Abstract
The century-long research on succession has bestowed us with a number of theories, but little agreement on what causes species replacements through time. The majority of studies has explored the temporal trends of individual species in plant and much less so in microbial communities, arguing that interspecific interactions, especially competition, play a key role in community organization throughout succession. In this experimental investigation of periphytic succession in re-circulating laboratory streams, we examined the density and the relative abundance of diatoms and soft algae for 35 days across gradients of low to high nutrient supply (nitrogen + phosphorus) and low to intermediate current velocity (10 vs. 30 cm·s−1). All algal species were classified into trophic groups and morphological guilds, both of which responded more strongly to nutrient than current velocity manipulations, as shown by regression analyses. We concluded that within the manipulated environmental ranges: (1) Succession was a gradient of stress tolerance, driven primarily by nutrient supply and secondarily, by current velocity. Nutrient supply had a qualitative effect in determining whether the contribution of species tolerant vs. sensitive to nutrient limitation would increase through time, while current velocity had a quantitative influence and affected only the rate of this increase. (2) The mechanism of algal succession at a functional level was a neutral coexistence, whereby the tolerant low profile guild maintained high density when overgrown by sensitive species, while sensitive species, constituting mostly the motile and high profile guilds, were neither facilitated nor inhibited by tolerant species but controlled by the environment. It is suggested that the mechanism of succession may depend on the level of biological organization with interspecific interactions giving way to neutral coexistence along the hierarchy from species to functional groups. Considering that the functional makeup is strictly environmentally defined, while species composition reflects local and regional species pools that may exhibit substantial geographic variability, succession is deterministic at a functional level but stochastic at a species level.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The study of ecological succession has over a century-long history and a record of prominent but frequently opposing theories, venturing to explain the mechanisms that drive species replacements through time. The emphasis has been either on the individual species and their environmental requirements or on the interactions among species, ranging from positive, e.g., facilitation, to negative, e.g., inhibition and competition [6, 7, 11, 14]. Although succession probably results from the operation of several mechanisms [33, 34, 42], competition for limiting resources has received the most attention due to a strong plant bias in the field [7, 23, 40].
Like plants, algae are controlled by resource supply [31]; however, unlike plant communities, the algal benthos functions as a spatially complex three-dimensional entity, where later recruits overgrow earlier colonizers and consume the resources coming from the water column (both nutrients and light). Unless capable of alternative ways of resource acquisition, such as heterotrophy or nitrogen fixation, earlier colonizers subsist on what is left from the overstory because internal nutrient recycling is insufficient for positive growth [39]. The proposed mechanism of succession in these 3D communities is tolerance [1, 25], which, however, can assume different forms. In the subtidal zone, early encrusting macroalgae coexisted with later overgrowth by turf-forming immigrants without experiencing reduction in growth and reproduction [1]. A combination of two mechanisms, including passive and active tolerance, described the successional process in stream microalgae [25]. Earlier stages were marked by coexistence of species with different growth forms and life history strategies, consistent with the passive tolerance model [34]. The successional mechanism switched to active tolerance in later stages when later immigrants, having better access to resources, suppressed the growth rates of early colonizers in both low and high current regimes [25]. However, the role of resource supply in these processes, alone or in conjunction with current velocity, remains elusive even though both nutrients and disturbance have been identified as drivers of vegetation dynamics [33] and resource supply, as a major constraint on algal succession in streams [15].
According to a recently proposed benthic model, a nutrient gradient generates biofilms of increasing thickness and diversity, where diatom coexistence is achieved via a still unexplored tradeoff between tolerance to resource limitation and spatial position in the biofilm [30]. If this hypothesis is true, then nutrient supply is expected to drive the biofilm successional changes, which would encompass not only the taxonomic identity but also the growth habit and nutrient requirements of the establishing colonizers. There are two separate and unrelated diatom functional classifications that are of relevance here, i.e., morphological and nutritional. Specifically, three diatom morphological guilds were distinguished based on species prominence from substrate and motility, i.e., low profile (short-statured species), high profile (tall-statured forms), and motile (fast-moving species) [29]. Additionally, two nutrient groups were identified, namely tolerant, with low resource requirements (i.e., oligotrophic, mesotrophic, and indifferent species) and sensitive, with high nutrient demands (i.e., meso-eutrophic to hyper-eutrophic forms) [30]. Although the low profile guild was shown to decrease and the other two guilds to increase in relative abundance along nitrate and phosphate gradients [29], it is still unknown to what extent the morphological guilds coincide with the trophic groups, but we expect a significant overlap. In particular, as eutrophic species require high resource levels, they must secure position in the overstory, which is beneficial for resource uptake in crowded environments and can be obtained either via specialized habits and/or late successional growth. Larger and colonial diatoms (high profile guild) extend above the substrate and understory forms, whereas biraphid diatoms (motile guild) avoid resource-depleted areas. Large, high profile, and motile forms have been documented in productive systems [8, 29, 35], which is not surprising since high amounts of nutrients are necessary to build colonial or individual biomass, i.e., a colony can encompass hundreds of cells and motile diatoms tend to be larger. Tolerant species, on the other hand, can survive under resource limitation, either environmental (low supply) or biotic (overgrowth); consequently, they need not invest in accumulating biomass, building extensive colonies or developing motility, and can remain small and low profile. Although the benthic model was developed for diatoms, its predictions should apply also to soft algae, which can be assigned the same growth morphologies and trophic groups as diatoms. Thus, one of the goals of this study is to expand the ideas of benthic coexistence to the entire algal periphyton.
