Abstract
We evaluated changes in the succession process in benthic algal communities of an afforested stream by comparing them with those in a grassland stream through analysis of temporal changes in composition, structure and functional traits in a 3-month experiment. We hypothesized that sunlight intensity reduction as a result of streamside implanted tree shading reduces the succession rate and community physiognomic complexity. We selected two streams with different riparian cover (grassland and pine afforestation) for installation of unglazed tiles as artificial substrates. The tiles were collected from each stream after 23, 45 and 73 days of exposure. Afforestation produced changes in algal succession, with lower biomass, lower diversity at the beginning of the experiment, and an increase in the proportion of small, low-profile, tolerant, unicellular and stalked algae, coinciding with the predominance of Achnanthidium. However, the grassland stream contained a higher proportion of intermediate-size algae, as well as a larger proportion of high-profile algae, coinciding with the expected results. In the afforested stream, succession proceeded toward the dominance of low-profile species, that were tolerant of low-light conditions. However, in grassland streams with higher sunlight availability, high-profile algae prevailed without the displacement of tolerant forms. Overall, our results indicate that algal communities in afforested stream remain structurally simpler.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Species replacement through time and the mechanisms involved in succession have long been studied in aquatic attached algal communities such as periphyton (Hudon and Bourget 1981; Hoagland et al. 1982; McCormick and Stevenson 1991; Tuji 2000; Passy and Larson 2011). Hoagland et al. (1982) proposed that the three-dimensional structure of periphytic communities through time indicates that their microsuccession is analogous to vascular plant succession, with changes in their vertical structure tending to be dominated by organisms of large stature (owing to cell size or long stalks), and the progressive decrease in succession rates. Tuji (2000) agreed that stalked species develop in later phases of succession, but proposed a new phase, with tangled-type and rosette-type species developing in the upper part of the biofilm, tangled among the stalks.
Some authors have related microalgal succession patterns with those occurring in plants communities (Odum 1969; Hoagland et al. 1982; Steinman and McIntire 1986). However, it is still not clear if stream periphyton follows the pattern from low-profile to high-profile species described for plants, or how environmental factors such as light, current, dissolved substances or nutrients interact in the three-dimensional spatial complexity, affecting succession (Dudley and D’Antonio 1991; Larson and Passy 2012). These factors may be altered by riparian vegetation changes, particularly when grasslands are afforested with perennial tree species without buffer strips.
Monoculture plantation of rapid growth trees in grassland ecosystems has become an accepted land use practice in semiarid areas around the world (Simberloff et al. 2010), resulting in profits for timber and labour production, and enhancing sequestration of carbon from the atmosphere. While those benefits are unquestionable, scientists have pointed out unintended negative effects of such practices, most of which are related to the biogeochemical and hydrological balance of afforested catchments and their potential effects on available water quality and quantity (Farley et al. 2005; Jackson et al. 2005; Mátyás and Sun 2014). However, there is a gap in knowledge regarding how aquatic biodiversity is affected. In Argentina, semiarid lands were extensively afforested with exotic evergreen pines during the 1970s as a result of a tax deferral plan implemented by the government. In our study area (Córdoba Province in central Argentina), up to 36,000 ha of mountain grasslands have been converted to pine plantations, which produced changes in the stream algal community (Cibils et al. 2015); however, the effects on succession patterns are still not well known.
Streamside implanted trees have reduced sunlight inputs to streams for years, diminishing the physiognomic options of members of periphytic algal communities. Structural complexity can only develop under ample light conditions (Lowe et al. 1986; Hill 1996; Lange et al. 2011), and light is important to community structure and functioning (Roberts et al. 2004); therefore, it is likely that the succession patterns of algal communities in the study area have been strongly altered. Large species and high-profile algae have a competitive advantage for light against non-motile, prostrate taxa (i.e., low-profile species) (Boston and Hill 1991; Hill 1996; Lange et al. 2011), which may show partial shade adaptation (Hill et al. 1995). Passy (2008) reported that algae with a differential position in the biofilm matrix may have different tolerance to nutrient limitations. In this sense, large and high-profile species may be considered sensitive to light limitations, whereas low-profile algae would be tolerant to lower light intensities. Passy and Larson (2011) proposed that direction and rate of succession are driven by the environment through its influence on sensitive forms. Hence, we expected succession in afforested streams to be limited by shade, which mainly affects sensitive species with higher light demands.
The rapid development and short generation times of benthic algae allow the study of succession in the field, which enables further analysis of the effects of land use changes on this process. Therefore, we evaluated the changes in the succession process in benthic algal communities of an afforested stream by comparison to those in a grassland stream through analysis of temporal changes in composition, structure and functional traits in a 3-month experiment. We hypothesized that reduced sunlight intensity as a consequence of streamside plantation of trees reduces the succession rate and community physiognomic complexity. We expected (1) greater differences in algal composition and structure with time in grassland streams than afforested streams, with grassland communities developing earlier and reaching higher values of biomass; and (2) a predominance of high-profile algae as late successional species in grassland streams compared to afforested streams, where only shade-tolerant low profile species would grow.
