Abstract
The Ohdaigahara subalpine plateau in Japan has recently suffered a reduction in primary forest land caused by an increasing population of sika deer (Cervus nippon). Deer have debarked many trees, causing dieback, gradually changing the primary forest first to light forest with a floor that is densely covered with sasa grass (Sasa nipponica) and then to S. nipponica grassland. To examine the effects of vegetative transformation on the dung-beetle community, we compared the diversity and abundance of dung-beetle assemblages in the primary forest, transition forest, and S. nipponica grassland using dung-baited pitfall traps. The species richness and species diversity (Shannon-Wiener index) were significantly highest in the primary forest and lowest in the S. nipponica grassland. The evenness (Smith-Wilson index) was highest in the primary forest and nearly equal in the transition forest and S. nipponica grassland. The abundance was apparently greater in the transition forest than in the primary forest and S. nipponica grassland. These results suggest that loss of primary forest resulting from an increasing deer population decreases the diversity of the dung-beetle community while increasing the abundance of dung beetles in the transition forest. Sika deer use transition forests and grasslands more frequently than primary forests as habitat, but an increase in dung supply there does not necessarily increase the diversity or abundance of dung-beetle assemblages.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The diversity of local dung-beetle communities is primarily influenced by vegetation cover, soil type and moisture, and resource (dung) availability (Doube 1987; Davis 2002). Hence dung-beetle communities might be expected to be impacted seriously by deforestation, which increases solar radiation at the soil surface and decreases ground moisture (Hanski 1989; Gill 1991; Estrada et al. 1998). High solar radiation accelerates the loss of moisture from dung mass, making dung unavailable for beetles. Furthermore, deforestation changes the species composition and abundance of dung-producing mammals, affecting the availability of dung for beetles (Cambefort and Walter 1991; Estrada et al. 1993, 1999). Since dung beetles have different preferences for dung of different mammals based on texture, the structure of dung-beetle communities is influenced by the local mammalian fauna. Many studies in tropical South America and Southeast Asia have demonstrated that deforestation caused by human activities (e.g. logging, pasture clearing, and plantation cutting) reduces the diversity of dung-beetle communities (Klein 1989; Estrada et al. 1998; Davis 2000; Davis et al. 2001; Estrada and Coates-Estrada 2002; Halffter and Arellano 2002; Horgan 2002; Medina et al. 2002). Deforestation has sometimes decreased the numbers of mammals producing dung for beetles (Estrada et al. 1993, 1998, 1999; Estrada and Coates-Estrada 2002). This suggests that although vegetation cover may be most important for determining the structure of dung-beetle communities (Halffter and Arellano 2002), mammalian fauna may substantially influence the diversity of dung-beetle communities.
The Ohdaigahara subalpine plateau on the Kii Peninsula in Japan has the southernmost spruce forest [Picea jezoensis (Sieb. et Zucc.) Carrière var. hondoensis (Mayr) Rehder] and the largest beech forest (Fagus crenata Blume) in western Japan (Ide and Kameyama 1972). However, the plateau has recently suffered deforestation caused by an increasing population of sika deer (Cervus nippon Temminck), which strip the bark of many coniferous trees, causing dieback (Akashi and Nakashizuka 1999; Yokoyama et al. 2001). Canopy loss increases light inside the forest, allowing the forest floor to become densely covered with sasa grass (Sasa nipponica Makino et Shibata), which grows well with more light (Kawahara and Tadaki 1978). S. nipponica is a major forage of sika deer because it is richer in protein than other forage plants (Yokoyama et al. 1996; Yokoyama and Shibata 1998a). Moreover, S. nipponica can tolerate heavy browsing by sika deer (Yokoyama and Shibata 1998b). The dense covering of S. nipponica inhibits conifer seedlings from growing and surviving on the forest floor (Yamamoto 1993). Seedlings are browsed by the sika deer (Takeda 1994). As a result, the plateau has been partly changed from coniferous forest to S. nipponica grassland (Yokota and Nakamura 2002). Accordingly, the sika deer have come to use the grassland as habitat more intensively than the coniferous forest (Maeji et al. 1999; Yajima et al. 2002).