Current is a major mechanical force in running waters with both positive and negative effects on periphyton. Faster currents stimulate algal metabolism and nutrient uptake [26, 43] and enhance the rates of mass transfer in dense mucilaginous diatom communities [2], which can explain why faster flows are frequently associated with greater periphytic photosynthesis and biomass [21, 24, 36]. However, current velocity constrains algal growth habit [3, 28] which, as already discussed, defines the spatial positioning within the biofilm and access to resources. Under higher velocities, species with extended habits and no means of attachment are disfavored, while low profile forms become dominant, indicating that ecological guilds that are sensitive to nutrient limitation are also prone to disturbance [29]. Although resource supply and flow disturbance are likely to act synergistically and define biofilm successional trajectories [4], experimental research is scarce.
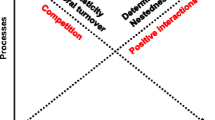
In this investigation, we propose and test a new model of succession in stream periphyton (both diatoms and soft algae), viewed as a gradient of species tolerance with direction, i.e., increasing or decreasing, determined primarily by nutrient supply and secondarily by flow disturbance at low to intermediate levels (Fig. 1a, b). Assuming that both tolerant and sensitive species are present in the immigrant pool, which is expected in organisms of generally unlimited dispersal, such as algae [5], successional patterns will diverge across nutrient regimes. Under low resource supply, succession will proceed toward dominance of tolerant algae (Fig. 1a) because only they will be able to grow and reproduce. As tolerant algae are expected to be mostly of low profile, the mature resource-limited community will be thin, composed of an understory only. Under high resource supply, where all species can maintain positive growth, succession is expected to culminate in the dominance of sensitive forms (Fig. 1b), which are much more numerous, accumulate substantial biomass, and form a thick canopy, but without eliminating the understory of tolerant forms, as suggested by the benthic model. Current velocity (at low to intermediate levels) is not expected to alter the direction of these successional trajectories because of the strong dependence of sensitive species on nutrient supply. As discussed, by enhancing nutrient uptake, faster currents can stimulate the growth of some species, such as tolerant forms as well as some motile and attached high profile species (e.g., Synedra and Gomphonema colonies or filamentous chlorophytes), but it can also eliminate species prone to scour, such as unattached high profile forms (e.g., filamentous Fragilaria and Melosira colonies). It is expected that tolerant low profile forms will benefit from faster currents across nutrient regimes, while the response of sensitive species should be more pronounced under resource limitation, i.e., positive in the motile guild but positive to negative in the high profile guild, depending on species composition. Therefore, it is hypothesized that low to intermediate current velocities exert comparatively minor effects on the establishment rates of both tolerant and sensitive forms, and consequently, on the rate of change of their ratio. Conversely, nutrient supply has a major influence and defines the directional change of this ratio, i.e., positive or negative (Fig. 1a, b).
Predicted changes in species tolerance through time of colonization as a function of nutrient regime and current velocity. Faster flow can accelerate the rate of establishment of tolerant species but, depending on species composition, it can have a positive vs. a negative effect on the establishment of sensitive species (attached vs. unattached forms). If higher velocity stimulates tolerant species to a greater extent than sensitive forms, it will generate higher tolerant-to-sensitive species ratios than slower currents across nutrient regimes. Conversely, if sensitive species benefit from higher currents more than tolerant species, the ratio will be lower than in slower currents. Thus, flow rate is expected to affect the rate of change of tolerant-to-sensitive species ratio (the shaded area) but not its direction, which should be positive under nutrient-depleted conditions (a) but negative under high resource supply (b)
It is thus envisioned that the succession of functional forms in the three-dimensional periphyton is environmentally controlled in accordance with the benthic model [30]. Nutrient supply sets the direction of succession, i.e., toward dominance of tolerant or sensitive species, whereas current velocity (at low to intermediate levels) selects among sensitive species and determines their densities. Coexistence of the two trophic groups throughout succession is hypothesized to be neutral with respect to each other but not a result of interspecific interactions, such as facilitation or competition (note that neutrality here is used to define the lack of species interactions but not species environmental responses, which are highly deterministic). It is proposed that facilitation can be ruled out if overstory sensitive species are capable of colonizing the substrate both early and late in the succession, while negative interactions can be ruled out if the density of the understory tolerant species is unaffected by the abundance of sensitive algae. To test these ideas, we ran a series of experiments in re-circulating channels, manipulating nutrient concentrations and current velocity and examining how composition of species, trophic groups, and morphological guilds changed through time.
Methods
Experiments were conducted in four 200 × 100 cm doughnut-shaped laboratory streams with an experimental trough measuring 80 cm in length, 12 cm in width, and 13 cm in depth. Eighty liters of modified Guillard’s WC media (see below) were re-circulated in each stream channel at a uniform current velocity (± 1 cm·s−1), maintained by adjusting a belt and multiple drive step pulleys attached to a motor and a water pump. Velocity was measured with a Marsh-McBirney model 2000 flowmeter (Mach-McBirney Inc., Frederick, MD, USA). Drop-in chillers (1/5 hp, TradeWind Chillers, Escondido, CA, USA) maintained room temperature (~20°C) in the high velocity channels, while the low velocity channels did not experience water temperature elevated above the room level. In each experimental trough, 4.9 × 4.9 cm unglazed porcelain tiles were placed equidistant from one another. A 250W metal halide lamp, positioned above each experimental trough, provided light on a 14:10 daily light/dark ratio at levels sufficient to saturate photosynthesis of attached algae, i.e., ~200 μmol·m−2·s−1 [17].
