Abstract
The loss of natural habitats is one of the main drivers of biodiversity decline. Anthropogenic land uses preserving biotic and abiotic conditions of the native ecosystem are more suitable to preserve the native biodiversity. In this study, we explored changes in species richness and composition in different land uses of the southern Atlantic forest, considering three independent factors: (1) canopy (presence–absence), (2) type of vegetation (native–exotic) and (3) livestock (presence–absence). We expected a gradient of response in the richness and composition of the native forest dung beetle community, from land uses preserving canopy and native vegetation to open land uses with exotic vegetation. Dung beetles were sampled in protected native forests and four land uses, using two potential food resources: human dung and carrion. The species richness and composition of each habitat, as well as differences in composition and the influence of factors over diversity, were then analyzed. As expected, our results showed that land uses preserving canopy and native vegetation maintain the dung beetle diversity of the native forest. Moreover, while the three factors analyzed influenced dung beetle diversity, canopy cover was the main driver of dung beetle diversity loss. The main conclusion of this study is that the conservation of canopy (either native or exotic) is determinant to preserve highly diverse dung beetle communities and subsequently, the ecological functions performed by this taxon. However, the ecophysiological mechanism behind the response of dung beetles to habitat disturbance is poorly understood.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the last decades, the destruction of natural habitats has been largely identified as one of the main drivers of biodiversity loss across all major taxonomic groups (Dirzo and Raven 2003). Particularly, the expansion of agricultural areas and tree plantations in highly diverse tropical and subtropical forests has had a major influence on biodiversity patterns (Davies and Margules 1998; Myers et al. 2000; Myers and Knoll 2001; Novacek and Cleland 2001; Arellano et al. 2008). Forest replacements influence the abundance of populations and the structure and composition of communities through changes in the availability of resources and microclimatic conditions (ecological niche) (Culot et al. 2013; Nichols et al. 2013; da Silva and Hernández 2016). While in general land uses influence biological diversity, previous studies have shown that anthropogenic lands uses preserving some of the biotic and abiotic conditions of the native ecosystem are more suitable for native species than those that drastically modify the original conditions (Pineda et al. 2005; Quintero and Roslin 2005; Nichols et al. 2007; Hernández and Vaz-de-Mello 2009; Filloy et al. 2010; Zurita and Bellocq 2012; Hernández et al. 2014; Filgueiras et al. 2015; da Silva and Hernández 2016; Gómez-Cifuentes et al. 2017).
Due to their high diversity and abundance (Ocampo and Hawks 2006; Spector 2006), their sensitivity to human disturbances (Verdú et al. 2007; Gardner et al. 2008; Tonelli et al. 2017), their relatively stable taxonomy (Philips et al. 2004), and their importance in ecosystem functioning (Hanski and Cambefort 1991; Andresen and Feer 2005; Nichols et al. 2008; Verdú et al. 2017), Scarabaeinae dung beetles are an excellent focal taxon to explore the influence of anthropogenic disturbances on populations, communities and ecosystem processes. Previous studies in the Atlantic forest (Hernández et al. 2014; Bogoni et al. 2016; Gómez-Cifuentes et al. 2017; Giménez Gómez et al. 2018) and other tropical and neotropical forests (Vulinec 2002; Quintero and Roslin 2005; Nichols et al. 2007; Alvarado et al. 2018) have shown that forest replacement influences dung beetle populations and communities through changes in vegetation structure, microclimate, soils and the availability of trophic resources. Moreover, changes in vegetation structure have probably an indirect effect on dung beetles through the alteration of microclimatic conditions, including radiant heat (Halffter et al. 1992; Verdú et al. 2007), light intensity, and air and soil temperature and humidity (Davis et al. 2002). Forest dung beetles have physiological restrictions and the large majority of species are sensitive to changes in the microclimate (Davis et al. 2000; Duncan and Byrne 2000). Also, changes in soil conditions (compaction, pH, relative humidity) impose new restrictions to forest dung beetle species because these beetles depend on soil-specific conditions, especially for nesting (Osberg et al. 1993; Sowig 1995; Nichols et al. 2013).
The Atlantic forest is one of the most diverse and threatened ecosystems worldwide (Mittermeier et al. 1998). Currently, it is characterized by highly heterogeneous landscapes, combining large tracks of native forest with agricultural areas, pastures and tree plantations. This area offers the opportunity to simultaneously explore changes in dung beetle diversity in a large variety of land uses within the same region (Izquierdo et al. 2008; Zurita and Bellocq 2012). Taking advantage of this heterogeneity, our main objective was to explore changes in species richness and composition between different land uses, considering three independent factors usually related to dung beetle diversity: (1) canopy (presence-absence), (2) type of vegetation (native-exotic) and (3) livestock (presence-absence). We expected a gradient of response in the richness and composition of the native forest dung beetle community, from land uses preserving canopy and native vegetation to open land uses with exotic vegetation.