Methods
Study area
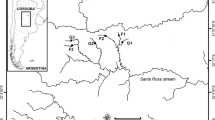
The study area included streams belonging to the headwaters of the Ctalamochita River, which are situated between 800 and 1500 m a.s.l. on the east side of the Córdoba Hills, Argentina. Vegetation varies according to altitude, with grassland developing between 1000 and 1500 m a.s.l. (mainly Festuca hieronymi Hack., Nasella spp., Schizachyrium condensatum (Kunth) Nees and Eragrostis airoides Nees; Cabido et al. 2003; Oggero and Arana 2012). Annual precipitation in the region reaches 725 mm, occurring mostly between spring and the end of summer (October–March) (Cabido et al. 2003), whereas the maximum temperature reaches 34 °C in summer (December–March), and decreases to as low as −5 °C in winter (June–September). The lithology is dominated by granite, but localized patches of metamorphic rocks (gneiss, schist, migmatite) are present. The study area is affected by anthropogenic activities, mainly livestock and afforestation with exotic pines, dominated by the slash pine Pinus elliottii Engelm. from North America (Plevich et al. 2002).
Experimental design and laboratory methods
We selected two streams with different riparian cover, one flowing entirely through grassland (31°58′47″S, 64°48′41″W, 1175 m a.s.l., drainage area 87 ha) and the other flowing entirely through a pine plantation (31°59′22″S, 64°48′44″W, 1144 m a.s.l., drainage area 89 ha). The experiment was conducted during the low flow season (July–September 2012). Water depth, current velocity, dominant substrate type and channel wet width were registered at the beginning of the experiment in four riffles each corresponding to the location of tiles in each stream. Depth, width and current velocity were measured with a digital water velocity meter (Global Flow probe FP101), while substrate type was visually assessed (Gordon et al. 1994) and assigned to a category proposed by Thomson et al. (2001). Some physicochemical parameters were measured three times at each stream during the experiment, coinciding with the extractions of tiles for algal community analysis. The PAR (photosynthetically active radiation) intensity was measured with a QSL–2100 Irradiance Sensor (Biospherical Instruments, Inc., San Diego, CA, USA) at midday along a 50 m reach in each stream. Water pH, conductivity and temperature were recorded with portable sensors. Total dissolved solids (TDS), carbonate, bicarbonate and nitrates were analyzed by the area of Hydrology, Department of Geology, National University of Río Cuarto, according to standard methods (APHA 1998). Carbonates and bicarbonates were measured by potentiometric titration with a Thermo Orion-selective electrode, while nitrates were determined by potentiometry using an ion selective electrode (Orion Model 9307), a reference electrode and an Orion potentiometer 710 A. To calibrate the potentiometer, six benchmarks (5, 10, 25, 50, 100 and 300 mg L−1 NO3 −) were used. The detection limit for NO3 − was 0.2 mg L−1 and the analytical error was 0.5%.
Thirty unglazed tiles of 7.5 × 7.5 cm were used as artificial substrates. Substrates were placed on the stream bottom at different riffles. Five tiles were collected from each stream at 23, 45 and 73 days of exposure. We considered this timescale to include several generations of algae (Hulot et al. 2000; Flöder and Hillebrand 2012). At each extraction date, tiles were taken to the laboratory and algae from the upper surface of the substrates were scrubbed off with a stiff nylon brush, after which they were rinsed with clean water and all the dislodged material was collected. The periphyton suspension was homogenized and fractionated in two subsamples. One fraction was filtered through a glass-fiber filter GF/C to extract and quantify Chl a by spectrophotometry (Nusch 1980). Next, each filter was dried at 60 °C for 48 h, weighed (to the nearest 0.01 mg), combusted at 500 °C for 1 h, and then re-weighed to determine the ash free dry mass (AFDM). The autotrophic index (AI) was then calculated from the Chl a and AFDM (AI = AFDM/Chl a) for each substrate replicate (Weber 1973). This value is indicative of the proportion of the community composed of heterotrophic (plus organic non-living matter) and autotrophic organisms. The AFDM represents the combined mass of heterotrophic and autotrophic biomass (and organic detritus), and Chl a reflects the autotrophic component (Biggs and Kilroy 2000). Biofilm is considered to be more heterotrophic for values higher than 400 (Collins and Weber 1978). Another aliquot was fixed with 4% formalin and used for species identification and density calculations. Algal communities were analyzed quantitatively at 400× magnification with organisms grouped taxonomically by genus. For each sample, three subsamples were counted following transects along the coverslip to determine cell densities (cells cm−2, based on Villafañe and Reid 1995) and taxa richness. The counting unit was an individual cell for unicellular organisms and coenobia, a 10 µm length for filaments and a 10 × 10 µm area for colonies. The biovolume was estimated by multiplying the cell densities by the average biovolume of each species according to the size of the analyzed populations and then fitting to the nearest geometric models. The results were given in µm3 cm−2 (Hillebrand et al. 1999).