We examined algal succession under different current and nutrient regimes. Streams were subjected to constant flows of either 10 or 30 cm·s−1 because these values were representative of low and high velocity, respectively, observed in natural streams and shown to cause significant differences in algal biomass accumulation, immigration, and emigration rates [38]. Additionally, nutrient concentration was varied across current regimes with either high (800 μmol N–NO3 and 50 μmol P–PO4) or low (20 μmol N–NO3 and 1.25 μmol P–PO4) modified Guillard’s WC media [16]. Modified WC media consisted of all constituents in their normal concentrations other than nitrate and phosphate, which were manipulated between treatments. The nutrient analyses of water samples, collected at the time of algal sampling, were performed with AutoAnalyzer III (SEAL Analytical Inc., Mequon, WI, USA). The average concentrations (μg·L −1) in our low nutrient treatments, i.e., NO −3 (343–407) and PO 3−4 (6.44–6.53), were within the ranges shown to limit algal communities [12, 18], while in the high nutrient treatments, i.e., NO −3 (7,730–9,123) and PO 3−4 (840–1,165), these ranges were greatly exceeded. Thus, there were four different treatments: low nutrients at 10 and 30 cm·s−1, referred to as 10-low and 30-low, respectively, and high nutrients at 10 and 30 cm·s−1, referred to as 10-high and 30-high, respectively. The limited number of channels did not allow for replication in each flow × nutrient treatment so the experiment itself was replicated three times in November and December 2006 and February 2007, with each experimental run lasting 35 days. Every third day throughout the runs, 24 L of water were replaced with new media.
All artificial streams were seeded once, at the beginning of the experiment, with algae suspended in 2 L of carbon-filtered water. Seed algae were collected by scraping rocks from several small streams in the Dallas–Fort Worth area with diverse physico-chemical conditions. Although there were some taxonomic differences among the three seed communities (Supplementary Table 1), there were no significant functional differences among them, such as in the number of tolerant vs. sensitive species (a 2 × 3 contingency table analysis, χ 2 = 0.43, p = 0.71) or in the number of species in each morphological guild (a 3 × 3 contingency table analysis, χ 2 = 3.35, p = 0.50). After seeding the streams and allowing for initial biofilm colonization, two tiles were randomly retrieved from each stream channel at days 7, 11, 14, 18, 21, 25, 28, and 35, referred to as day of colonization in Figs. 3, 4, and 5. Tiles were taken from the same locations within each channel for each sampling period, scraped with a razor blade and a toothbrush until visibly clean, and returned back into the channels, but never retrieved again for the duration of the experiment. Biomass from the two tiles was consolidated, suspended in carbon filtered water, and preserved in 4% buffered formalin solution. After each sample was uniformly mixed by pulse sonification, a subsample was placed into a Palmer-Maloney counting cell to obtain algal density estimates using a light microscope at 400× magnification. For diatom species identification, the material was acid-digested, washed, and mounted in Naphrax® mounting medium (Brunel Microscopes Ltd., Chippenham, Wiltshire, UK). At least 300 frustules were counted and identified for each sample at 1000× magnification. Counts were converted to density of cells per surface area of tiles (no. of cells·cm−2).
Algae were placed into morphological guilds, i.e., low profile, high profile, and motile, following Passy [29], and further classified as either “sensitive” or “tolerant”, according to Passy [30]. Although the original guilds were described for diatoms only, soft algae can be placed in the same functional groups, and here, we expanded our definition of guilds to include all photosynthetic periphyton. Specifically, short-statured algae, either immobile, including adnate, prostrate, erect, and solitary cells, small colonies and cenobia, or slowly moving diatoms, constituted the low profile guild; tall-statured unicellular, colonial, or filamentous algae extending above the substrate, the high profile guild; and fast-moving biraphid diatoms or flagellated soft algae, the motile guild. Algae requiring high nutrient concentrations for growth and reproduction, e.g., meso-eutrophic to hypereutrophic, were considered sensitive, while those proliferating under low nutrient levels, e.g., indifferent, oligotrophic, or mesotrophic, were viewed as tolerant (Supplementary Table 1). A ratio of tolerant to sensitive species was calculated for each sample by summing the density of all tolerant species and dividing by the sum of density of all sensitive species.
The correspondence between the two functional classifications was examined by t test comparisons of tolerant vs. sensitive algal density in each ecological guild (e.g., Fig. 2). Temporal fluctuation across treatments in the density of low profile species establishing early in succession was taken as an indication of the outcome of overgrowth (e.g., Fig. 3a). Specifically, positive or sustained growth of the understory would suggest a weak effect, while a density decline would provide strong evidence for negative interactions with the overstory and subsequent inhibition. Temporal density patterns of the high profile and motile guilds were perceived as a response to the ambient environment rather than biotic interactions as these guilds form the overstory or can move freely and thus avoid locally unfavorable conditions, such as those caused by nutrient overconsumption or shading. Following McCormick and Stevenson [25], temporal trends in relative abundance were used to infer successional status, i.e., early, mid-, or late successional species. Guilds displaying a relative decline vs. a relative increase through time were classified as pioneers vs. late successional colonizers, achieving maximum relative abundance early vs. late in succession (e.g., Fig. 4). Guilds with a mid-successional peak in relative abundance were considered mid-successional colonizers. Pioneer performance of a guild would suggest that its establishment was not facilitated by another guild.