Materials and methods
Study area and experimental design
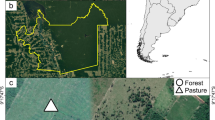
This study was performed in the southern Atlantic forest of Argentina (Fig. 1), a semi-deciduous forest with average precipitations of 2000 mm distributed throughout the year and an average temperature of 25 °C in summer and 15 °C in winter (Oliveira-Filho and Fontes 2000). Landscapes are composed of large tracks of continuous native forest in protected areas (Parque Nacional Iguazú, Parque Provincial Urugua-í, etc.), exotic tree plantations (mainly Pinus taeda) with and without livestock (silvo-pastoral systems), open pastures with livestock and exotic grasses, small-scale annual crops (such as corn and tobacco) and Yerba mate (Ilex paraguariensis) plantations (Izquierdo et al. 2008; Zurita and Bellocq 2012). Dung beetles were sampled during the 2016 spring (November–December), the time of the year with the highest activity of dung beetles in the region (Hernández and Vaz-de-Mello 2009).
The objective of this study was to explore the individual influence of the following three factors: (1) canopy cover (presence-absence), (2) type of vegetation (native-exotic) and (3) livestock (presence-absence) (Table 1), on dung beetle diversity. To achieve this objective, within the study area, we selected four different land uses, differing in the above-mentioned three factors (five replicates of each land use): (A) mature pine plantations (10–12 years old) (Pinus taeda) (pine plantations; exotic canopy cover without livestock); (B) native forest with livestock (agroforestry parklands; native canopy cover with livestock); (C) pine plantations (Pinus taeda) with livestock (silvo-pastoral systems; exotic canopy cover with livestock); and (D) deforested areas of pastures with livestock (open pastures without canopy). Additionally, five replicates of native continuous forest were sampled in protected areas (Parque Nacional Iguazú and Parque Provincial Urugua-í). A detailed description of the land uses selected and the native forest can be found in Online Appendix S1. The distance between sampling sites (25 in total) was at least 1 km to guarantee independence among samples. To increase regional representativeness, sampling sites were clustered in four areas separated by a minimum distance of 30 km. All land uses were represented in all areas.
Dung beetle sampling
A grid of 150 m × 150 m was established in each sampling site (25 in total, five in the native forest and five in each land use). Each grid contained 16 traps separated by 50 m, to minimize the interference between traps (16 traps × 25 sites = 400 traps) (Larsen and Forsyth 2005; Tshikae et al. 2013). Traps consisted of a plastic container (12 cm in diameter and depth) filled with water, neutral detergent and salt to avoid the decomposition of individuals, without interfering with attraction (Nichols et al. 2007). In each replicate, eight traps were baited with human excrement and eight with rotten meat, to capture both necrophagous and coprophagous species (Halffter and Matthews 1966; Spector 2006). Human excrements and rotten meat are the most commonly used baits in neotropical studies to attract the majority of dung beetle species (Audino et al. 2014, da Silva and Hernández 2016). Four sampling periods of 72 h (12 days) were carried out, collecting the material and renewing the bait in each period. All samples were preserved in 70% alcohol until further processing and identification of specimens at species or genus levels using taxonomic guides and the assistance of specialists (Vaz-de-Mello, personal communication). Collected individuals were deposited at the Scarabaeidae Collection of the Instituto de Biología Subtropical—Iguazú (IBSI Sca), Misiones, Argentina.
Data analysis
To estimate sample coverage, the estimator of the sample coverage of the reference sample was calculated on INEXT (Chao et al. 2016). At community level, to compare species richness between habitats, a non-parametric Kruskal–Wallis test and a pairwise post hoc comparison were performed using the ‘conover.test’ package in R (Dinno 2017; R Core Team 2017).
To explore differences in dung beetle species composition between land uses and the native forest, a non-metric multi-dimensional scaling (NMDS) analysis using Log (x + 1) transformation and Bray–Curtis index was performed (Clarke, 1993). The Log (x + 1) transformation was used to improve the visualization of points in the Fig. 3. The statistical differences between the groups formed by the NMDS were compared using a nonparametric permutation-based multivariable analysis of variance (PERMANOVA). The P value was estimated after 999 permutations. Both NMDS and PERMANOVA were performed with PRIMER 6 + Permanova software (Clarke and Gorley 2006).