Data analysis
Environmental variables were compared between grassland and afforested streams using one-way ANOVAs for those registered at the beginning of the experiment in four riffles at each stream, and repeated measures ANOVA for those measured three times at each stream. We evaluated differences in benthic algal communities between riparian vegetation types and time of exposure using non-metric multidimensional scaling (NMDS) based in the Bray–Curtis dissimilarity matrix, and performed permutational multivariate analysis of variance (PERMANOVA, Anderson 2001; McArdle and Anderson 2001) using distance matrices (Bray–Curtis) to statistically test differences between groups, with 999 permutations. To determine if groups differed in terms of their centroids not being induced by differences in variances, we used analysis of multivariate homogeneity of group dispersions (PERMDISP, Anderson 2001). Additionally, SIMPER (similarity percentages) analysis was performed to determine which species contributed most to the dissimilarity between sampling units (Clarke and Warwick 2001; Quinn and Keough 2002). All statistical analyses described above were performed in R version 3.0.1, using vegan library (Oksanen et al. 2013; R Core Team 2013).
Structural attributes of algal communities [density, richness, and Shannon diversity (H′) and evenness indices (J′)] were calculated. Shannon’s diversity index was calculated from the biovolume data as follows:
where, pi is the contribution of the ith species to the total biovolume of the community and S is species number (Hillebrand and Sommer 2000). Evenness was calculated as H′/H′max. Additionally, genera were grouped in categories of functional traits according to Cibils et al. (2015): size, morphological guild, resources requirement, attachment mechanism and life-form (Table S1). Taxa were placed into five size classes, consisting of those with different biovolumes from class 1, which included smaller species, to class 5, which contained larger species. Morphological guild was defined according to the access of taxa to resources and vulnerability to disturbances. In particular, short-statured algae constituted the low-profile guild, while tall-statured algae extending above the substrate were considered the high-profile guild and fast-moving biraphid diatoms or flagellated soft algae were considered the motile guild. Resource requirements were defined related to the tolerance or sensitivity of species to nutrients and light limitation. Algae requiring high resource levels for growth and reproduction are considered sensitive, while those proliferating under low resource levels are viewed as tolerant. The attachment mechanism determines the spatial position in biofilm and the ability to withstand disturbances. Adnates were algae firmly attached by their valve face or by their girdle view. Algae with mucilage pads secrete a small mucilage pad at one end of the valve to attach to the substrate, allowing them to stand upright. Species with mucilage stalks grow attached to surfaces by the stalks, which can be simple (one cell) or can link several cells forming arbuscular colonies. In species assigned to the holdfast category, filaments are attached by an initial cell. Unattached included species without any specific mechanism of attachment, because they float or move freely. Life-form categories are related to biofilm architecture, and the response of a community to environmental factors changes if it is dominated by unicellular forms or filamentous and colonial organisms. Diatoms which usually form chains or rosette or arbuscular aggregates, were categorized as colonies, even when they were not real colonies.
Structural attributes, Chl a, AFDM, AI variables and the proportion of algae corresponding to different functional trait categories were compared by repeated measures ANOVA. This was used to determine if differences between riparian vegetation types and time × riparian vegetation type interactive effects were significant during succession. ANOVAs were performed using InfoStat, which implements an interface of platform R to estimate general and mixed linear models (Di Rienzo et al. 2011, 2012). Validation of assumptions of the models was performed reviewing standardized residuals vs. predicted, the normal Q–Q plot of standardized residuals and the Shapiro–Wilks test. Variables that did not meet ANOVA assumptions were natural logarithm transformed. The DGC test (Di Rienzo–Guzman–Casanoves), which is a hierarchical method that controls type I error while maintaining acceptable power, was used for multiple comparisons.
Results
Environmental variables measured to characterize grasslands and afforested streams are shown in Table 1. The PAR intensity was 70% lower in afforested streams. Additionally, afforested streams showed higher conductivity than grassland streams, although ANOVA was marginally significant. Time had no effect on the variables measured in triplicated in each stream (P > 0.05). For variables measured only once at the beginning of the experiment, there might not have been differences throughout the experimental period based on previous studies in the area (see Cibils et al. 2015; Márquez et al. 2015).