Observed changes in ln-transformed density throughout succession across the four treatments averaged over the three runs. The fits were generated by the following models with all parameters significant at 0.000001 ≤ p ≤ 0.005. a Low profile-tolerant guild; 10-low, \( y = a + bx + c{x^{{2}}} \), r 2 = 0.97 (a = 6.34, b = 0.49, c = −0.008); 30-low, y = a + bx, r 2 = 0.94 (a = 7.18, b = 0.24); 10-high, no significant model; and 30-high, \( y = a + bx + c{x^{{2}}} \), r 2 = 0.96 (a = 8.80, b = 0.33, c = −0.007). b Low profile-sensitive guild; 10-low, \( y = a + bx + c{x^{{2}}} \), r 2 = 0.92 (a = 6.84, b = 0.26, c = −0.005); 30-low: y = a + bx, r 2 = 0.96 (a = 6.68, b = 0.11); 10-high, \( y = a + bx + c{x^{{2}}} \), r 2 = 0.96 (a = 7.72, b = 0.318, c = −0.004); and 30-high, \( y = a + bx + c{x^{{2}}} \), r 2 = 0.97 (a = 6.89, b = 0.40, c = −0.006). c High profile guild; 10-low, \( y = a + bx + c{x^{{2}}} \), r 2 = 0.99 (a = 7.49, b = 0.35, c = −0.005); 30-low, y = a + bx, r 2 = 0.96 (a = 7.68, b = 0.17); 10-high, \( y = a + b/x \) , r 2 = 0.95 (a = 16.20, b = −25.92); and 30-high: y = a + b/x, r 2 = 0.94 (a = 16.54, b = −33.57). d Motile guild; 10-low, no significant model; 30-low, y = a + bx 2, r 2 = 0.93 (a = 8.01, b = 0.003); 10-high, \( y = a + b/x + c/{x^{{2}}} \), r 2 = 0.88 (a = 12.69, b = 61.92, c = −436.99); and 30-high, \( y = a + b/x + c/{x^{{2}}} \), r 2 = 0.98 (a = 13.03, b = 73.68, c = −557.73). For each guild, the equality of density means across treatments was tested with a repeated measures two-way ANOVA with a temporal blocking factor using all data points (N = 96). The effect of current velocity was not significant (p > 0.05) for any of the four guilds, while the effect of nutrient supply was not significant for the low profile-tolerant guild (p = 0.53; a), but highly significant for the other three guilds at 0.000001 < p < 0.005 (b–d). all = mean density across all days of colonization for each guild with 95% CI
Observed changes in guild relative abundance throughout succession across the four treatments, averaged over the three runs. The fits were generated by the following models with all parameters significant at 0.00002 ≤ p ≤ 0.04. a 10-low, low profile-sensitive guild, \( y = a + bx + c{x^{{2}}} \), r 2 = 0.97 (a = 0.23, b = −0.01, c = 0.0002); low profile-tolerant guild, \( y = a + bx + c{x^{{2}}} \), r 2 = 0.86 (a = 0.04, b = 0.05, c = −0.0008); high profile guild, no significant model; and motile guild, \( y = a + bx + c{x^{{2}}} \), r 2 = 0.96 (a = 0.41, b = −0.03, c = 0.0005). b 10-high, low profile-sensitive guild, y = a + bx 2.5, r 2 = 0.89 (a = 0.06, b = 0.00003); low profile-tolerant guild, y = a + b/x, r 2 = 0.96 (a = −0.04, b = 1.47); high profile guild, \( y = a + bx + c{x^{{2}}} \), r 2 = 0.92 (a = 0.09, b = 0.05, c = −0.001); and motile guild, y = a + bx, r 2 = 0.60 (a = 0.41, b = −0.007). c 30-low, low profile-sensitive guild, \( y = a + bx + c{x^{{2}}} \), r 2 = 0.88 (a = 0.23, b = −0.01, c = 0.0002); low profile-tolerant guild, \( y = a + bx + c{x^{{2}}} \), r 2 = 0.87 (a = −0.06, b = 0.06, c = −0.0009); high profile guild, y = a + bx, r 2 = 0.55 (a = 0.39, b = −0.007); and motile guild, \( y = a + bx + c{x^{{2}}} \), r 2 = 0.95 (a = 0.36, b = −0.025, c = 0.0005). d 30-high, low profile-sensitive guild, \( y = a + bx + c{x^{{2}}} \), r 2 = 0.95 (a = 0.20, b = −0.02, c = 0.0005); low profile-tolerant guild, \( y = a + b/x + c/{x^{{2}}} \), r 2 = 0.92 (a = 0.12, b = −2.44, c = 22.92); high profile guild, \( y = a + b/x + c/{x^{{2}}} \), r 2 = 0.88 (a = 0.24, b = 8.34, c = −53.66); and motile guild: no significant model
The relationships of tolerant-to-sensitive species ratio, guild density, and guild relative abundance with day of colonization were analyzed using regression analysis with the curve-fitting software TableCurve 2D 5.01 (SYSTAT Software Inc., Richmond, CA). Only biologically meaningful models were considered, including linear, power, and polynomial functions, capturing a variety of responses, including monotonic, decelerating (e.g., saturation), peaks, etc. The model with the best fit was selected. To determine the major successional trends, the data points in all regression analyses were calculated as averages of the three runs. Notably, averaging did not change the relationships but made them stronger by reducing the residual variation. Tests of equality of means across trophic groups and guilds included t tests and a repeated measures two-way ANOVA with nutrient and current regimes as factors and time as a blocking factor (SYSTAT version 11). These tests were performed on the full datasets to preserve the degrees of freedom.
