Abstract
Introduction
Recent studies provide a convincing support that the presence of cancer cells in the body leads to the alteration of volatile organic compounds (VOCs) emanating from biological samples, particularly of those closely related with tumoral tissues. Thus, a great interest emerged for the study of cancer volatilome and subsequent attempts to confirm VOCs as potential diagnostic biomarkers.
Objectives
The aim of this study was to determine the volatile metabolomic signature of bladder cancer (BC) cell lines and provide an in vitro proof-of-principle that VOCs emanated into the extracellular medium may discriminate BC cells from normal bladder epithelial cells.
Methods
VOCs in the culture media of three BC cell lines (Scaber, J82, 5637) and one normal bladder cell line (SV-HUC-1) were extracted by headspace-solid phase microextraction and analysed by gas chromatography-mass spectrometry (HS-SPME/GC–MS). Two different pH (pH 2 and 7) were used for VOCs extraction to infer the best pH to be used in in vitro metabolomic studies.
Results
Multivariate analysis revealed a panel of volatile metabolites that discriminated cancerous from normal bladder cells, at both pHs, although a higher number of discriminative VOCs was obtained at neutral pH. Most of the altered metabolites were ketones and alkanes, which were generally increased in BC compared to normal cells, and alcohols, which were significantly decreased in BC cells. Among them, three metabolites, namely 2-pentadecanone, dodecanal and γ-dodecalactone (the latter only tentatively identified), stood out as particularly important metabolites and promising volatile biomarkers for BC detection. Furthermore, our results also showed the potential of VOCs in discriminating BC cell lines according to tumour grade and histological subtype.
Conclusions
We demonstrate that a GC–MS metabolomics-based approach for analysis of VOCs is a valuable strategy for identifying new and specific biomarkers that may improve BC diagnosis. Future studies should entail the validation of volatile signature found for BC cell lines in biofluids from BC patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Bladder cancer (BC) is one of the most common and deadly malignancies affecting the urinary system, worldwide (Burger et al. 2013). Its incidence continues to rise and mortality rates have not lessened over the years due, mainly, to the fact that early detection methods are still lacking, hampering therapeutic success (Huang et al. 2011). To improve diagnostic strategies, several potential markers have been proposed over the years or are under investigation, with some of them already validated and approved for clinical use (Chan et al. 2015; Miremami and Kyprianou 2014). Nevertheless, for early detection purposes, most do not offer advantage over classical diagnostic methods (Ku et al. 2012), and its high cost seriously limits their use in routine practice (Miremami and Kyprianou 2014). Hence, there is an urgent need to discover more accurate, disease-specific, easily detectable and cost-effective biomarkers, overcoming limitations of current diagnostic methods, as these often detect malignancy at more advanced stage, which minimizes effective therapeutic strategies, impairing patient survival (Rodrigues et al. 2016a).
The investigation on volatiles and its potential as diagnosis biomarkers started with the observation that distinct diseases might be detected through specific odours in urine, exhaled breath or sweat (Kwak and Preti 2011), which, in turn, correlated with different volatile organic compounds (VOCs) patterns. In cancer, it was demonstrated that dogs can be trained to diagnose bladder (Willis et al. 2004), colorectal (Peng et al. 2010), lung and breast cancers (McCulloch et al. 2006) as well as melanoma (Pickel et al. 2004), by sensing patients, supporting the hypothesis that volatiles might be potential biomarkers for cancer detection. Thus, a great interest emerged for the study of cancer volatilome and subsequent attempts to confirm VOCs as potential diagnostic biomarkers (Shirasu and Touhara 2011; Di Lena et al. 2016; Monteiro et al. 2014). This new approach relies on the fact that VOCs are final products of cellular metabolism (Di Lena et al. 2016), which are released through cell membranes. Evidently, tumour cells will emanate a distinct pattern of VOCs due to specific genetic alterations or protein modifications that affect oxidative stress and, consequently, peroxidation of membrane components (Di Lena et al. 2016).
VOCs analysis presents several advantages, including simple and fast sample preparation compared to other molecules that need a derivatization process (e.g., amino acids and fatty acids), thus preventing loss of metabolites; non-invasive collection (easily detected in exhaled breath or voided urine); and enable high-throughput screening and analysis of numerous compounds across different samples (Nunes de Paiva et al. 2014). Considering in vitro VOCs studies, this approach may help understand the source of VOCs as cell metabolomics display more controllable experimental designs and easier interpretation of the data (Rodrigues et al. 2016a) due to the absence of confounding factors such as diet and microbiota (Davis et al. 2011; Lauridsen et al. 2007). These factors significantly influence VOC profiles in in vivo studies and VOCs unrelated to cancer itself may arise as discriminative metabolites (Lauridsen et al. 2007). Therefore, analysis of the in vitro volatile metabolome offers a great advantage over animal and human biological matrices as it provides an unchanged metabolic signature originated directly from cells. Nevertheless, in vitro studies present some limitations (Leon et al. 2013), especially the fact that cultured cells cannot reproduce the cancer microenvironment, the relationships between cancer cells and other surrounding cells or the immune system, thus the cancer-host interactions are lost (Rodrigues et al. 2016b).
Herein, we aimed to discover volatiles that might discriminate cancerous from non-cancerous bladder cell lines, through an untargeted in vitro metabolomic study. We chose this approach because, to the best of our knowledge, a rather limited number of studies have focused on BC urinary volatilome (Jobu et al. 2012; Cauchi et al. 2016). Moreover, there are only a few reports on VOCs as BC discriminative metabolites (Pasikanti et al. 2010a, b, 2013; Putluri et al. 2011), although no integrated BC metabolomic study has performed VOCs profiling in in vitro cell lines, which makes this work pivotal in that matter. Furthermore, VOCs were analysed due to their potential as biomarkers for BC detection and the relatively easy translatability to other biological matrices (such as urine, blood or exhaled breath) (Schmidt and Podmore 2015), through headspace-solid phase microextraction/gas chromatography–mass spectrometry (HS-SPME/GC–MS). This technique requires simpler and faster sample preparation, which is an advantage in a clinical environment. The overarching goals of the present work were, firstly, the evaluation of two different pH (pH 2 and 7) to infer which pH is optimum for VOCs extraction (to be applied in future in vitro metabolomic studies), and secondly, to evaluate the volatilome of three different tumorigenic bladder cell lines and of a non-tumorigenic cell line, in search of significantly different VOCs profiles among them.