The change from dense forest into S. nipponica grassland may affect the community structure of organisms inhabiting the Ohdaigahara subalpine plateau. For example, Hino (2000) has shown that a dense population of sika deer decreases the diversity and abundance of bird communities because the deer heavily graze undergrowth foliage that forms nesting sites for birds and cause loss of canopy trees through trunk barking. However, despite the widely held view that the structure of dung-beetle communities is strongly affected by changes in vegetation and the composition and abundance of mammals, no study on dung-beetle community has been made in Ohdaigahara.
This paper examines how the diversity of the dung-beetle community has been affected by the reduction in primary forest caused by an increasing population of sika deer on the Ohdaigahara subalpine plateau. We compared the diversity of dung-beetle assemblages in primary forest, transition forest with floor dominated by S. nipponica, and S. nipponica grassland using dung-baited pitfall traps. We referred to data on sika deer habitat use and the distribution of deer fecal pellets, and evaluated the relative importance of vegetation and dung supply as factors that determine the diversity of the dung-beetle community.
Methods
Study site
This study was carried out on the Ohdaigahara subalpine plateau (34°12′N, 136°06′E) at an altitude of 1,400–1,695 m on the Kii Peninsula, Japan. The climate is characterized by cool temperatures (annual mean 6.4°C) and high precipitation (annual average >4,500 mm) (Japan Forest Technology Association 2001). The plateau is covered with snow from mid-December to late March. The primary vegetation of the plateau is evergreen coniferous forest dominated by spruce (P. jezoensis var. hondoensis) in the eastern area and a mixed forest dominated by beech (F. crenata) and fir (Abies homolepis Sieb. et Zucc.) in the western area (Ide and Kameyama 1972). More than half the coniferous trees, such as spruce, fir, hemlock (Tsuga diversifolia Masters) and cypress (Chamaecyparis obtusa Endl.), have been debarked by sika deer. In particular, 75.9% of spruce trees suffer debarking with a resultant mortality ranging from 17.8–25.9% (Yokoyama et al. 2001). The population density of sika deer on the plateau in 1996 and 1997 was estimated at 17.5–30.9 individuals km−2 (Maeji et al. 1999).
The Ohdaigahara subalpine plateau harbors several species of large or medium-sized mammals that produce dung for dung beetles, including sika deer, Japanese serow [Capricornis crispus (Temminck)], Japanese monkey [Macaca fuscata (Blyth)], red fox [Vulpes vulpes (L.)], raccoon dog [Nyctereutes procyonoides (Gray)], badger [Meles meles (L.)], black bear [Selenarctos thibetanus (Cuvier)], wild boar (Sus scrofa L.), and marten [Martes melampus (Wagner)]. Sika deer are the most abundant (E. Shibata, unpublished data).
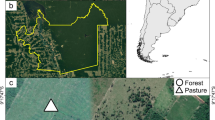
Three sampling sites comprising different types of vegetation were selected (Fig. 1). Site A is primary or dense mixed forest (1,560 m a.s.l.) dominated by beech and fir with the forest floor covered mainly with sasa grass (S. nipponica) and perennial herbs (Skimmia japonica Thunberg). The crown coverage at 1 m is 77% according to image analysis of hemispheric photographs (LIA for Win32; Yamamoto 2003). Site B is grassland (1,680 m a.s.l.) where azalea shrubs (Rhododendron quinquefolium Bisset et Moor) are distributed sparsely and S. nipponica covers the floor densely. The crown coverage is 20%. Until the 1980s, this area was forest dominated by spruce, but it has suffered deforestation resulting from an increasing population of sika deer (Yokota and Nakamura 2002). Site C is transition forest between mixed forest and grassland (1,650 m a.s.l.) dominated by spruce and beech with a forest floor covered densely with S. nipponica. The crown coverage is 77%, which is similar to the primary or dense mixed forest.
