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-019-03731-8