Results and Discussion
The present results show that, throughout succession, nutrient supply controlled the density and relative abundance of tolerant and sensitive species, while the role of current velocity, at low to intermediate levels, was secondary. The null hypothesis that the density of each ecological guild across all samples (N = 96) comprised equal proportions of tolerant vs. sensitive species was rejected by individual t tests at p < 0.000001 (Fig. 2). There were 48 tolerant diatoms, six tolerant soft algae, 79 sensitive diatoms, and 39 sensitive soft algae found in this study (Supplementary Table 1). Density of the low profile guild (mean of 66%, 95% CI = 60–73%) was derived primarily from tolerant diatoms, density of the motile guild (mean of 91%, 95% CI = 89–92%), from sensitive diatoms, while that of the high profile guild, from sensitive diatoms and soft algae (mean of 97%, 95% CI = 96–98%). Although tolerant forms, both diatoms and soft algae had a significantly greater contribution to the density of the low profile guild; some sensitive soft algae, e.g., Scenedesmus spp. (Supplementary Table 1), too reached high numbers in that guild (Figs. 2 and 3a, b). As the low profile-sensitive soft algae tended to be late successional colonizers in conditions of overgrowth (i.e., under nutrient enrichment, Fig. 4b, d), we can tentatively conclude that in securing overstory location, sensitive diatoms rely primarily on growth habits, i.e., high profile and motile, while sensitive soft algae utilize both growth habit (high profile) and successional appearance (late). However, further investigations across broader environmental gradients, associated with greater algal biodiversity, are necessary to confirm this conjecture.
Previous research showed that diatom guilds behave distinctly along nutrient gradients [29] and suggested that tolerant and sensitive species have differential position in the 3D biofilm [30]. However, this investigation is the first to formally link growth form with nutrient requirements and corroborate the prediction of the benthic model that sensitive species would exhibit extended growth habit or late successional appearance affording them an overstory position with unimpeded resource access [30]. We further demonstrate that motility, which is another means of obtaining beneficial position, is a nearly exclusive feature of sensitive species. Finally, we reveal that all algae, not just diatoms, are subject to the same tradeoff between trophic demand and spatial position, but there may be a difference between diatoms and soft algae in the way they acquire beneficial position.
The ratio of tolerant to sensitive algal density, averaged over the three experimental runs, was near 1 or >1 under nutrient limitation but always lower than 1 in high nutrient regimes, indicating a shift in dominance along the nutrient gradient (Fig. 5). As colonization progressed, the relative contribution of tolerant species increased under nutrient-limited conditions but decreased under high nutrient supply. Current velocity did not alter these successional trends but affected the rate of change and the magnitude of the tolerant-to-sensitive species ratio. At higher current velocity, tolerant species took over the nutrient-limited community faster and to a much greater extent (Fig. 5a). In nutrient-replete conditions, sensitive species dominated across current regimes but tolerant species maintained greater relative density at higher velocity (Fig. 5b). These findings are consistent with the proposed model (Fig. 1a, b) with the difference that the real trends were generally non-linear. With the exception of 30-low, the rate of change in the tolerant-to-sensitive species ratio was dependent on the time of colonization—it was fast early in succession but leveled off at mid- to late succession, meaning that the densities of both groups tended to stabilize over time. Similarly, algal succession in a nitrogen-limited desert stream was marked by a temporally increasing relative biovolume of tolerant species, attaining complete dominance on control substrates [32]. However, tolerant species in the desert stream exhibited positive trends of relative biovolume on nitrogen-enriched substrates due to resource depletion within the thick periphytic mat. As nutrient addition in the present experiments was 16 times higher than in the aforementioned investigation, it is conceivable that internal resource limitation was not severe, evident in the declining relative contribution of tolerant species.