Map of Ohdaigahara subalpine plateau showing location of three trapping sites. A Dense mixed forest or primary forest, B S. nipponica grassland, C transition forest with forest floor densely covered with S. nipponica grass. Dashed lines indicate trails and solid lines indicate contour lines (in 100-m intervals)
Sampling methods
Dung beetles were sampled using dung-baited pitfall traps designed by Hoga (1982). Plastic containers (130 mm diameter, 95 mm deep) containing 150 ml of 50% ethylene glycol as a preserving fluid were buried to the rim in the ground. A plastic cup (85 mm diameter, 45 mm deep) containing 35 g of fresh deer dung was suspended with wire at the mouth of the container. Sampling was conducted at the three sites at the end of each month from May 2001 to November 2001. Three traps were buried in the soil at 20-m intervals on a transect line at each site for 7 days with no rebaiting. A voucher collection of dung beetles was deposited at Department of Biological Sciences, Nara Women’s University.
Dung-beetle diversity
Species diversity in a community takes two components into account: species richness (number of species in sample) and evenness (degree to which species are equally abundant) (Magurran 1988). The Shannon-Wiener index H′ was used in the present study to measure species diversity
where s is the number of species, p i is the proportion of total sample belonging to species i. Species richness was estimated by the rarefaction method (Krebs 1999). This method estimates the number of species expected in a random sample \(\left( {E\left( {\hat S_n } \right)} \right)\) of n individuals taken from a collection
where S is the number of species in the sample, N i is the number of individuals of species i, N is the total number of individuals in the collection, and n is the value of the sample size chosen for standardization (n≤N). The Smith-Wilson index Evar was used to measure evenness (Smith and Wilson 1996):
where n i and n j are the numbers of individuals in species i and j, respectively, in the sample, and S is the number of species in the entire sample. This index is nearly independent of species richness and is sensitive to both rare and common species in the community (Krebs 1999; note that his equation should be altered to the above).
Calculation and statistics
We calculated the species richness and its standard deviation predicted by the rarefaction method, the Shannon-Wiener measure H′, bootstrap mean H′ based on 5,000 iterations and its 99% confidence limit, and the Smith-Wilson measure Evar using the software EcoMeth (Kenney and Krebs 2003). Bootstrap procedure for Evar was not conducted because appropriate software was not available. Differences in the abundance of dung beetles among the primary forest, transition forest and S. nipponica grassland were tested by two-way factorial ANOVA, after the original data were transformed by Box-Cox method (Sokal and Rohlf 1995). Statistical tests were performed with the software BIOMstat (Rohlf and Slice 1999) or SPSS (SPSS 2002).
Results
Species composition
A total of 2,299 dung beetles belonging to two species of Geotrupidae and ten species of Scarabaeidae were captured (Table 1). Several traps, including three traps in the transition forest in July, were lost owing to natural disturbances. Hence, the abundance of each species is expressed not as the actual number of captures, but instead as the sum of mean numbers of captures per trap in a sampling episode. As a whole, the dominant species was Aphodius superatratus Nomura and Nakane, followed by A. igai Nakane. These two species comprised 73.4% of all captures.
The composition of abundant species differed among the three habitats. In the primary forest, A. igai comprised 50% of the captures, and three species, A. unifasciatus Waterhouse, A. superatratus, and A. isaburoi Nakane, made up 35% of the captures. In the transition forest, A. superatratus was most abundant followed by A. igai and A. unifasciatus; these species accounted for 85% of the captures. In the S. nipponica grassland, A. superatratus was dominant followed by A. igai; these two species comprised 89% of the captures.
Diversity of dung-beetle assemblages
The primary forest had the highest species richness followed by the transition forest, and the S. nipponica grassland (Table 1). The differences between the habitats were significant based on the expected species richness at 400 individuals estimated using the rarefaction method (Scheffé test, P<0.01; Sokal and Rohlf 1995). Figure 2 shows rarefaction curves for the three habitats. Expected species richness was highest in the primary forest and lowest in the S. nipponica grassland for all sample sizes.
Figure 3 shows species rank–abundance curves for the three habitats; the slope of rank–abundance curves reflects diversity (Magurran 1988). The transition forest and S. nipponica grassland show similar species rank–abundance slopes, and accordingly, similar evenness (Smith-Wilson index, Evar). On the other hand, the primary forest has a shallower slope than the other habitats, demonstrating the highest evenness (Table 1).