Up to 88 genera were identified and counted, with 50% being diatoms, 20% Cyanobacteria, 20% Chlorophyta, 10% Charophyta, and one genus of Euglenozoa (Table S1). NMDS revealed differentiation of communities from grasslands and afforested streams (Fig. 1, stress = 0.12, PERMANOVA, F 1,24 = 8.30, P = 0.001) and differences between assemblages present at each extraction date (PERMANOVA, F 1,24 = 6.14, P = 0.001). In addition, differentiations were not due to differences in dispersion within groups (PERMDISP, F 1,25 = 0.001, P = 0.97). According to SIMPER, the genera that made the greatest contributions to differences between vegetation type and extraction dates were Fragilaria, Achnanthidium, Phormidium and Gomphonema, which are shown in Fig. 2 with two other genera that presented a relative abundance higher than 5% on some extraction dates (Encyonema and Cymbella). The mean density and biovolume of these genera at each stream type and designation to functional traits categories are shown in Table 2. Fragilaria capucina Desmazières and Achnanthidium minutissimum (Kützing) Czarnecki were the most abundant species in the study (Fig. 2). In the grassland stream, the proportion of Fragilaria decreased and that of Achnanthidium increased with time, while in afforested stream Achnanthidium also increased, dominating at the end of the experiment (Fig. 2). As the predominance of Fragilaria in the grassland stream was decreasing, some stalked species become more important, such as Cymbella cymbiformis C.Agardh, Gomphonema pumilum (Grunow) Reichardt & Lange-Bertalot and Gomphoneis herculeana, the latter having long and thick stalks. In this stream, there were also some important filamentous algae, including Bulbochaete, or mucilaginous colonies such as Tetraspora. Some frequent taxa in the grassland stream, but absent from the afforested stream were: Aphanothece, Bulbochaete, Euastrum, and among diatoms Aulacoseira granulata (Ehrenberg) Simonsen, Craticula cuspidata (Kützing) D.G.Mann, small fragilarioids and G. herculeana (Ehrenberg) Cleve.
Genera making the greatest contribution to differences between vegetation type and extraction date according to SIMPER (similarity percentages) and with relative abundance (pi) higher than 5% at some extraction dates in grassland (a) and afforested (b) streams. Ency: Encyonema, Achi: Achnanthidium, Gomp: Gomphonema, Frag: Fragilaria, Cym: Cymbella, Phor: Phormidium
In relation to structural variables, density and richness increased with time, but were not affected by riparian vegetation (Table 3; Fig. 3). However, the grassland stream had 12 more species at the end of the experiment. Diversity and evenness values were lower at the beginning of the experiment in the afforested stream, but increased at 45 days, reaching values similar to grassland communities. Chl a values increased with time in both streams, but higher values were observed in the grassland stream at the end of the experiment, while in the afforested stream it was higher at the second extraction date. AFDM varied similarly in the grassland and afforested streams, showing lower values at 23 days and higher at 45 days. As a result, the autotrophic index was more variable in the grassland stream, and at the end of the experiment the AI was low in the grassland stream (mean value = 71), but remained high in the afforested stream (mean value = 169).
Structural variables of algal communities developed on substrates of grassland (open symbols) and afforested streams (filled symbols) collected at 23, 45 and 73 days. For each variable, the mean values and standard error are shown. a Algal abundance, b richness, c Shannon diversity, d evenness, e Chl a, chlorophyll a values, f AFDM, ash free dry mass, g Ln AI, natural logarithm of autotrophic index
Streamside afforestation primarily affected the temporal dynamics of algal functional traits. The proportion of small (c1) and large algae (c5), respectively, increased and decreased with time in the afforested stream (Table 4; Fig. 4). In contrast, these size classes did not changed during succession in the grassland stream, where mid-size algae (c2) predominated (Tables 2, 4; Fig. 4). The succession of morphological guilds was also affected by afforestation, with low-profile algae increasing and high-profile algae decreasing with time. No temporal changes were observed in the algal community of the grassland stream, where high-profile algae dominated (Tables 2, 4; Fig. 4). Sensitive species were predominant in the afforested stream at 23 days, and were replaced by tolerant species. In the grassland stream, there was a higher proportion of tolerant species at the beginning of the experiment, but a similar proportion of tolerant and sensitive species at 73 days (Tables 2, 4; Fig. 4). Algae with different attachment mechanisms changed with time in both streams. Stalked algae increased in both the grassland and afforested stream. Unattached forms were dominant at 23 days in the afforested stream, while algae with pad mucilage predominated in the grassland stream, and both forms decreased with time (Tables 2, 4; Fig. 4). The proportions of algae from different life-form categories also changed with time depending on streamside vegetation (Tables 2, 4; Fig. 4). In the afforested stream, unicellular algae increased and filamentous forms decreased. In the grassland stream, the proportion of unicellular forms was lower than in the afforested stream, but they also increased with time, while filamentous forms comprised <1%. Colonial forms decreased with time in the grassland stream and were kept in low proportion in the afforested stream. Coenobial algae (e.g., Scenedesmus, Pediastrum) presented a low proportion throughout the experimental period in both streams.