2 Methods
2.1 Chemicals
Minimum essential medium eagle (MEM) and Ham’s F-12K nutrient mixture powder, both supplemented with l-glutamine, sodium chloride (NaCl, ≥ 99.5%), sodium hydroxide (NaOH, ≥ 97%), Folin–Ciocalteu’s phenol reagent, bovine serum albumin (BSA), 4-fluorobenzaldehyde (98%), and pure standards for 2-methyl-2-butanol (≥ 99.5%), α-terpineol (≥ 98.5%), 2-nonanone (≥ 99.5%), 4-methylbenzaldehyde (≥ 97%), benzaldehyde (≥ 99.5%), acetophenone (≥ 99.5%), cyclohexanone (≥ 99%), 2-pentadecanone (98%), geranylacetone (≥ 97%), nonanal (≥ 99.5%), dodecanal (≥ 95%), 1-phenylethanol (≥ 98%), menthol (≥ 99%), phenol (≥ 99.5%), benzoic acid (≥ 99.5%), (2E,4E)-deca-2,4-dienal (≥ 85%), isopentanol (≥ 98.5%) and octanol (≥ 99.7%) were obtained from Sigma-Aldrich (St. Louis, MO, USA). Penicillin, streptomycin and trypsin were purchased from Invitrogen (Karlsruhe, Germany); fetal bovine serum (FBS) was purchased from PAA laboratories GmbH (Colbe, Germany); DMSO (99.0%) and hydrochloric acid (HCl) were obtained from Merck (Darmstadt, Germany); and methanol (99.9%) from VWR (Leuven, Belgium). All chemicals were of analytical grade and were dissolved in deionized water unless otherwise indicated.
2.2 Cell lines and culture conditions
The BC cell lines J82 (TCC; grade III/IV, stage pT3), Scaber (SCC; grade III/IV, stage pT4) and 5637 (TCC; grade II), and the non-tumorigenic SV-HUC-1 were obtained from American Type Culture Collection (ATCC; Manassas, VA, USA). General characteristics of all bladder cell lines are summarized in Table 1. All cancer cell lines were cultured in MEM, whereas SV-HUC-1 was cultured in Ham’s F-12K medium under the conditions suggested by the supplier in order to preserve their characteristics after in vitro passages. Both medium were prepared as indicated by the manufacturer and supplemented with 10% FBS and 50 units/mL penicillin/50 µg/mL streptomycin. To minimize possible effects of the used medium on metabolic footprints, the culture conditions were kept constant throughout the entire study. All cell lines were routinely tested for Mycoplasma spp. contamination (PCR Mycoplasma Detection Set, Clontech Laboratories).
2.3 Collection of VOCs from extracellular medium
The experiments were carried out during 5 passages (passages 4 to 8 in the case of cancer cell lines and passages 6 to 10 in the case of SV-HUC-1), with triplicates for each passage, after an adaptation stage of at least 3 passages for all cell cultures. After cells’ growth to near confluence was achieved, medium from each T75 culture flask was discarded and 15 mL of fresh medium was added and incubated in a humidified incubator supplied with 5% CO2 at 37 °C for 48 h. Subsequently, the extracellular medium was transferred to falcons on ice and centrifuged for 4 min at 2000×g at 4 °C. The supernatant was divided into 2 aliquots of 7 mL, for further samples analysis at two different pH, and stored at − 80 °C until analysis (Leon et al. 2013). T75 culture flasks containing only medium (blanks) were treated in an identical manner. Blanks were submitted to the same collection and storage conditions.
2.4 Sample preparation for HS-SPME/GC–MS analysis
Stored samples were thawed slowly in ice to minimize the loss of volatile compounds. Each sample (2 mL) was transferred to a 10 mL glass vial, capped with a polytetrafluoroethylene (PTFE) septum and a screw cap, containing 0.59 g NaCl and 10 µL of internal standard (IS) 4-fluorobenzaldehyde (Millipore, Bedford, MA, USA) with final concentration of 10 µg/mL. The purpose of IS addition to each sample was to monitor the performance of the chromatographic analysis (injection issues and retention time deviation).
As aforementioned, medium of each cell line and blanks were analysed at two different pH (pH 2 and 7) in order to evaluate which pH is optimum for VOCs extraction. For pH 7 samples, there was no need to adjust the pH (median pH 7.252 ± 0.088). In the case of pH 2 samples, 70–75 µL of a 5 M HCl solution was added to adjust the pH (median 2.076 ± 0.045). Quality control (QCs) samples were prepared as a pool of all samples in the study and divided into aliquots to avoid the repeated freezing and thawing (Leon et al. 2013).
2.5 HS-SPME/GC–MS analysis: equipment and conditions
The HS-SPME (Zhang and Pawliszyn 1993) was performed using a Combi-PAL autosampler (Varian Pal Autosampler, Switzerland) and the Cycle Composer software (CTC Analytics System Software, Switzerland). Headspace volatiles were extracted by exposing a 65 µm film thickness polydimethylsiloxane/divinylbenzene (PDMS/DVB) fiber (Supelco Inc., Bellefonte, PA), previously conditioned at 250 °C for 30 min. The HS-SPME method was developed and optimized previously by our group for urine samples (Monteiro et al. 2014) because the forthcoming goal is the application of this method in urine samples from BC patients. The HS was generated after 5 min of incubation and 20 min of extraction, at 45 °C. Samples were randomly injected in the HS-SPME/GC–MS.
The chromatographic analysis of the VOCs extracted from the extracellular medium were performed on a SCIONTM SQ (single quadrupole) 436-GC–MS system (Bruker Daltonics, Fremont, CA) equipped with a SCION SQ mass detector and a Bruker Daltonics MS workstation software (version 8.2). A capillary column Rxi-5Sil MS (30 m × 0.25 mm × 0.25 µm) from Restek was used for the chromatographic separation. Helium C-60 (Gasin, Portugal) was used as the carrier gas at a constant flow rate of 1.0 mL/min. The injection was in splitless mode and the injector temperature was 230 °C (held for 20 min.). As for the oven temperature, it was held for 1 min at 40 °C, followed by an increase at a rate of 5 °C/min until reaching 250 °C (held for 5 min) and finally an increase of 5 °C/min to 300 °C (held for 1 min). The MS detector was operated in EI mode. Data acquisition was performed in full scan mode with a mass range between 40 and 350 m/z at a scan rate of 6 scan/s. A QC sample was repeatedly analysed under the same conditions, on every 15 samples, in order to assess analytical reproducibility.
2.6 GC–MS data pre-processing
VOCs identification in HS-SPME/GC–MS chromatograms of extracellular medium was performed in two steps. First, the peak spectrum was checked against the mass spectra from the NIST mass spectral library (2014) and the Kovats retention index (RI). Only a reverse match of 700 or above was considered in the compound identification, whereas compounds for which no satisfactory match was found were listed as “unknown i” (i = 1, 2, 3 …) according to their increasing retention times (RTs). Next, the compounds identification was confirmed through comparison of their RTs with the RTs obtained from pure standards, namely menthol, 1-phenylethanol, α-terpineol, 2-methyl-2-butanol, isopentanol, octanol, phenol, (2E,4E)-deca-2,4-dienal, 4-methylbenzaldehyde, benzaldehyde, nonanal, dodecanal, benzoic acid, geranylacetone, acetophenone, 2-nonanone, cyclohexanone, and 2-pentadecanone (Supplementary Table S1). The Human Metabolome Database (HMDB) (Wishart et al. 2007) was also consulted to obtain further information about the compounds found, when available, such as the matrices where they were previously found.