Shannon-Wiener index (H′) was highest in the primary forest, intermediate in the transition forest, and lowest in the S. nipponica grassland (Table 1). The differences were statistically significant because the 99% confidence limits of bootstrap mean H′ in one habitat always exclude bootstrap mean H′ in the other habitats.
Monthly species richness and abundance of dung beetles
The species richness of dung beetles decreased at each site as the season progressed (Fig. 4a). The grassland had the poorest species richness in all months, while the primary forest had the highest species richness in all months except September. More dung beetles were caught in May and June at all sites (Fig. 4b). After July, the number remained very low in the primary forest and S. nipponica grassland. In the latter, especially, no more than one dung beetle was trapped after August. In the transition forest, however, the abundance increased slightly in August through September. A two-way ANOVA (with the July data excluded because there were none for the transition forest) revealed that the pattern of monthly abundance significantly differed among the sites (P<0.001 for site × month interaction; Table 2). The transition forest exceeded the other sites in abundance until September and was nearly equal to the primary forests in October and November. The S. nipponica grassland showed lower abundance through the season except in June.
Discussion
Twelve species were recorded in the present study. The collection includes almost all the subalpine species recorded for western Japan (Masumoto and Ochi 1985). Only two species, A. gotoi Nomura and Nakane and A. hasegawai Nomura and Nakane, were not captured. Since there has been no record of these species from the Ohdaigahara subalpine plateau, it is probable that neither of them inhabits the area.
The diversity of dung-beetle assemblages measured as species richness, Evar, and H′ was significantly or substantially higher in the primary forest than the S. nipponica grassland and transition forest. Data were lost for the transition forest in July, so the species richness might be underestimated there. However, the possibility seems unlikely because the species that were trapped in the primary forest and the S. nipponica grassland in July were caught in the transition forest in other months. The grassland had the lowest species richness in all months, while the primary forest had the highest richness except in September. Thus, the diversity of dung beetle assemblages can be regarded as highest in the primary forest and lowest in the S. nipponica grassland.
The transition forest did not show an edge effect, i.e. species richness was not greater at the boundary between two adjacent habitats (Odum 1971). No species were collected only from the S. nipponica grassland, which suggests that species specializing in open lands were not present. Any possible edge effect was minimized by the absence of species specific to open habitats; there is no mixture of species that inhabit the grassland and species that inhabit the forest to increase species richness at the boundary (i.e. transition forest) between the two adjacent habitats. There are two reasons for the absence of species specializing in open lands. One is a topological reason—the Ohdaigahara subalpine plateau is surrounded by dense forests, and thus hardly receives immigrations of dung beetles from open lands. The other is a historical reason—since the primary vegetation of the plateau is dense coniferous and dense mixed forest (Ide and Kameyama 1972), no dung beetle specializing in open lands is likely to maintain its population.
Although the pattern of monthly abundance of dung beetles significantly differed among the habitats, the abundance tended to be greatest in the transition forest, intermediate in the primary forest, and lowest in the S. nipponica grassland. Transition forests and grasslands are used more intensively by sika deer from spring to autumn than primary forests (Maeji et al. 1999; Yajima et al. 2002). Furthermore, the mean number of fecal pellets during May 2001 and June 2001 was higher in the S. nipponica grassland (666.4/10 m2; min=98, max=1,825, n=10) (M. Matsumura, unpublished data) than the primary forest (24.5/10 m2, min=0, max=125, n=15) (T. Hino, unpublished data). This suggests a higher deer population density in the grassland than the primary forest. Additionally, during the censuses of sika-deer fecal pellets, no dung of other mammals was found. Thus, an increase in dung supply by sika deer does not necessarily increase the abundance of dung beetles.