Observed changes in tolerant-to-sensitive species ratio throughout succession across the four treatments, averaged over the three runs. The fits were generated by the following models with all parameters significant at 0.0003 ≤ p ≤ 0.02. a Low nutrients, 10-low, y = a + b/x, r 2 = 0.62 (a = 2.02, b = −10.77) and 30-low: y = a + bx, r 2 = 0.84 (a = −0.76, b = 0.16). b High nutrients, 10-high, y = a + b/x, r 2 = 0.90 (a = −0.11, b = 3.18) and 30-high, y = a + b/x, r 2 = 0.88 (a = −0.08, b = 3.32)
The high profile and motile morphological guilds were nearly synonymous with the sensitive nutrient group (Fig. 2), while the low profile guild comprised mostly tolerant but also some sensitive species. To avoid redundancy, subsequent analyses were carried out on guilds only with the understanding that they reflected the dynamics of the respective nutrient groups. An exception was made for the low profile guild which was split into tolerant and sensitive species. All guilds, composed primarily of sensitive species, including low profile-sensitive, high profile, and motile, were stimulated by nutrient enrichment, evident in the substantially higher densities throughout succession and the significantly greater mean density in high than in low nutrient treatments (repeated measures two-way ANOVA, Fig. 3b–d). The low profile-tolerant guild had a higher initial density in high nutrient concentrations, varying comparatively little throughout succession (Fig. 3a). Conversely, this guild underwent strong changes in the low nutrient treatments, starting with lower densities and ending with higher densities than in nutrient-replete communities of the same successional stage. This means that for tolerant species, nutrient supply was beneficial only early in succession, possibly by stimulating growth and with this, immigration and settlement. However, later in succession, when these species became established, the nutrient amount was of little consequence. Notably, the density of the low profile-tolerant guild remained stable or showed a quadratic response, declining only at the last day of succession (Fig. 3a), which suggests that despite substantial overgrowth by sensitive species in high nutrient regimes, tolerant forms could sustain high biomass. Furthermore, the mean density of the low profile-tolerant guild remained constant across treatments (repeated measures two-way ANOVA, Fig. 3a), indicating that overall, tolerant species were comparatively indifferent to environmental variability and presence of the other guilds, which confirms the predictions of the benthic model [30]. Contrary to our expectations, none of the sensitive guilds showed a density decline under low nutrient supply (except for a single point in 10-low in Fig. 3b), probably because the nutrient levels were not as extremely low as seen in many natural nutrient limited streams [30]. Under high nutrient supply, all sensitive guilds increased (e.g., low profile-sensitive, Fig. 3b) or plateaued at mid-succession (e.g., high profile guild, Fig. 3c) or early succession (e.g., motile guild, Fig. 3d). The absence of a density decrease through time in all guilds (the decline in the motile guild past day 11 was not significant) points to a lack of negative interactions at a guild level.
The effect of current velocity was weaker and temporally dependent. Under nutrient-deficient conditions, high velocity initially slowed down biomass accumulation but toward the end of succession, all guilds exhibited nearly the same or greater density at high than at low current velocity (Fig. 3a–d). This can be explained with the opposing impacts of current velocity on algal immigration and nutrient uptake [38]. In 30-low, all guilds showed lower density during early colonization possibly as a result of flow-impeded immigration, important at early stages of succession. In contrast, late in succession, all guilds in 30-low reached or surpassed the density of their 10-low counterparts because faster currents reduce local nutrient depletion, becoming more pronounced in crowded mature biofilms (Fig. 3). Under nutrient-replete conditions, the differences in guild density between the two current regimes were smaller but higher velocities tended to generate larger biomass, i.e., in the low profile-tolerant and motile guilds. These findings indicate that under nutrient enrichment, where algal densities were much higher and immigration was unlikely to be limited, current velocity was an overall positive force (Fig. 3).
Reproduction governs the outcome of succession in the periphyton [25], but, as seen here, temporal dynamics were a function of both nutrient supply and species tolerance. Tolerant species exhibited sustained growth and uniform average density across nutrient regimes, while sensitive guilds accumulated significantly lower biomass under nutrient limitation than in conditions of plentiful resources. Active tolerance was proposed as a mechanism of succession in the periphyton at a species level [25]. The results presented here suggest that at the level of functional forms, including morphological guilds and trophic groups, this mechanism was neutral coexistence, that is, tolerant and sensitive forms proliferated regardless of each other’s presence because they had differential tolerance coupled with differential biofilm position. Neutral coexistence does not equate with tolerance sensu Connell and Slatyer [7], viewed as the transition of species with different life histories, e.g., short-lived are replaced by long-lived species, or less tolerant by more tolerant forms [33]. Under the neutral coexistence model, no replacement of the functional groups occurs but rather there is overgrowth of tolerant by sensitive groups and subsequent coexistence. Thus, the overall direction and rate of change during succession in the periphyton were set by the environment through its influence on sensitive species. Although interspecific interactions can be important at a species level [25], they did not transfer to the functional level, encompassing a number of functionally redundant species. These findings showed that the long-standing view of succession as an emergent community property [6, 27] was not supported at a functional level in the periphyton. This was consistent with the view of Drury and Nisbet [11], emphasizing the characteristics of the organism rather than those of the community. They further illuminated that different mechanisms of succession might become prevalent at different levels of biological organization.
Succession has been viewed as predictable [27] to unpredictable [41] and in the lotic environment, as only marginally predictable [13]. Here, we argue that in the spatially complex periphyton, succession is a dialectic process—deterministic at the level of functional groups, but stochastic at the level of individual species. The relative importance of the tolerant vs. sensitive group in community organization is predictable because it is set exclusively by the environment. In contrast, the composition of each group is stochastic as it depends on interspecific interactions as well as on the local and regional species pools, which are geographically variable.