All raw data files obtained from HS-SPME/GC-SQ/MS were exported as CDF files and pre-processed in order to convert instrumental data sets into a manageable format for data analysis and remove any bias such as background, noise and RT fluctuations over a set of samples. Data pre-processing was performed using the software MZmine 2.2 (Pluskal et al. 2010) and consisted in baseline correction, peak detection, chromatogram deconvolution, alignment and normalization. The parameters used in these steps were set as follow: RT range 2.8–34.0 min; m/z range 50–350; MS data noise level 1.0 × 104; m/z tolerance 0.5 or 5 ppm; chromatogram baseline level 1.0 × 103; peak duration range 0.02–0.30 min. After pre-processing steps, artefact peaks such as GC contaminants from the chromatographic column (e.g., cyclosiloxanes, siloxanes and phthalates) were manually removed from data matrix, as well as all peaks with relative standard deviation (RSD) ≥ 30% across all QCs, as an indicator of poor reproducibility and repeatability of the analysis. The mean of the chromatogram’s area of correspondent triplicates of each cell line was determined and data was subsequently normalized by total area (TA) by dividing each mean peak area by the total mean peak area of the respective chromatogram. The resulting m/z-RT pairs, sample names and normalized peak areas were subjected to statistical analysis.
2.7 Statistical analysis
The final matrix was imported to SIMCA-P 13.0.3 (Umetrics, Umea, Sweden) and scaled to pareto (Par) (Worley and Powers 2013). Principal component analysis (PCA) was first applied to detect trends and outliers, followed by partial least squares discriminant analysis (PLS-DA) and orthogonal PLS-DA (OPLS-DA), used to discriminate between classes and identify the volatile signature associated with a specific sample class. OPLS-DA is a modification of the PLS-DA method which removes the Y-uncorrelated variation in X, thus improving interpretability. In this study, OPLS-DA was only used for assessment of covariance of X-variables, while PLS-DA was used for prediction purposes. Model robustness was evaluated in terms of R2X (variance explained by the X matrix), R2Y (variance explained by the Y matrix) and Q2 (goodness of prediction or prediction power), a set of parameters obtained by sevenfold cross validation in SIMCA-P 13.0.3 software. All PLS-DA models were validated through permutation test (200 permutations) comparing the goodness of fit (R2 and Q2) of the original model with the goodness of fit of several models where the order of Y-observations was randomly permuted. The variables (m/z-RT pairs) responsible for group separation were assessed through inspection of OPLS-DA S-line plots and the corresponding variable importance in projection (VIP) of each variable. Only variables with VIP > 1 were considered as important for group discrimination.
The individual statistical significance of relevant compounds identified in the S-line plots was assessed using the unpaired Mann–Whitney test, in GraphPad Prism version 6 (GraphPad Software, San Diego, CA, USA). For each model, the discriminative compounds were considered statistically significant when p value < 0.05 (confidence level 95%). Bonferroni correction (Aickin and Gensler 1996) was used to adjust p values for multiple comparisons by setting the significance cut-off to α/n, in which α = 0.05 and n represents the number of compounds simultaneously tested in statistical analysis. Furthermore, for each significant compound, the effect size (calculated as described in Berben et al. 2012) and the area under the curve (AUC) (GraphPad Software, San Diego, CA, USA) were determined. The same statistical analysis was performed using media blanks at both pH in order to provide insight into the potential impact that the two different media (MEM and Ham’s F-12K) may have on the VOCs profiles.
3 Results and discussion
In this study, the volatile exometabolome of three bladder cancer cell lines (5637, J82 and Scaber) and one normal bladder epithelial cell line (SV-HUC-1) was analysed to find VOCs profiles that might discriminate between cancerous and non-cancerous bladder epithelial cells. Importantly, the cancer cell lines included in this study are classified according to histological subtype and grade, which allowed for extending our investigation on the metabolites responsible for discriminating BC cell lines according to tumour grade and histological subtype. The 5637 and J82 cell lines represent transitional cell carcinoma (TCC), which is the most common form of BC, whereas Scaber represents squamous cell carcinoma (SCC) subtype of BC. Moreover, 5637 cell line is low-grade (LG), whereas J82 and Scaber represent high-grade (HG) BC (Table 1).
3.1 Effect of pH on volatile exometabolome of BC cell lines
The extracellular medium of all samples was analysed considering a neutral pH (pH 7) and an acidic one (pH 2) to evaluate which pH would result in a more efficient VOCs extraction. Neutral pH was the natural pH of bladder cells’ medium, and thus no alteration was introduced. In turn, pH 2 was chosen because it is widely acknowledged that tumour microenvironment is acidic due to increased lactate production (Ferreira 2010). Overall, a variety of chemical compounds was detected in both pH 2 and pH 7, such as alcohols, aldehydes, ketones, alkanes and carboxylic acids. The HS-SPME/GC–MS analysis of samples at pH 7 and pH 2 enabled detection of 191 and 206 chromatography peaks, respectively, 107 of them were common to both pHs (Supplementary Fig. S1). Of note, the number of peaks detected at pH 7 and pH 2 corresponded to the total number of peaks detected in the chromatograms before pre-processing and the removal of peaks with RSD ≥ 30%. Acidification predominantly favoured the detection of more acidic compounds, such as organic acids (acetic, hexanoic, heptanoic, octanoic, nonanoic and decanoic acids, benzoic acid), and esters, namely methyl nonanoate and methyl decanoate. The general characteristics of the VOCs identified in the extracellular medium, such as retention time (RT), retention indexes (RI), characteristic ions (m/z), identification method (NIST and standards), HMDB identification and matrices or cellular locations where they have been previously found are summarized in Supplementary Table S1 for both pH 2 and pH 7. The QC samples confirmed the analytical reproducibility at both pH values, since all QCs were closely clustered in the PCA scores scatter plot (Supplementary Fig. S2).
Multivariate statistical analysis unveiled 60 VOCs significantly altered at pH 7, considering all comparison groups (i.e., cancerous vs. non-cancerous cell lines, HG vs. LG cancer cell lines and TCC vs. SCC cell lines), whereas pH 2 revealed a lower number of statistically significant compounds (26 VOCs) for the same comparison groups, 18 of them in common with pH 7. This suggests that neutral pH provides a more complete profiling of volatile exometabolome, despite some complementarity of pH 2 volatilome profiling (γ-dodecalactone, 1-butanol, (2E,4E)-deca-2,4-dienal, benzoic acid and α-methylstyrene).