So far, we have proceeded under the assumption that the number of dung beetles caught in dung-baited traps reflects the abundance of dung beetles in a habitat. One might question this assumption, raising the possibility that, if a large number of dung pellets are deposited, pellets would hinder dung-baited traps from attracting dung beetles, or if there are few pellets around the traps, more dung beetles would be attracted to the traps than expected. However, Lobo et al. (1998) have demonstrated that the number of trapped beetles was in accordance with estimated population sizes of beetles in three pastures with different-sized flocks of sheep. Hence, our assumption may be acceptable.
Halffter and Arellano (2002) have noted that vegetation cover rather than dung supply is important for determining the structure and diversity of a dung-beetle community in a tropical region of Mexico. In the Ohdaigahara subalpine plateau, the abundance of dung beetles seems to be influenced by ground moisture, which is determined by solar radiation and transpiration (Horgan 2002). The dense coniferous and mixed forests should have very moist ground because of high annual precipitation (>4,500 mm) and low solar radiation. This ground condition is unlikely to favor dung beetles because it decreases the survivorship of the offspring in brood chambers in the soil (Vessby and Wiktelius 2003). On the other hand, the transition forest should have moderately moist ground because Sasa grasses have high transpiration rates (Takagi et al. 1999; Kitamura et al. 2000). In fact, Furusawa et al. (2001) have demonstrated experimentally that soil moisture measured by matrix potential at a depth of 6 cm was significantly lower in the forest floor covered with S. nipponica grass than in the forest floor without grass in the Ohdaigahara subalpine plateau. The low abundance of dung beetles in the S. nipponica grassland is probably due to high solar radiation, which dries dung mass at a faster rate. Furthermore, the low abundance in the grassland may be attributable to the inability of forest dung beetles, such as A. igai, A. unifasciatus, A. eccoptus Bates, A. isaburoi and Oxyomus ishidai Nakane in the present study (Masumoto and Ochi 1985), to extend their activity into open land (Howden and Nealis 1975; Peck and Forsyth 1982; Doube 1983; Nummelin and Hanski 1989; Estrada et al. 1998; Halffter and Arellano 2002).
The reduction in primary forest caused by debarking by sika deer and the resultant dieback of coniferous trees has resulted in loss of diversity in the dung-beetle community as a result of an increase in S. nipponica grassland on the Ohdaigahara subalpine plateau. Although dung beetles are most abundant in transition forests with a floor densely covered with S. nipponica grass, the community structure is simpler, showing reduced evenness. The increasing population of sika deer supplies more dung for beetles, but the increased dung supply does not necessarily increase the abundance or diversity of dung beetles. Instead, sika deer reduce the primary forest by debarking, which in turn decreases the diversity of the subalpine dung-beetle community.
References
Akashi N, Nakashizuka T (1999) Effect of bark-stripping by sika deer (Cervus nippon) on population dynamics of a mixed forest in Japan. For Ecol Manage 113:83–89
Cambefort Y, Walter P (1991) Dung beetles in tropical forests in Africa. In: Hanski I, Cambefort Y (eds) Dung beetle ecology. Princeton University Press, Princeton, pp 198–210
Davis AJ (2000) Does reduced-impact logging help preserve biodiversity in tropical rainforests? A case study from Borneo using dung beetles (Coleoptera: Scarabaeoidea) as indicators. Env Ent 29:467–475
Davis ALV (2002) Dung beetle diversity in South Africa: influential factors, conservation status, data inadequacies and survey design. Afr Entom 10:53–65