Variation in relative abundance (pi) of the different categories of functional traits of algae developed on substrates of grassland and afforested streams collected at 23, 45 and 73 days. For each variable, mean values and standard error are shown. a Size classes in afforestation, b size classes in grassland, c morphological guild in afforestation, d morphological guild in grassland, e resources requirements in afforestation, f resources requirements in grassland, g attachment mechanism in afforestation, h attachment mechanism in grassland, i life form in afforestation, j life form in grassland. Categories with a proportion <1% were not plotted (i.e., c3, c4, adnates, holdfast, coenobial). References: size classes: c1 <99 µm3, c2 100–299 µm3, c3 300–599 µm3, c4 600–1499 µm3, c5 >1500 µm3. Morphological guilds: low-profile: short-statured algae; high-profile: tall-statured algae; motile: fast-moving biraphid diatoms or flagellated soft algae. Resources requirements: sensitive: algae requiring high resource levels; tolerant: low resource requirements
Discussion
Our study revealed that afforestation of grassland streams changes the succession dynamic of algal communities. We expected higher differences with time in the grassland stream, and we indeed found earlier growth and higher biomass in these algal assemblages. Most structural attributes showed different trajectories under pine afforestation (i.e., richness, diversity, evenness, biomass as Chl a and the autotrophic index) compared to grassland streams. Regarding functional traits, succession in the afforested stream was differentiated by an increase in the proportion of small, low-profile, tolerant, unicellular and stalked algae, which coincided with the predominance of Achnanthidium. Instead, a higher proportion of intermediate-size algae was registered in grassland streams, as well as an increase in the proportion of high-profile algae, coinciding with the expected results, which is coincident with the results reported by Lange et al. (2011). These findings for the grassland stream can be compared with the increase in structural complexity described for microalgal communities (Hoagland et al. 1982; Steinman and McIntire 1986; Hill 1996; Lange et al. 2011), macroalgae (Branco et al. 2005) and plants of tropical and deciduous forests (Terborgh 1985; Guariguata and Ostertag 2001).
Communities from grassland streams reached higher density and biomass. Similarly, Villeneuve et al. (2010) reported an increase in periphyton biomass and algal density at higher light intensities, and other researchers have found that colonization is slower at low light intensities and temperature (Stevenson 1996; Díaz Villanueva and Modenutti 2004). Accordingly, even when algal density increased through time in the afforested stream, it was at a lower rate compared to grassland. Furthermore, some authors proposed that algae could compensate for low light intensities by increasing their cellular Chl a content (Wellnitz and Ward 2000; Roberts et al. 2004). This could explain the high values of Chl a in the afforested stream at 45 days. This compensation mechanism was also inferred in a previous experiment in the area, in which similar values of Chl a concentration and AFDM were found between grassland and afforested streams in a 30-day community (Principe et al. 2015).
Temporal changes in periphyton growth can be conceptualized in three phases after a major flood event: colonization, exponential growth and sloughing (Biggs 1996; Biggs and Stokseth 1996). At the beginning, there is low biomass and high diversity. Over time, biomass and cell density increase until reaching a plateau, while diversity decreases, although some processes such as detachment can alter these phases (Roemer et al. 1984; Boulêtreau et al. 2006). In our reference grassland stream, density, richness and Chl a increased with time. However, in the afforested stream we noticed a decrease in these variables, but an increase in diversity with time given that, at the beginning of the experiment, the total biovolume of the community was dominated by Phormidium, which was subsequently dislodged and replaced by diatoms. This resulted in a more equitable and therefore more diverse community. The results obtained with structural variables could reflect differences in the succession process with different riparian vegetation, with a longer accrual phase occurring in grassland streams. Other researchers have reported altered duration of phases given differences in hydrological (Artigas et al. Artigas et al. 2012) or pollution (Duong et al. 2007) regimes. Our results could also reflect inhibition of the growth of microorganisms given the release of substances from large needle packs present year round in afforested streams (Bärlocher and Oertli Bärlocher and Oertli 1978a, b). Our experiment coincided with the low discharge period (i.e., winter-early spring dry season), which may have led to higher ambient leachate concentrations from this detritus accumulation. In addition, the physical wearing down by needle packs accumulated on the algal communities could occur in afforested streams, though further experiments are needed to fully understand these effects.
The identity of dominant taxa was heavily altered by afforestation. The most abundant species were Fragilaria capucina and A. minutissimum, which is in agreement with the results of other succession studies (Sekar et al. 2002; Díaz Villanueva and Modenutti 2004; Villeneuve et al. 2010). A. minutissimum has been cited as an early colonizer (Korte and Blinn 1983; Roemer et al. 1984; Stevenson et al. 1991), though in our study its proportion increased with time, both in the grassland and afforested streams, possibly because of its high immigration rate (Stevenson et al. 1991). The increase through time of some stalked species in the grassland stream (C. cymbiformis, G. pumilum, G. herculeana) is in agreement with the results proposed by other authors (Hoagland et al. 1982; Tuji 2000), especially that of G. herculeana, which has long and thick stalks. In this stream, there were also important filamentous algae such as Bulbochaete, and mucilaginous colonies such as Tetraspora. In agreement with these results, Wu et al. (1999) highlighted the importance of filamentous algae and stalks as structural components of the assemblage during the final stages of succession. Roemer et al. (1984) mentioned that the mucilage of diatoms affects community structure, since it allows the attachment of other algae (e.g., A. minutissimum) and vertical stratification of the community. Similarly, Tuji (2000) noted that stalks could be a secondary substrate for the colonization of algae loosely attached in the final stage of succession. In the afforested stream, the community succeeded to one dominated by A. minutissimum, which is likely explained by low sunlight intensities that usually prevent the settlement of larger algae (Lamberti et al. 1989; Lange et al. 2011), together with the shade-tolerance characteristics of A. minutissimum (Johnson et al. 1997; Díaz Villanueva and Modenutti 2004). This behavior is analogous to that of non-pioneer species able to germinate and establish under forest shade (Swaine and Whitmore 1988).