3.2 Volatile exometabolome signature of BC versus normal cell lines
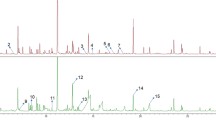
PCA showed a clear separation between the extracellular medium of each cancer cell line and the one from the normal cell line, at pH 7 (Fig. 1a). For pH 2, a less efficient separation was found since the discrimination according to BC grade is not clear (Fig. 1b). Pairwise PCA and PLS-DA were performed to assess the set of VOCs responsible for separation of each cancer cell line from normal cell line (SV-HUC-1). A representative PLS-DA score scatterplot is depicted in Fig. 2a, showing a clear separation between Scaber and SV-HUC-1 with a good prediction ability (Q2 0.969). The statistical validation of the PLS-DA model by permutation testing (Fig. 2b) unveiled no over fitting since all blue Q2 values to the left are lower than the original points to the right. Based on the analysis of the OPLS-DA S-line plot (Fig. 2c), 37 VOCs were found to vary significantly (8 alcohols, 2 aldehydes, 5 alkanes, 1 benzothiazole, 5 ketones and 16 unknowns) in Scaber compared with SV-HUC-1 at pH 7 (Table 2 and Supplementary Table S2). Receiver operating characteristic (ROC) curves were computed for all significantly altered VOCs, unveiling a perfect performance (AUC = 1) for several metabolites of which 2-pentadecanone (Fig. 2d), α-terpineol, 2-methylbutan-2-ol, 1-phenylethanol, and the tentatively identified 2-phenylpropan-2-ol and 2-hydroxy-2-methyl-1-phenylpropan-1-one are the most significantly altered metabolites between Scaber and normal cell line. The statistical models obtained for pH 2 unveiled a similar performance to discriminate between Scaber and SV-HUC-1 (Supplementary Table S5). However, the number of discriminant VOCs found at pH 2 was lower compared with pH 7 and most of them were in common, with exception of benzoic acid, 2-methyl-2-heptanol, 1-butanol, α-methylstyrene (the last 3 only tentatively identified) and unknown 25 (λ in Table 2 and Supplementary Table S2).
PCA scores scatter plots obtained for the HS-SPME/GC–MS chromatograms of the normal cell line SV-HUC-1 (n = 5, dark red inverted triangle) and the cancer cell lines 5637 (n = 5, low-grade, TCC, yellow circle), J82 (n = 5, high-grade, TCC, green square) and Scaber (n = 5, high-grade, SCC, blue triangle) extracellular medium at pH 7 (a) and pH 2 (b). The ellipses indicate the 95% confidence limit of the model. R2X is the variance explained by the X matrix
a PLS-DA scores scatter plot obtained for the HS-SPME/GC–MS chromatograms of the normal cell line (SV-HUC-1, n = 5, dark red inverted triangle) and Scaber (n = 5, blue triangle) extracellular medium at pH 7. b Statistical validation of the PLS-DA model by permutation testing (200 permutations). c OPLS-DA S-line plot obtained for the same pairwise comparison with identification of most important VOCs for class discrimination: 1 isopentanol, 2 unknown 2 (RT 8.29 min), 3 4-methylheptan-2-one, 4 unknown 4 (RT 10.33 min), 5 1-phenylethan-1-ol, 6 2-phenylpropan-2-ol, 7 unknown 10 (RT 13.68 min), 8 unknown 19 (RT 24.08 min), 9 2-tridecanone, 10 2-pentadecanone. d Example of assessment of the diagnostic performance through ROC analysis obtained for 2-pentadecanone (AUC = 1.000), unknown 1 (AUC = 0.960) and phenol (AUC = 0.920). LV number of latent variables, Q2 predictive power, R2X variance explained by the X matrix, R2Y variance explained by the Y matrix
Regarding J82 versus SV-HUC-1, 40 VOCs with statistically significant differences were found, considering both pHs (Table 2), from which 4-methylbenzaldehyde, cyclohexanone, 2-pentadecanone, the tentatively identified 2-phenylpropan-2-ol, phenol, dodecane, 4-methylheptan-2-one, γ-dodecalactone and unknown 24 present the highest magnitude of variation and unveiled a perfect performance (AUC = 1). The comparison 5637 versus SV-HUC-1 showed statistically significant differences in 42 VOCs, considering both pHs as well (Table 2 and Supplementary Table S2), namely nonanal, 1-phenylethanol, 4-methylnonane, dodecane, γ-dodecalactone, and unknowns 10 and 24 (AUC = 1).
Of a total of 58 discriminative VOCs at pH 7, 16 VOCs (highlighted in shaded lines in Table 2 and Supplementary Table S2) were found altered and displayed the same trend (either increased or decreased) in all BC volatile exometabolomes. For example, benzaldehyde (Fig. 3a), 2-nonanone (Fig. 3b), 4-methylheptan-2-one, dodecane 2,3-dimethylhexane, hexadecane and tetradecane were found significantly increased in cancer cells medium, whereas 2-phenylpropanol and isopentanol were significantly decreased in cancer cells medium compared to SV-HUC-1 medium. These results are suggestive of a common regulatory mechanism in 5637, J82 and Scaber cell lines. An alternative explanation is that, compared to the normal bladder cells, BC cell lines are incubated in a growth medium with different carbon source composition, and therefore those VOCs may display the same trend due to media-associated differences. In this regard, multivariate analysis of media blanks (Supplementary Table S4) unveiled that only 5 (identified or tentatively identified) of those 16 altered VOCs were affected by the medium composition, namely butanol, 2-phenylpropan-2-ol, dodecane, tetradecane and 4-methylheptanone (α in Table 2 and Supplementary Table S2). Further studies are required to clarify the possible additive effects of culture media on the alterations observed for these VOCs.
From those 16 VOCs, isopentanol, 2-phenylpropan-2-ol, 2,3-dimethylhexane, hexadecane, and tetradecane have not been reported for other cancer types so far, either in in vitro studies or in biological samples, which may indicate a potential specificity towards BC. The remaining VOCs have been found as potential biomarkers in previous cancer studies, including colorectal cancer (CRC) (Silva et al. 2011), melanoma (Abaffy et al. 2010) and lung cancer (Filipiak et al. 2014; Ligor et al. 2009; Bajtarevic et al. 2009). Similarly to the results obtained in this study, an in vitro study observed higher levels of 2-nonanone in the extracellular medium of liver cancer cell line HepG2 but, unlike BC cells, benzaldehyde was decreased in HepG2 extracellular medium (Mochalski et al. 2013).
Importantly, ketone 2-pentadecanone was found consistently altered in all three cancer cells medium (though with different trends among BC cells) but was not detectable in blanks, suggesting its origin from cellular metabolism. Hence, 2-pentadecanone may constitute one of the most important and promising compounds in BC volatile signature, despite being already reported in two other in vitro cancer studies (Hu et al. 2010; Zimmermann et al. 2007).
Regarding pH 2, only 26 VOCs were found significantly altered in BC exometabolome, of which 18 were in common to those extracted at pH 7, presenting the same trend of change. Therefore, only the 8 VOCs exclusively found significantly altered at pH 2 were considered for further interpretation (Supplementary Table S1). Among these, 1-butanol and α-methylstyrene (tentatively identified) were found significantly decreased in all BC volatile exometabolomes, whereas benzoic acid presented a different trend of alteration (Table 2) being significantly increased in 5637 cell line and decreased in J82 and Scaber cell lines. From those VOCs, only α-methylstyrene has not been found in other diseases yet, thus it might be a good candidate as BC volatile biomarker.