The temporal dynamics of guild relative abundance revealed that the successional appearance of tolerant and sensitive species depended on nutrient supply (Fig. 4). In general, guilds with significant successional trends (i.e., with non-zero regression slopes) peaked in mid- to late succession under beneficial conditions but early otherwise. For example, a late successional maximum in relative abundance was observed in the low profile-tolerant guild in low nutrient treatments and in the low profile-sensitive guild in high nutrient treatments, while the high profile guild culminated in mid-succession under nutrient enrichment. Current velocity had an occasional effect on guild successional status, varying from early to no successional preference, e.g., the high profile guild in 30-low vs. 10-low and the motile guild in 10-high vs. 30-high.
The overall lack of successional fidelity in the morphological guilds, capable of performing as both early and late successional forms, suggests that the presence of a guild was not necessary for the establishment of another guild, which rules out facilitation as a mechanism of succession in the periphyton at a guild level (at least using the present guild classification). This observation further sheds light on the unresolved debate in benthic ecology as to whether periphyton follows the plant successional type from low to high profile species [27] or not, fueled by conflicting reports of larger and colonial algae being both initial and late colonizers [20, 37]. According to the present study, algae of any growth form can start a succession, if present in the immigrant pool, because they have either an adaptation for coping with subsequent overgrowth (e.g., tolerance) or specialized growth habits. However, the progression of this succession depends on nutrient supply (tolerant vs. sensitive species dominance) and regional species pool (e.g., low profile-sensitive, high profile, or motile guild dominance in resource-rich environments). These ideas were supported by the present experimental study but need to be verified in natural environments.
Our results indicated that nutrient supply had a qualitative effect on algal communities, while the effect of current velocity at low to intermediate levels was quantitative. Nutrient supply determined functional group composition, while current velocity influenced group density. At the maximum velocity of our experiments, i.e., 30 cm·s−1, it is conceivable that current was mostly a stimulating rather than a destructive force, considering that faster currents promote carbon assimilation and phosphorus uptake in the periphyton [22, 24, 43]. Admittedly, greater velocities can alter community organization in a way indistinguishable from resource limitation. For instance, in a natural stream, thalweg velocity of about 60 cm·s−1 reduced the diatom biofilm diversity and led to an overwhelming dominance of Achnanthidium minutissimum, while the neighboring low-velocity margin communities hosted a number of sensitive high profile species [28]. These observations imply that current velocity exhibits a threshold behavior within its natural range with positive influences switching to negative at the threshold. Conversely, the effects of eutrophication are generally positive, expressed in a greater algal biomass and diversity [9, 19, 44], although excessive nutrient inputs from human activities can create hyper-eutrophic conditions and cause a biodiversity decline [10]. Therefore, eutrophication too displays a threshold behavior which, however, becomes evident beyond the natural ranges of this process.
In conclusion, succession in the benthic biofilm led to dominance of tolerant or sensitive species, depending on resource supply but not on current velocity at low to intermediate levels. The two trophic groups persisted via neutral coexistence, whereby tolerant species showed sustained growth despite overgrowth by sensitive species. Sensitive species were neither facilitated nor inhibited by tolerant forms, and by being of extended growth form, motile, or late successional dominants, they had an advantage in resource acquisition. The mechanism of succession probably shifts across the levels of biological organization—competition may emerge as an important process when individual species are concerned but not at the level of functional groups.
References
Airoldi L (2000) Effects of disturbance, life histories, and overgrowth on coexistence of algal crusts and turfs. Ecology 81:798–814
Biggs BJF, Goring DG, Nikora VI (1998) Subsidy and stress responses of stream periphyton to gradients in water velocity as a function of community growth form. J Phycol 34:598–607
Biggs BJF, Hickey CW (1994) Periphyton responses to a hydraulic gradient in a regulated river in New Zealand. Freshw Biol 32:49–59
Biggs BJF, Stevenson RJ, Lowe RL (1998) A habitat matrix conceptual model for stream periphyton. Arch Hydrobiol 143:21–56
Cermeño P, Falkowski PG (2009) Controls on diatom biogeography in the ocean. Science 325:1539–1541
Clements FE (1916) Plant succession. Carnegie Institute of Washington, Washington
Connell JH, Slatyer RO (1977) Mechanisms of succession in natural communities and their role in community stability and organization. Am Nat 111:1119–1144
DeNicola D, de Eyto E, Wemaere A, Irvine K (2006) Periphyton response to nutrient addition in 3 lakes of different benthic productivity. J N Am Benthol Soc 25:616–631
Dodds WK, Smith VH, Lohman K (2002) Nitrogen and phosphorus relationships to benthic algal biomass in temperate streams. Can J Fish Aquat Sci 59:865–874