The observed trend in the afforested stream was toward the predominance of small, low-profile, tolerant species, coinciding with other studies that showed a mature community dominated by low-profile algae forming a thin and simple layer under low resource availability (Hudon and Bourget 1981, 1983; Passy and Larson 2011; Hlúbiková et al. 2014; Cibils et al. 2015). In contrast, all species could grow in high sunlight availability, as occurred in the grassland stream, with predominance of high-profile species that accumulated higher amounts of biomass (i.e., Chl a concentration) and formed a superior thick layer without eliminating tolerant forms (Passy 2008; Passy and Larson 2011). We also observed that communities from the grassland stream reached higher algal biomass and richness, which was expected, and supported the assumption that reduced sunlight intensity as a result of trees planted along streams reduces the succession rate and community physiognomic complexity.
References
Anderson MJ (2001) A new method for non-parametric multivariate analysis of variance. Austral Ecol 26:32–46
APHA (1998) Standard methods for examination of water and wastewater, 20th edn. American Public Health Association, American Water Works Association and the Water and Environment Federation, Washington, DC
Artigas J, Fund K, Kirchen S, Morin S, Obst U, Romaní AM, Sabater S, Schwartz T (2012) Patterns of biofilm formation in two streams from different bioclimatic regions: analysis of microbial community structure and metabolism. Hydrobiologia 695:83–96
Bärlocher F, Oertli JJ (1978a) Colonization of conifer needles by aquatic hyphomycetes. Can J Bot 56:57–62
Bärlocher F, Oertli JJ (1978b) Inhibitors of aquatic hyphomycetes in dead conifer needles. Mycologia 70:964–974
Biggs BJF (1996) Patterns in benthic algae of streams. In: Stevenson RJ, Bothwell ML, Lowe RL (eds) Algal ecology: freshwater benthic ecosystems. Academic Press Inc, San Diego, pp 31–56
Biggs BJF, Kilroy C (2000) Stream periphyton monitoring manual. Publishers NIWA for the New Zealand Ministry for the Environment, New Zealand
Biggs BJF, Stokseth S (1996) Hydraulic habitat suitability for periphyton in rivers. Regul rivers 12:251–261
Boston HL, Hill WR (1991) Photosynthesis-light relations of stream periphyton communities. Limnol Oceanogr 36:644–656
Boulêtreau S, Garabétian F, Sauvage S, Sánchez-Pérez JM (2006) Assessing the importance of a self-generated detachment process in river biofilm models. Freshw Biol 51:901–912
Branco CCZ, Branco LHZ, Moura MO, Bertusso FR (2005) The succession dynamics of a macroalgal community after a flood disturbance in a tropical stream from São Paulo State, southeastern Brazil. Revista Brasil Bot 28:267–275
Cabido D, Cabido M, Garre SM, Gorgas JA, Miatello R, Rambaldi S, Ravelo A, Tassile JL (2003) Regiones Naturales de la Provincia de Córdoba, Serie C. Publicaciones Técnicas, Agencia Córdoba, Córdoba
Cibils L, Principe R, Márquez J, Gari N, Albariño R (2015) Functional diversity of algal communities from headwater grassland streams: how does it change following afforestation? Aquat Ecol 49:453–466
Clarke KR, Warwick RM (2001) A further biodiversity index applicable to species lists: variation in taxonomic distinctness. Mar Ecol Prog Ser 216:265–278
Collins GB, Weber CT (1978) Phycoperiphyton (algae) as indicators of water quality. T Am Microsc Soc 97:36–43
Di Rienzo JA, Macchiavelli RE, Casanoves F (2011) Modelos lineales mixtos: aplicaciones en InfoStat, 1a edn. Grupo Infostat, Córdoba
Di Rienzo JA, Casanoves F, Balzarini MG, Gonzalez L, Tablada M, Robledo CW (2012) InfoStat versión 2012. Grupo InfoStat, FCA, Universidad Nacional de Córdoba, Argentina. http://www.infostat.com.ar
Díaz Villanueva V, Modenutti B (2004) Experimental analysis of grazing by the mayfly Meridialaris chiloeensis on different successional stages of stream periphyton. Internat Rev Hydrobiol 89:263–277
Dudley TL, D’Antonio CM (1991) The effects of substrate texture, grazing, and disturbance on macroalgal establishment in streams. Ecology 72:297–309
Duong TT, Feurtet-Mazel A, Coste M, Dang DK, Boudou A (2007) Dynamics of diatom colonization process in some rivers influenced by urban pollution (Hanoi, Vietnam). Ecol Ind 7:839–851
Farley KA, Jobbágy EG, Jackson RB (2005) Effects of afforestation on water yield: a global synthesis with implications for policy. Glob Change Biol 11:1565–1576