In general, VOCs belonging to ketones and alkanes chemical classes were highly released by BC cell lines, whereas alcohols were significantly decreased in BC cells extracellular medium, compared to the normal cell line medium. These results may be related with either the synthesis of fatty acids for membrane formation or β-oxidation of fatty acids for energy production, amino acids metabolism, oxidative stress and inflammation process (Calenic 2013; Hakim et al. 2012). Thus, one may hypothesise that 1-octanol might be associated with synthesis of a medium-chain fatty acid, namely caprylic acid (C8:0), for energy production. The presence of ketones in the extracellular medium of cancer cells may result from β-oxidation of branched-chain fatty acids. The metabolism of the branched chain fatty acid, nonanoic acid, may result in 2-nonanone production in BC cells, as well as the metabolism of C15:0 long-chain fatty acids may originate 2-pentadecanone in J82 cells, analogous to what has been demonstrated for other ketone (3-heptanone) (Erhart et al. 2009). It is acknowledged that cancer cells use β-oxidation as an alternative pathway to generate sufficient energy to sustain its higher energetic demands (Al-Zoughbi et al. 2014). Indeed, an increase in fatty acid β-oxidation has already been associated with several cancer types, including BC (Rodrigues et al. 2016a) as well as those from the kidney (Hakimi et al. 2016), prostate (Liu 2006) and pancreas (Khasawneh et al. 2009). Regarding simple alkanes (such as dodecane, tetradecane and hexadecane) and benzaldehyde, it has been described that they result from lipid peroxidation and their levels increase during inflammation and oxidative stress caused by an increase of ROS levels (Phillips et al. 2000; Silva et al. 2011; Poli et al. 2010), a hallmark of cancer. The higher oxidative stress status of cancer cells may explain the higher levels of alkanes and benzaldehyde released by BC cells in this study. Indeed, aldehydes are known biomarkers of oxidative stress and tissue damage (Fuchs et al. 2010; Poli et al. 2010), which makes them promising cancer biomarkers.
3.3 Volatile exometabolome signature of low-grade versus high-grade cancer cell lines
A more specific study was performed comparing LG cancer line 5637 and HG BC cell lines J82 and Scaber to search for a VOC signature with potential to assess tumour aggressiveness. Overall, 30 VOCs were found to be significantly altered at pH 7, being most of them decreased in HG cancer cells compared to 5637 cell line culture medium, such as acetophenone (Fig. 4a), α-terpineol, nonanal, and the tentatively identified 2-phenylpropan-2-ol, 4-methylnonane and 6-methylheptan-2-one (with an AUC = 1), among others (Table 3). VOCs that were found increased in HG cancer cells medium comprise dodecanol, dodecanal and γ-nonalactone (Table 3). Considering pH 2, only 2 of the 8-specific pH 2 VOCs were significantly altered, namely α-methylstyrene and unknown 25 (Table 3 and Supplementary Table S3).
Boxplots of a acetophenone, extracted at pH 7 and found significantly altered between the HG cancer cell lines, J82 (n = 5) and Scaber (n = 5), and the LG cancer cell line 5637 (n = 5) extracellular medium; b 2-pentadecanone and c γ-dodecalactone, two VOCs extracted at pH 7 and pH 2, respectively, and found significantly altered between the cancer cell lines J82 (HG TCC, n = 5) and Scaber (HG SCC, n = 5) extracellular medium. **p value of 7.90 × 10−3; ***p value of 7.00 × 10−4
These results suggest that VOCs levels change in a grade-dependent manner. From the compounds that contributed for discrimination between HG and LG cancer cell lines, cyclohexanone, methyl isobutyl ketone, styrene, dodecane, nonanal and benzaldehyde were already reported as significantly altered in previous in vitro (Bartolazzi et al. 2010; Chen et al. 2007; Sponring et al. 2009; Mochalski et al. 2013; Filipiak et al. 2010), ex vivo human tissues (Abaffy et al. 2010; Filipiak et al. 2014) and exhaled human breath cancer studies (Altomare et al. 2013; Poli et al. 2010; Rudnicka et al. 2011; Wang et al. 2014; Filipiak et al. 2014). More importantly, dodecanal was found in urine of BC patients whereas it was not detected in controls (Jobu et al. 2012), thus being considered a potential biomarker for BC by the authors. Nevertheless, no cancer grade association was evaluated.
To the best of our knowledge, this is the first study demonstrating the potential of VOCs in discriminating BC cells with different grades, supporting the well-known idea that HG cancer cells have different energetic needs compared to LG cancer cells. Indeed, it is known that as tumours progress to more advanced stages, energetic demands of cancer cells increase to support DNA replication, protein synthesis and production of new components (such as those required for membrane formation). Energy production is also essential for cancer cells to survive in more hostile conditions (e.g., under oxidative stress) and to be able to migrate and adapt in other tissues (metastases formation) (Ferreira 2010; Hanahan and Weinberg 2011). However, despite the lack of BC metabolomic studies addressing VOCs, some in vitro, serum and urinary studies have described metabolic differences between LG BC and HG BC (for a review see Rodrigues et al. 2016a).
3.4 Volatile exometabolome signature of TCC versus SCC cancer cell lines
Because the two HG BC cell lines J82 and Scaber represent different subtypes of BC (TCC and SCC, respectively), we sought to determine which VOCs significantly differ between them. From the pH 7 analysis, we found that 14 VOCs were significantly altered in J82 compared to Scaber (Table 3). Most of those VOCs were decreased in J82, except for 2-pentadecanone (Fig. 4b), 1-phenylethanol, α-terpineol, benzaldehyde, geranylacetone and unknown 17 (Table 3 and Supplementary Table S3). The pH 2 analysis resulted in 3 significantly altered VOCs, in addition to those in common with pH 7, namely γ-dodecalactone (Fig. 4c), (2E,4E)-deca-2,4-dienal and benzoic acid (AUC = 1), which were increased in the extracellular medium of J82 compared to the one of Scaber (Table 3). Interestingly, γ-dodecalactone demonstrated specificity towards TCC cells. Similar to 2-pentadecanone, γ-dodecalactone was not detected in blanks, thus unveiling great potential as candidate biomarker.
Some of the discriminative VOCs between TCC and SCC cell lines, namely nonanal, 2-pentadecanone, n-butyl acetate, cyclohexanone, and phenol have not been associated to cancer subtypes so far. Remarkably, the results obtained herein demonstrate that different histological subtypes of BC cells present distinct VOC profiles. Hence, we can assume that VOCs change in a subtype-dependent manner, as well. TCC and SCC cell lines might present distinct genetic characteristics that could be associated with up- or down-regulation of different metabolic enzymes, favouring the production or consumption of certain compounds over others. Nevertheless, further investigation is essential to better understand the differences in the volatile exometabolomes of these two subtypes of BC.