Donar C, Stoermer EF, Brenner M (2009) The Holocene paleolimnology of Lake Apopka, Florida. Nova Hedwigia 135:57–70
Drury WH, Nisbet ICT (1973) Succession. J Arnold Arbor 54:331–368
Earl SR, Valett HM, Webster JR (2006) Nitrogen saturation in stream ecosystems. Ecology 87:3140–3151
Fisher SG (1990) Recovery process in lotic ecosystems: limits of successional theory. Environ Manage 14:725–736
Gleason HA (1917) The structure and development of the plant association. Bull Torrey Bot Club 44:463–481
Grimm NB (1994) Disturbance, succession and ecosystem processes in streams: a case study from the desert. In: Giller PS, Hildrew AG, Raffaelli DG (eds) Aquatic ecology: scale, pattern and process. Blackwell Science Ltd, London, pp 93–112
Guillard RRL (1975) Culture of phytoplankton for feeding marine invertebrates. In: Smith WL, Chantey MH (eds) Culture of marine invertebrate animals. Plenum Publishers, New York, pp 29–60
Hill W (1996) Effects of light. In: Stevenson RJ, Bothwell ML, Lowe RL (eds) Algal ecology: freshwater benthic ecosystems. Academic, San Diego, pp 121–148
Hill WR, Fanta SE (2008) Phosphorus and light colimit periphyton growth at subsaturating irradiances. Freshw Biol 53:215–225
Hillebrand H, Gruner DS, Borer ET, Bracken MES, Cleland EE, Elser JJ, Harpole WS, Ngai JT, Seabloom EW, Shurin JB et al (2007) Consumer versus resource control of producer diversity depends on ecosystem type and producer community structure. Proc Natl Acad Sci USA 104:10904–10909
Hoagland KD, Roemer SC, Rosowski JR (1982) Colonization and community structure of two periphyton assemblages, with emphasis on the diatoms (Bacillariophyceae). Am J Bot 69:188–213
Hondzo M, Wang H (2002) Effects of turbulence on growth and metabolism of periphyton in a laboratory flume. Water Resour Res 38:1277–1286
Horner RR, Welch EB, Seeley MR, Jacoby JM (1990) Responses of periphyton to changes in current velocity, suspended sediment and phosphorus concentration. Freshw Biol 24:215–232
Huston M, Smith T (1987) Plant succession: life history and competition. Am Nat 130:168–198
Keithan ED, Lowe RL (1985) Primary productivity and spatial structure of phytolithic growth in streams in the Great Smoky Mountains National Park, Tennessee. Hydrobiologia 123:59–67
McCormick PV, Stevenson RJ (1991) Mechanisms of benthic algal succession in lotic environments. Ecology 72:1835–1848
Munk WH, Riley GA (1952) Absorption of nutrients by aquatic plants. J Mar Res 11:215–240
Odum EP (1969) Strategy of ecosystem development. Science 164:262–270
Passy SI (2001) Spatial paradigms of lotic diatom distribution: a landscape ecology perspective. J Phycol 37:370–378
Passy SI (2007) Diatom ecological guilds display distinct and predictable behavior along nutrient and disturbance gradients in running waters. Aquat Bot 86:171–178
Passy SI (2008) Continental diatom biodiversity in stream benthos declines as more nutrients become limiting. Proc Natl Acad Sci USA 105:9663–9667
Passy SI (2010) A distinct latitudinal gradient of diatom diversity is linked to resource supply. Ecology 91:36–41
Peterson CG, Grimm NB (1992) Temporal variation in enrichment effects during periphyton succession in a nitrogen-limited desert stream ecosystem. J N Am Benthol Soc 11:20–36
Pickett STA, Cadenasso ML, Meiners SJ (2009) Ever since Clements: from succession to vegetation dynamics and understanding to intervention. Appl Veg Sci 12:9–21
Pickett STA, Collins SL, Armesto JJ (1987) Models, mechanisms and pathways of succession. Bot Rev 53:335–371
Pringle CM (1990) Nutrient spatial heterogeneity: effects on community structure, physiognomy, and diversity of stream algae. Ecology 71:905–920
Rinke K, Robinson CT, Uehlinger U (2001) A note on abiotic factors that constrain periphyton growth in alpine glacier streams. Int Rev Hydrobiol 86:361–366
Steinman AD, McIntire CD (1986) Effects of current velocity and light energy on the structure of periphyton assemblages in laboratory streams. J Phycol 22:352–361
Stevenson RJ (1996) The stimulation and drag of current. In: Stevenson RJ, Bothwell ML, Lowe RL (eds) Algal ecology: freshwater benthic ecosystems. Academic, San Diego, pp 321–340
Stevenson RJ, Glover R (1993) Effects of algal density and current on ion transport through periphyton communities. Limnol Oceanogr 38:1276–1281
Tilman D (1985) The resource-ratio hypothesis of plant succession. Am Nat 125:827–852
Van Hulst R (1979) On the dynamics of vegetation: Markov chains as models of succession. Vegetatio 40:3–14
Walker LR, Chapin FS (1987) Interactions among processes controlling successional change. Oikos 50:131–135
Whitford LA, Schumacher GJ (1961) Effect of current on mineral uptake and respiration by a freshwater alga. Limnol Oceanogr 6:423–425
Yallop M, Hirst H, Kelly M, Juggins S, Jamieson J, Guthrie R (2009) Validation of ecological status concepts in UK rivers using historic diatom samples. Aquat Bot 90:289–295
Acknowledgments
We gratefully acknowledge the financial support from UT Arlington Research Enhancement Grant No. 14-7487-30 and Norman Hackerman Advanced Research Program Grant No. 003656-0054-2009 to SP and Environmental Protection Agency GRO Fellowship for Graduate Environmental Study No. F6E61489 to CL. We thank an anonymous reviewer for the helpful suggestions that improved the clarity of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 311 kb)
Rights and permissions
About this article
Cite this article
Passy, S.I., Larson, C.A. Succession in Stream Biofilms is an Environmentally Driven Gradient of Stress Tolerance. Microb Ecol 62, 414–424 (2011). https://doi.org/10.1007/s00248-011-9879-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-011-9879-7