Flöder S, Hillebrand H (2012) Species traits and species diversity affect community stability in a multiple stressor framework. Aquat Biol 17:197–209
Gordon ND, Mcmahon TA, Finlayson BL (1994) Stream hydrology, an introduction for ecologists. Wiley, New York
Guariguata MR, Ostertag R (2001) Neotropical secondary forest succession: changes in structural and functional characteristics. Forest Ecol Manag 148:185–206
Hill WR (1996) Effects of light. In: Stevenson RJ, Bothwell ML, Lowe RL (eds) Algal ecology: freshwater benthic ecosystems. Academic Press Inc, San Diego, pp 121–148
Hill WR, Ryon MG, Schilling EM (1995) Light limitation in a stream ecosystem: responses by primary producers and consumers. Ecology 76:1297–1309
Hillebrand H, Sommer U (2000) Diversity of benthic microalgae in response to colonization time and eutrophication. Aquat Bot 67:221–236
Hillebrand H, Dürselen CD, Kirschtel D, Pollingher U, Zohary T (1999) Biovolume calculation for pelagic and benthic microalgae. J Phycol 35:403–424
Hlúbiková D, Novais MH, Dohet A, Hoffmann L, Ector L (2014) Effect of riparian vegetation on diatom assemblages in headwater streams under different land uses. Sci Total Environ 475:234–247
Hoagland KD, Roemer SC, Rosowski JR (1982) Colonization and community structure of two periphyton assemblages, with emphasis on the diatoms (Bacillariophyceae). Am J Bot 69:188–213
Hudon C, Bourget E (1981) Initial colonization of artificial substrate: community development and structure studied by scanning electron microscopy. Can J Fish Aquat Sci 38:1371–1384
Hudon C, Bourget E (1983) The effect of light on the vertical structure of epibenthic diatom communities. Bot Mar 26:317–330
Hulot FD, Lacroix G, Lescher-Moutoué F, Loreau M (2000) Functional diversity governs ecosystem response to nutrient enrichment. Nature 405:340–344
Jackson RB, Jobbágy EG, Avissar R, Roy SB, Barrett D, Cook CW, Farley KA, Le Maitre DC, McCarl BA, Murray BC (2005) Trading water for carbon with biological carbon sequestration. Science 310:1944–1947
Johnson RE, Tuchman NC, Peterson CG (1997) Changes in the vertical microdistribution of diatoms within a developing periphyton mat. J N Am Benthol Soc 16:503–519
Korte VL, Blinn DW (1983) Diatom colonization on artificial substrata in pool and riffle zones studied by light and scanning electron microscopy. J Phycol 19:332–341
Lamberti GA, Gregory SV, Ashkenas LR, Steinman AD, McIntire CD (1989) Productive capacity of periphyton as a determinant of plant–herbivore interactions in streams. Ecology 70:1840–1856
Lange K, Liess A, Piggott JJ, Townsend CR, Matthaei CD (2011) Light, nutrients and grazing interact to determine stream diatom community composition and functional group structure. Freshw Biol 56:264–278
Larson CA, Passy SI (2012) Taxonomic and functional composition of the algal benthos exhibits similar successional trends in response to nutrient supply and current velocity. FEMS Microbiol Ecol 80:352–362
Lowe RL, Golladay SW, Webster JR (1986) Periphyton response to nutrient manipulation in streams draining clearcut and forested watersheds. J N Am Benthol Soc 5:221–229
Márquez J, Cibils L, Principe R, Albariño R (2015) Stream macroinvertebrate communities change with grassland afforestation in central Argentina. Limnologica 53:17–25
Mátyás C, Sun G (2014) Forests in a water limited world under climate change. Environ Res Lett 9:1–10
McArdle BH, Anderson MJ (2001) Fitting multivariate models to community data: a comment on distance-based redundancy analysis. Ecology 82:290–297
McCormick PV, Stevenson RJ (1991) Mechanisms of benthic algal succession in lotic environments. Ecology 72:1835–1848
Nusch EA (1980) Comparison of different methods for chlorophyll and phaeopigment determination. Arch Hydrobiol 14:14–36
Odum EP (1969) Strategy of ecosystem development. Science 164:262–270
Oggero A, Arana M (2012) Inventario de la biodiversidad de plantas vasculares del sur de la zona serrana de Córdoba, Argentina. Hoehnea 39:171–199
Oksanen J, Guillaume Blanchet F, Kindt R, Legendre P, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Henry M, Stevens H, Wagner H (2013) Vegan: community ecology package. R package version 2.0–8
Passy SI (2008) Continental diatom biodiversity in stream benthos declines as more nutrients become limiting. Proc Natl Acad Sci USA 105:9663–9667