4 Conclusions
This study demonstrates that VOC extraction at pH 7 provides a more complete profiling of the volatile exometabolome of BC cell lines, and, thus, it may constitute the first choice for subsequent in vitro metabolomic studies. Nevertheless, extraction at pH 2 clearly complements BC cell lines volatile signature, providing additional VOCs of interest. More importantly, we found that VOC levels differed when the exometabolomes of BC and normal cell lines were compared, indicating that BC presents a distinct volatilome. We also observed that VOC levels change depending on cancer grade and histological subtype. Remarkably, 2-pentadecanone and the tentatively identified γ-dodecalactone seem to be particularly important compounds, due to their cellular origin, as well as dodecanal, since it was already found in urine of BC patients (Jobu et al. 2012). These results definitively establish a proof-of-concept for the discrimination among different human BC cell lines through VOCs analysis using HS-SPME/GC–MS technique. Nevertheless, due to current shortage of information about the metabolic pathways underlying VOCs production, this study cannot provide comprehensive information on the biochemical processes involving VOCs that are affected in BC. Therefore, in vivo VOCs translation through analysis of human samples is mandatory to achieve validation of the complete BC volatilome signature.
Abbreviations
- AUC:
-
Area under the curve
- BC:
-
Bladder cancer
- ES:
-
Effect size
- GC:
-
Gas chromatography
- GC-SQ:
-
Gas chromatography-single quadrupole
- HMDB:
-
Human metabolome database
- HS-SPME:
-
Headspace solid-phase microextraction
- MS:
-
Mass spectrometry
- OPLS-DA:
-
Orthogonal partial least squares-discriminant analysis
- PCA:
-
Principal component analysis
- PLS-DA:
-
Partial least squares-discriminant analysis
- QCs:
-
Quality controls
- RI:
-
Retention index
- SCC:
-
Squamous cell carcinoma
- TA:
-
Total area
- TCC:
-
Transitional cell carcinoma
- VOCs:
-
Volatile organic compounds
- VIP:
-
Variable importance in projection
References
Abaffy, T., Duncan, R., Riemer, D. D., Tietje, O., Elgart, G., Milikowski, C., et al. (2010). Differential volatile signatures from skin, naevi and melanoma: A novel approach to detect a pathological process. PLoS ONE, 5(11), e13813. https://doi.org/10.1371/journal.pone.0013813.
Aickin, M., & Gensler, H. (1996). Adjusting for multiple testing when reporting research results: The Bonferroni vs Holm methods. American Journal of Public Health, 86(5), 726–728.
Altomare, D. F., Di Lena, M., Porcelli, F., Trizio, L., Travaglio, E., Tutino, M., et al. (2013). Exhaled volatile organic compounds identify patients with colorectal cancer. British Journal of Surgery, 100(1), 144–150. https://doi.org/10.1002/bjs.8942.
Al-Zoughbi, W., Huang, J., Paramasivan, G. S., Till, H., Pichler, M., Guertl-Lackner, B., et al. (2014). Tumor macroenvironment and metabolism. Seminars in Oncology, 41(2), 281–295. https://doi.org/10.1053/j.seminoncol.2014.02.005.
Bajtarevic, A., Ager, C., Pienz, M., Klieber, M., Schwarz, K., Ligor, M., et al. (2009). Noninvasive detection of lung cancer by analysis of exhaled breath. BMC Cancer, 9, 348. https://doi.org/10.1186/1471-2407-9-348.
Bartolazzi, A., Santonico, M., Pennazza, G., Martinelli, E., Paolesse, R., D’Amico, A., et al. (2010). A sensor array and GC study about VOCs and cancer cells. Sensors and Actuators B: Chemical, 146(2), 483–488. https://doi.org/10.1016/j.snb.2009.11.046.
Berben, L., Sereika, S. M., & Engberg, S. (2012). Effect size estimation: Methods and examples. International Journal of Nursing Studies, 49(8), 1039–1047. https://doi.org/10.1016/j.ijnurstu.2012.01.015.
Burger, M., Catto, J. W., Dalbagni, G., Grossman, H. B., Herr, H., Karakiewicz, P., et al. (2013). Epidemiology and risk factors of urothelial bladder cancer. European Urology, 63(2), 234–241. https://doi.org/10.1016/j.eururo.2012.07.033.
Calenic, B. (2013). Volatile organic compounds expression in different cell types: An in vitro approach. International Journal of Clinical Toxicology, 1, 43–51.
Cauchi, M., Weber, C. M., Bolt, B. J., Spratt, P. B., Bessant, C., Turner, D. C., et al. (2016). Evaluation of gas chromatography mass spectrometry and pattern recognition for the identification of bladder cancer from urine headspace. Analytical Methods, 8(20), 4037–4046. https://doi.org/10.1039/C6AY00400H.
Chan, E. C., Pasikanti, K. K., Hong, Y., Ho, P. C., Mahendran, R., & Raman Nee Mani, L. et al. (2015). Metabonomic profiling of bladder cancer. Journal of Proteome Research, 14(2), 587–602. https://doi.org/10.1021/pr500966h.
Chen, X., Xu, F., Wang, Y., Pan, Y., Lu, D., Wang, P., et al. (2007). A study of the volatile organic compounds exhaled by lung cancer cells in vitro for breath diagnosis. Cancer, 110(4), 835–844. https://doi.org/10.1002/cncr.22844.
Davis, V. W., Bathe, O. F., Schiller, D. E., Slupsky, C. M., & Sawyer, M. B. (2011). Metabolomics and surgical oncology: Potential role for small molecule biomarkers. Journal of Surgical Oncology, 103(5), 451–459. https://doi.org/10.1002/jso.21831.
Di Lena, M., Porcelli, F., & Altomare, D. F. (2016). Volatile organic compounds as new biomarkers for colorectal cancer: A review. Colorectal Disease, 18(7), 654–663. https://doi.org/10.1111/codi.13271.
Erhart, S., Amann, A., Haberlandt, E., Edlinger, G., Schmid, A., Filipiak, W., et al. (2009). 3-Heptanone as a potential new marker for valproic acid therapy. Journal of Breath Research, 3(1), 016004. https://doi.org/10.1088/1752-7155/3/1/016004.
Ferreira, L. M. (2010). Cancer metabolism: The Warburg effect today. Experimental and Molecular Pathology, 89(3), 372–380. https://doi.org/10.1016/j.yexmp.2010.08.006.
Filipiak, W., Filipiak, A., Sponring, A., Schmid, T., Zelger, B., Ager, C., et al. (2014). Comparative analyses of volatile organic compounds (VOCs) from patients, tumors and transformed cell lines for the validation of lung cancer-derived breath markers. Journal of Breath Research, 8(2), 027111. https://doi.org/10.1088/1752-7155/8/2/027111.
Filipiak, W., Sponring, A., Filipiak, A., Ager, C., Schubert, J., Miekisch, W., et al. (2010). TD-GC-MS analysis of volatile metabolites of human lung cancer and normal cells in vitro. Cancer Epidemiology and Prevention Biomarkers, 19(1), 182–195. https://doi.org/10.1158/1055-9965.epi-09-0162.