Davis AJ, Holloway JD, Huijbregts H, Krikken J, Kirk–Spriggs AH, Sutton SL (2001) Dung beetles as indicators of change in the forests of northern Borneo. J Appl Ecol 38:593–616
Doube BM (1983) The habitat preference of some bovine dung beetles (Coleoptera: Scarabaeidae) in Hluhluwe Game Reserve, South Africa. Bull Entom Res 73:357–371
Doube BM (1987) Spatial and temporal organization in communities associated with dung pats and carcasses. In: Gee JHR, Giller PS (eds) Organization of communities: past and present. Blackwell, Oxford, pp 255–280
Estrada A, Coates-Estrada R (2002) Dung beetles in continuous forest, forest fragments and an agricultural mosaic habitat island at Los Tuxtlas, Mexico. Biodiv Conserv 11:1903–1918
Estrada A, Halffter G, Coates-Estrada R, Meritt D (1993) Dung beetles attracted to mammalian herbivore (Alouatta palliata Gray) and omnivore (Nasua narica Linnaeus) dung in the tropical rain forest of Los Tuxtlas, Mexico. J Trop Ecol 9:45–54
Estrada A, Coates-Estrada R, Dadda AA, Cammarano P (1998) Dung and carrion beetles in tropical rain forest fragments and agricultural habitats at Los Tuxtlas, Mexico. J Trop Ecol 14:577–593
Estrada A, Anzures AD, Coates-Estrada R (1999) Tropical rain forest fragmentation, howler monkey (Alouatta palliata) and dung beetles at Los Tuxtlas, Mexico. Am J Primatol 48:253–262
Fujioka M (2001) A list of Japanese Lamellicornia. Kogane, suppl 1. Japanese Society of Scarabaeoideans, Tokyo
Furusawa H, Araki M, Hino T (2001) Effects of sika deer and sasa on water potential in surface soil—a case study at Ohdaigahara (in Japanese with English summary). Appl For Sci 10:31–36
Gill BD (1991) Dung beetles in tropical American forests. In: Hanski I, Cambefort Y (eds) Dung beetle ecology. Princeton University Press, Princeton, pp 211–229
Halffter G, Arellano L (2002) Response of dung beetle diversity to human-induced changes in a tropical landscape. Biotropica 34:144–154
Hanski I (1989) Dung beetles. In: Lieth H, Werger MJA (eds) Tropical rain forest. Elsevier, London, pp 489–511
Hino T (2000) Bird community and vegetation structure in a forest with a high density of sika deer. Jpn J Ornithol 48:197–204
Hoga A (1982) A report on ecological studies of a dung beetle Geotrupes auratus (in Japanese). Environmental Management Bureau of Kyoto, Japan
Horgan FG (2002) Shady field boundaries and the colonization of dung by coprophagous beetles in Central American pastures. Agric Ecosys Environ 91:25–36
Howden HF, Nealis VG (1975) Effects of clearing in a tropical rain forest on the composition of the coprophagous scarab beetles fauna (Coleoptera). Biotropica 7:77–83
Ide H, Kameyama A (1972) Vegetation of Ohdaigahara (in Japanese). Appl Bot Sociol 1:1–48
Japan Forest Technology Association (2001) A field guide to the nature of the Ohsugi Valley and the Ohdaigahara subalpine plateau (in Japanese). Kinki-Chugoku Branch of the Japan Forestry Agency, Osaka
Kawahara T, Tadaki Y (1978) Studies on sasa communities (III): relationship between light intensity and biomass of Sasa nipponica (in Japanese with English summary). J Jpn For Soc 60:244–248
Kenney AJ, Krebs CJ (2003) EcoMeth: programs for ecological methodology, 2nd edn, ver. 6.1.1. Exeter Software, New York
Kitamura K, Nakai Y, Sakamoto T, Terashima T, Shirai T (2000) Effects of forest disturbance on transpiration properties of sasa grass community (in Japanese). North For Jpn 52:215–218
Klein BC (1989) Effects of forest fragmentation on dung and carrion beetle communities in central Amazonia. Ecology 70:1715–1725
Krebs CJ (1999) Ecological methodology, 2nd edn. Addison-Wesley/Longman, Menlo Park
Lobo JM, Lumaret J-P, Jay-Robert P (1998) Sampling dung beetles in the French Mediterranean area: effects of abiotic factors and farm practices. Pedobiologia 42:252–266