Passy SI, Larson CA (2011) Succession in stream biofilms is an environmentally driven gradient of stress tolerance. Microb Ecol 62:414–424
Plevich J, Nuñez C, Cantero J, Demaestri M, Viale S (2002) Biomasa del pastizal bajo diferentes densidades de pino (Pinus elliottii). Agroforestería en las Américas 9:19–23
Principe R, Márquez J, Cibils Martina L, Jobbágy EG, Albariño R (2015) Pine afforestation changes more strongly community structure than ecosystem functioning in grassland mountain streams. Ecol Indic 57:366–375
Quinn GP, Keough MJ (2002) Experimental design and data analysis for biologists. Cambridge University Press, Cambridge
R Core Team (2013) R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. http://www.R-project.org/
Roberts S, Sabater S, Beardall J (2004) Benthic microalgal colonization in streams of differing riparian cover and light availability. J Phycol 40:1004–1012
Roemer SC, Hoagland KD, Rosowski JR (1984) Development of a freshwater periphyton community as influenced by diatom mucilages. Can J Bot 62:1799–1813
Sekar R, Nair KVK, Rao VNR, Venugopalan VP (2002) Nutrient dynamics and successional changes in a lentic freshwater biofilm. Freshw Biol 47:1893–1907
Simberloff D, Nuñez MA, Ledgard NJ, Pauchard A, Richardson DM, Sarasola M, Vanwilgen VW, Zalba SM, Zenni RD, Bustamante R et al (2010) Spread and impact of introduced conifers in South America: lessons from other southern hemisphere regions. Austral Ecol 35:489–504
Steinman AD, McIntire CD (1986) Effects of current velocity and light energy on the structure of periphyton assemblages in laboratory streams. J Phycol 22:352–361
Stevenson RJ (1996) An introduction to algal ecology in freshwater benthic habitats. In: Stevenson RJ, Bothwell ML, Lowe RL (eds) Algal ecology: freshwater benthic ecosystems. Academic Press Inc, San Diego, pp 3–30
Stevenson RJ, Peterson CG, Kirschtel DB, King CC, Tuchman NC (1991) Density-dependent growth, ecological strategies and effects of nutrients and shading on benthic diatom succession in streams. J Phycol 27:59–69
Swaine MD, Whitmore TC (1988) On the definition of ecological species groups in tropical rain forests. Vegetatio 75:81–86
Terborgh J (1985) The vertical component of plant species diversity in temperate and tropical forests. Am Natur 126:760–776
Thomson JR, Taylor MP, Fryirs KA, Brierley GJ (2001) A geomorphological framework for river characterization and habitat assessment. Aquat Conserv 11:373–389
Tuji A (2000) Observation of developmental processes in loosely attached diatom (Bacillariophyceae) communities. Phycol Res 48:75–84
Villafañe VE, Reid FMH (1995) Métodos de microscopía para la cuantificación del fitoplancton. In: Alveal K, Ferrario ME, Oliveira EC, Sar E (eds) Manual de Métodos Ficológicos. Universitaria, Concepción, Chile, pp 169–185
Villeneuve A, Montuelle B, Bouchez A (2010) Influence of slight differences in environmental conditions (light, hydrodynamics) on the structure and function of periphyton. Aquat Sci 72:33–44
Weber CI (1973) Biological monitoring of the aquatic environment. In: Cairns J Jr, Dickson KL (eds) Biological methods for the assessment of water quality, ASTM STP 528. American Society of Testing and Materials, Philadelphia, USA, pp 46–60
Wellnitz TA, Ward JV (2000) Herbivory and irradiance shape periphytic architecture in a Swiss alpine stream. Limnol Oceanogr 45:64–75
Wu Y, Antoine SE, Bowker DW (1999) Colonisation of epilithic diatom population of the river Taff, South Wales, UK. Limnologica 29:174–185
Acknowledgements
We thank the two reviewers, two editors and the editorial coordinator, who greatly improved the quality of the manuscript. This work was funded by the Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT, Grant PICT 1113), Ministerio de Ciencia y Tecnología de la provincia de Córdoba (MinCyT Córdoba, Grant GRF 08), and Secretaría de Ciencia y Técnica Universidad Nacional de Río Cuarto. LCM and JM were supported by fellowships of Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Argentina.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Cibils-Martina, L., Principe, R.E., Márquez, J.A. et al. Succession of algal communities in headwaters: a comparison of pine afforested and natural grassland streams. Ecol Res 32, 423–434 (2017). https://doi.org/10.1007/s11284-017-1455-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11284-017-1455-2