Fuchs, P., Loeseken, C., Schubert, J. K., & Miekisch, W. (2010). Breath gas aldehydes as biomarkers of lung cancer. International Journal of Cancer, 126(11), 2663–2670. https://doi.org/10.1002/ijc.24970.
Hakim, M., Broza, Y. Y., Barash, O., Peled, N., Phillips, M., Amann, A., et al. (2012). Volatile organic compounds of lung cancer and possible biochemical pathways. Chemical Reviews, 112(11), 5949–5966. https://doi.org/10.1021/cr300174a.
Hakimi, A. A., Reznik, E., Lee, C. H., Creighton, C. J., Brannon, A. R., Luna, A., et al. (2016). An integrated metabolic atlas of clear cell renal cell carcinoma. Cancer Cell, 29(1), 104–116. https://doi.org/10.1016/j.ccell.2015.12.004.
Hanahan, D., & Weinberg, R. A. (2011). Hallmarks of cancer: The next generation. Cell, 144(5), 646–674. https://doi.org/10.1016/j.cell.2011.02.013.
Hu, Y. J., Qiu, Y. H., Chen, E. G., Ying, K. J., Yu, J., & Wang, P. (2010). Determination of volatile organic compounds in lung cancer cell lines and lung cancer tissue. Zhejiang Da Xue Xue Bao Yi Xue Ban, 39(3), 278–284.
Huang, Z., Lin, L., Gao, Y., Chen, Y., Yan, X., Xing, J., et al. (2011). Bladder cancer determination via two urinary metabolites: A biomarker pattern approach. Molecular & Cellular Proteomics. https://doi.org/10.1074/mcp.M111.007922.
Jobu, K., Sun, C., Yoshioka, S., Yokota, J., Onogawa, M., Kawada, C., et al. (2012). Metabolomics study on the biochemical profiles of odor elements in urine of human with bladder cancer. Biological and Pharmaceutical Bulletin, 35(4), 639–642.
Khasawneh, J., Schulz, M. D., Walch, A., Rozman, J., Hrabe de Angelis, M., Klingenspor, M., et al. (2009). Inflammation and mitochondrial fatty acid beta-oxidation link obesity to early tumor promotion. Proceedings of the National Academy of Sciences USA, 106(9), 3354–3359, https://doi.org/10.1073/pnas.0802864106.
Ku, J. H., Godoy, G., Amiel, G. E., & Lerner, S. P. (2012). Urine survivin as a diagnostic biomarker for bladder cancer: A systematic review. BJU International, 110(5), 630–636. https://doi.org/10.1111/j.1464-410X.2011.10884.x.
Kwak, J., & Preti, G. (2011). Volatile disease biomarkers in breath: A critique. Current Pharmaceutical Biotechnology, 12(7), 1067–1074.
Lauridsen, M., Hansen, S. H., Jaroszewski, J. W., & Cornett, C. (2007). Human urine as test material in 1H NMR-based metabonomics: Recommendations for sample preparation and storage. Analytical Chemistry, 79(3), 1181–1186. https://doi.org/10.1021/ac061354x.
Leon, Z., Garcia-Canaveras, J. C., Donato, M. T., & Lahoz, A. (2013). Mammalian cell metabolomics: Experimental design and sample preparation. Electrophoresis, 34(19), 2762–2775. https://doi.org/10.1002/elps.201200605.
Ligor, M., Ligor, T., Bajtarevic, A., Ager, C., Pienz, M., Klieber, M., et al. (2009). Determination of volatile organic compounds in exhaled breath of patients with lung cancer using solid phase microextraction and gas chromatography mass spectrometry. Clinical Chemistry and Laboratory Medicine, 47(5), 550–560. https://doi.org/10.1515/cclm.2009.133.
Liu, Y. (2006). Fatty acid oxidation is a dominant bioenergetic pathway in prostate cancer. Prostate Cancer and Prostatic Diseases, 9(3), 230–234. https://doi.org/10.1038/sj.pcan.4500879.
McCulloch, M., Jezierski, T., Broffman, M., Hubbard, A., Turner, K., & Janecki, T. (2006). Diagnostic accuracy of canine scent detection in early- and late-stage lung and breast cancers. Integrative Cancer Therapies, 5(1), 30–39. https://doi.org/10.1177/1534735405285096.
Miremami, J., & Kyprianou, N. (2014). The promise of novel molecular markers in bladder cancer. International Journal of Molecular Sciences, 15(12), 23897–23908. https://doi.org/10.3390/ijms151223897.
Mochalski, P., Sponring, A., King, J., Unterkofler, K., Troppmair, J., & Amann, A. (2013). Release and uptake of volatile organic compounds by human hepatocellular carcinoma cells (HepG2) in vitro. Cancer Cell International, 13(1), 72. https://doi.org/10.1186/1475-2867-13-72.
Monteiro, M., Carvalho, M., Henrique, R., Jeronimo, C., Moreira, N., de Lourdes Bastos, M., et al. (2014). Analysis of volatile human urinary metabolome by solid-phase microextraction in combination with gas chromatography-mass spectrometry for biomarker discovery: Application in a pilot study to discriminate patients with renal cell carcinoma. European Journal of Cancer, 50(11), 1993–2002. https://doi.org/10.1016/j.ejca.2014.04.011.
Nunes de Paiva, M. J., Menezes, H. C., & de Lourdes Cardeal, Z. (2014). Sampling and analysis of metabolomes in biological fluids. Analyst, 139(15), 3683–3694. https://doi.org/10.1039/c4an00583j.
Pasikanti, K. K., Esuvaranathan, K., Ho, P. C., Mahendran, R., Kamaraj, R., Wu, Q. H., et al. (2010a). Noninvasive urinary metabonomic diagnosis of human bladder cancer. Journal of Proteome Research, 9(6), 2988–2995. https://doi.org/10.1021/pr901173v.
Pasikanti, K. K., Esuvaranathan, K., Hong, Y., Ho, P. C., Mahendran, R., Raman Nee Mani, L., et al (2013). Urinary metabotyping of bladder cancer using two-dimensional gas chromatography time-of-flight mass spectrometry. Journal of Proteome Research, 12(9), 3865–3873. https://doi.org/10.1021/pr4000448.
Pasikanti, K. K., Norasmara, J., Cai, S., Mahendran, R., Esuvaranathan, K., Ho, P. C., et al. (2010b). Metabolic footprinting of tumorigenic and nontumorigenic uroepithelial cells using two-dimensional gas chromatography time-of-flight mass spectrometry. Analytical and Bioanalytical Chemistry, 398(3), 1285–1293. https://doi.org/10.1007/s00216-010-4055-3.
Peng, G., Hakim, M., Broza, Y. Y., Billan, S., Abdah-Bortnyak, R., Kuten, A., et al. (2010). Detection of lung, breast, colorectal, and prostate cancers from exhaled breath using a single array of nanosensors. British Journal of Cancer, 103(4), 542–551. https://doi.org/10.1038/sj.bjc.6605810.