Maeji I, Yokoyama S, Shibata E (1999) Population density and range use of sika deer, Cervus nippon, on Mt. Ohdaigahara, central Japan. J For Res 4:235–239
Magurran AE (1988) Ecological diversity and its measurement. Croom Helm, London
Masumoto K, Ochi T (1985) Scarabaeidae (Geotrupinae, Hybosorinae, Scarabaeinae, Aphodiinae) (in Japanese). In: Uéno S, Kurosawa Y, Sato M (eds) The coleoptera of Japan in color, vol II. Hoikusha, Osaka, pp 348–379
Medina CA, Escobar F, Kattan GH (2002) Diversity and habitat use of dung beetles in a restored Andean landscape. Biotropica 34:181–187
Nummelin M, Hanski I (1989) Dung beetles of the Kibale Forest, Uganda: comparison between virgin and managed forests. J Trop Ecol 5:349–352
Odum EP (1971) Fundamentals of ecology, 3rd edn. W. B. Saunders, Philadelphia
Peck SB, Forsyth A (1982) Composition, structure and competitive behaviour in a guild of Ecuadorian rain forest dung beetles (Coleoptera: Scarabaeidae). Can J Zool 60:1624–1634
Rohlf FJ, Slice DE (1999) BIOMstat for Windows, ver. 3.3. Execter Software, New York
Smith B, Wilson JB (1996) A consumer’s guide to evenness indices. Oikos 76:70–82
Sokal RR, Rohlf FJ (1995) Biometry, 3rd edn. W. H. Freeman, New York
SPSS (2002) SPSS base 11.5 syntax reference guide. SPSS, Chicago
Takagi K, Tsuboya T, Takahashi H, Inoue T (1999) Effect of the invasion of vascular plants on heat and water balance in the Sarobetsu Mire, Northern Japan. Wetlands 19:246–254
Takeda A (1994) Establishment and regeneration of seedlings of Picea jezoensis var. hondoensis forest on Mt. Ohdaigahara (in Japanese). In: Report on the conservation of spruce forest on Mt. Ohdaigahara (1989–1993). Environment Agency, Japan, pp 14–23
Vessby K, Wiktelius S (2003) The influence of slope aspect and soil type on immigration and emergence of some northern temperate dung beetles. Pedobiologia 47:39–51
Yajima K, Yamamoto Y, Maeji I, Kurosaki T, Yokota T, Sato H, Shibata E (2002) Seasonal changes in home range of female sika deer (Cervus nippon) on Mt. Ohdaigahara, central Japan (in Japanese with English Summary). Nagoya Univ For Sci 21:1–7
Yamamoto S (1993) Gap characteristics and gap regeneration in a subalpine coniferous forest on Mt. Ontake, central Honshu, Japan. Ecol Res 8:277–285
Yamamoto K (2003) LIA for Win32. http://hp.vector.co.jp/authors/VA008416
Yokota T, Nakamura S (2002) Time sequence of Sasa-type grassland enlargement on Mt. Ohdaigahara, Nara (in Japanese). Nara Bot 24–25:15–18
Yokoyama S, Shibata E (1998a) Characteristics of Sasa nipponica grassland as a summer forage resource for sika deer on Mt. Ohdaigahara, central Japan. Ecol Res 13:193–198
Yokoyama S, Shibata E (1998b) The effects of sika-deer browsing on the biomass and morphology of a dwarf bamboo, Sasa nipponica, in Mt. Ohdaigahara, central Japan. For Ecol Manage 103:49–56
Yokoyama S, Koizumi T, Shibata E (1996) Food habits of sika deer assessed by fecal analysis in Mt. Ohdaigahara, central Japan. J For Res 1:161–164
Yokoyama S, Maeji I, Ueda T, Ando M, Shibata E (2001) Impact of bark stripping by sika deer, Cervus nippon, on subalpine coniferous forests in central Japan. For Ecol Manage 140:93–99
Acknowledgements
We thank M. Matsumura, S. Nakamura, and H. Furusawa for their help in field surveys, T. Hino for providing unpublished data, and K. Tsukamoto for identifying some dung-beetle species. This study was financially supported in part by the Ministry of Environment, Japan, and Grants-in-Aid for Scientific Research (no.14206019 and no.14654155) from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Kanda, N., Yokota, T., Shibata, E. et al. Diversity of dung-beetle community in declining Japanese subalpine forest caused by an increasing sika deer population. Ecol Res 20, 135–141 (2005). https://doi.org/10.1007/s11284-004-0033-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11284-004-0033-6