Phillips, M., Cataneo, R. N., Greenberg, J., Gunawardena, R., Naidu, A., & Rahbari-Oskoui, F. (2000). Effect of age on the breath methylated alkane contour, a display of apparent new markers of oxidative stress. The Journal of Laboratory and Clinical Medicine, 136(3), 243–249. https://doi.org/10.1067/mlc.2000.108943.
Pickel, D., Manucy, G. P., Walker, D. B., Hall, S. B., & Walker, J. C. (2004). Evidence for canine olfactory detection of melanoma. Applied Animal Behaviour Science, 89(1), 107–116. https://doi.org/10.1016/j.applanim.2004.04.008.
Pluskal, T., Castillo, S., Villar-Briones, A., & Oresic, M. (2010). MZmine 2: Modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC Bioinformatics, 11, 395. https://doi.org/10.1186/1471-2105-11-395.
Poli, D., Goldoni, M., Corradi, M., Acampa, O., Carbognani, P., Internullo, E., et al. (2010). Determination of aldehydes in exhaled breath of patients with lung cancer by means of on-fiber-derivatisation SPME-GC/MS. Journal of Chromatography B, 878(27), 2643–2651. https://doi.org/10.1016/j.jchromb.2010.01.022.
Putluri, N., Shojaie, A., Vasu, V. T., Vareed, S. K., Nalluri, S., Putluri, V., et al. (2011). Metabolomic profiling reveals potential markers and bioprocesses altered in bladder cancer progression. Cancer Research, 71(24), 7376–7386. https://doi.org/10.1158/0008-5472.can-11-1154.
Rodrigues, D., Jeronimo, C., Henrique, R., Belo, L., de Lourdes Bastos, M., de Pinho, P. G., et al. (2016a). Biomarkers in bladder cancer: A metabolomic approach using in vitro and ex vivo model systems. International Journal of Cancer, 139(2), 256–268. https://doi.org/10.1002/ijc.30016.
Rodrigues, D., Monteiro, M., Jeronimo, C., Henrique, R., Belo, L., Bastos, M. L., et al. (2016b). Renal cell carcinoma: A critical analysis of metabolomic biomarkers emerging from current model systems. Translational Research. https://doi.org/10.1016/j.trsl.2016.07.018.
Rudnicka, J., Kowalkowski, T., Ligor, T., & Buszewski, B. (2011). Determination of volatile organic compounds as biomarkers of lung cancer by SPME-GC-TOF/MS and chemometrics. Journal of Chromatography B, 879(30), 3360–3366. https://doi.org/10.1016/j.jchromb.2011.09.001.
Schmidt, K., & Podmore, I. (2015). Current challenges in volatile organic compounds analysis as potential biomarkers of cancer. Journal of Biomarkers. https://doi.org/10.1155/2015/981458.
Shirasu, M., & Touhara, K. (2011). The scent of disease: Volatile organic compounds of the human body related to disease and disorder. The Journal of Biochemistry, 150(3), 257–266. https://doi.org/10.1093/jb/mvr090.
Silva, C. L., Passos, M., & Camara, J. S. (2011). Investigation of urinary volatile organic metabolites as potential cancer biomarkers by solid-phase microextraction in combination with gas chromatography-mass spectrometry. British Journal of Cancer, 105(12), 1894–1904. https://doi.org/10.1038/bjc.2011.437.
Sponring, A., Filipiak, W., Mikoviny, T., Ager, C., Schubert, J., Miekisch, W., et al. (2009). Release of volatile organic compounds from the lung cancer cell line NCI-H2087 in vitro. Anticancer Research, 29(1), 419–426.
Wang, C., Ke, C., Wang, X., Chi, C., Guo, L., Luo, S., et al. (2014). Noninvasive detection of colorectal cancer by analysis of exhaled breath. Analytical and Bioanalytical Chemistry, 406(19), 4757–4763. https://doi.org/10.1007/s00216-014-7865-x.
Willis, C. M., Church, S. M., Guest, C. M., Cook, W. A., McCarthy, N., Bransbury, A., et al. (2004). Olfactory detection of human bladder cancer by dogs: Proof of principle study. BMJ, 329, 712.
Wishart, D. S., Tzur, D., Knox, C., Eisner, R., Guo, A. C., Young, N., et al. (2007). HMDB: The Human Metabolome Database. Nucleic Acids Research, 35(Database issue), D521–D526. https://doi.org/10.1093/nar/gkl923.
Worley, B., & Powers, R. (2013). Multivariate analysis in metabolomics. Current Metabolomics, 1(1), 92–107. https://doi.org/10.2174/2213235x11301010092.
Zhang, Z., & Pawliszyn, J. (1993). Headspace solid-phase microextraction. Analytical Chemistry, 65(14), 1843–1852. https://doi.org/10.1021/ac00062a008.
Zimmermann, D., Hartmann, M., Moyer, M. P., Nolte, J., & Baumbach, J. I. (2007). Determination of volatile products of human colon cell line metabolism by GC/MS analysis. Metabolomics, 3(1), 13–17. https://doi.org/10.1007/s11306-006-0038-y.
Acknowledgements
This work received financial support from the European Union (FEDER funds POCI/01/0145/FEDER/007728) and National Funds (FCT/MEC, Fundação para a Ciência e Tecnologia and Ministério da Educação e Ciência) under the Partnership Agreement PT2020 UID/MULTI/04378/2013. This study is a result of the project NORTE-01-0145-FEDER-000024, supported by Norte Portugal Regional Operational Programme (NORTE 2020), under the PORTUGAL 2020 Partnership Agreement (DESignBIOtecHealth - New Technologies for three Health Challenges of Modern Societies: Diabetes, Drug Abuse and Kidney Diseases), through the European Regional Development Fund (ERDF). C.J.’s research is funded by a research grant from Research Center of Portuguese Oncology Institute of Porto (FB-GEBC-27) and S.M.-R. is a PhD fellow from Fundação para a Ciência e Tecnología (FCT SFRH/BD/112673/2015). M.C. acknowledges FCT through the project UID/Multi/04546/2013.
Author information
Authors and Affiliations
Contributions
DR was responsible for the execution of the experimental work and data analysis. AMA supported cell culture and data analysis. JP helped with the statistical analysis of the data. SM-R, CJ and RH kindly provided the cell lines used in the study and gave conceptual advice. PGP, MLB and MC designed and supervised the study. DR wrote the manuscript with input from MC. All authors critically commented on and approved the final submitted version of the paper.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests in relation to the work described.
Research involving human and animals participants
This article does not contain any studies with human participants or animals.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rodrigues, D., Pinto, J., Araújo, A.M. et al. Volatile metabolomic signature of bladder cancer cell lines based on gas chromatography–mass spectrometry. Metabolomics 14, 62 (2018). https://doi.org/10.1007/s11306-018-1361-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11306-018-1361-9