Finally, to explore the individual influence of canopy cover (presence-absence), livestock (presence-absence) and vegetation type (native-exotic) on the composition of dung beetle assemblages on the different land uses, we made a supplementary PERMANOVA (Bray–Curtis index as a dissimilarity) using the ‘Adonis’ function in R package vegan (Oksanen et al. 2017; R Core Team 2017). We used the replicate data to perform the PERMANOVA and constructed a model with canopy cover, livestock and vegetation type as explanatory variables (factors). The Adonis function provides a statistic (F), a measure of the “effect size” (R2) and a P-value (P) for each factor. Factors with P-value below 0.05 are considered significant and higher R2 indicates higher explanatory power.
Results
We collected 14,712 individuals corresponding to 47 species of 16 genera of the subfamily Scarabaeinae. Of the 47 species, 27 were captured in the native forest, 24 in the pine plantations, 31 in the agroforestry parklands, 24 in the silvo-pastoral systems and 19 in the open pastures. Canthon quinquemaculatus Castelnau was the most abundant species in the native forests, agroforestry parklands and silvo-pastoral systems, Eurysternus caribaeus Herbst in pine plantations, and Eutrichillum hirsutum Boucomont in open pastures (Table 2). Only eight species were found in all habitats: Canthon conformis Harold, C. quinquemaculatus, Coprophanaeus cyanescens Olsoufieff, Deltochilum aff. komareki Balthasar, Dichotomius sericeus Harold, Eurysternus parallelus Castelnau, E. hirsutum, Onthophagus tristis Harold; and only 15 species were found in one habitat (five in native forests, one in pine plantations, five in agroforestry parklands, one in silvo-pastoral systems and three in open pastures). Finally, the sampling effort captured more than 98% of species in all the habitats (Online Appendix S2).
When comparing species richness, significant differences were found between habitats (K-W, H = 11.30, N = 4, P = 0.0212). All habitats with canopy cover (native forests, pine plantations, agroforestry parklands and silvo-pastoral systems) showed similar and higher richness compared to open pastures (Fig. 2).
Species richness of dung beetles (whiskers, median and outliers) in the native forest (NF) and four land uses (PP: pine plantations, AP: agroforestry parklands, SS: silvo-pastoral systems and OP: open pastures) in the southern Atlantic forest of Argentina. Different letters indicate significant differences with P < 0.05 (Conover post hoc pairwise test)
When comparing species composition among land uses, the NMDS clearly separated the open pastures from the native forest and the rest of the land uses (2D Stress: 0.08) (Fig. 3). Also, the PERMANOVA analysis showed that significant differences exist between all land uses (PERMANOVA, F = 12.348, df = 4, P = 0.001), with the only exception of agroforestry parklands and silvo-pastoral systems (Table 3). Finally, the supplementary PERMANOVA (through the Adonis function) showed that the three factors proposed explained differences in dung beetle compositions among habitats and the native forest, but in a different magnitude: canopy cover presence had a higher effect (P = 0.001, R2 = 0.541), then was the livestock presence (P = 0.002, R2 = 0.094) and finally the vegetation type (P = 0.046, R2 = 0.043) (Table 4).
Non-metric multi-dimensional scaling (NMDS) based on the dung beetle community composition in the native forest and four land uses in the southern Atlantic forest of Argentina. Triangles: native forests (NF), squares: pine plantations (PP), inverted triangles: agroforestry parklands (AP), dots: silvo-pastoral systems (SS) and diamonds: open pastures (OP)
Discussion
We expected that the land uses preserving canopy and native vegetation would also preserve the dung beetle diversity of the native forest (both regarding richness and composition). Our results partially support this hypothesis since both the richness and composition of species were similar to the native forest in land uses with canopy cover (agroforestry parklands, pine plantations and silvo-pastoral systems) and strongly different in open habitats (open pastures). While the presence of cows and the vegetation type had a significant effect, explaining differences in species composition, the presence of canopy was, by far, the primary factor preserving native dung beetle communities in studied land uses. Previous studies carried out in the Atlantic forest have explored the influence of canopy cover, microclimate conditions and diversity of resources on the response of dung beetles to habitat disturbance (Hernández et al. 2014; da Silva and Hernández 2016; Bogoni et al. 2016; Gómez-Cifuentes et al. 2017; Giménez Gómez et al. 2018); however, this is the first study considering canopy cover, livestock and vegetation type at the same time to explain the response of dung beetle communities to intensive land uses.
A large number of previous studies have shown that the replacement, fragmentation and degradation of tropical and subtropical forests by intensive and semi-intensive land uses (e.g., cattle raising, tree plantations and agriculture) change dung beetle abundance (Sánchez-de-Jesús et al. 2016), richness and composition (Halffter and Arellano 2002; Scheffler 2005; Nichols et al. 2007; Gardner et al. 2008; Neita and Escobar 2012; Peyras et al. 2012; Audino et al. 2014; Hernández et al. 2014; Gómez-Cifuentes et al. 2017). In general, the magnitude of these changes has been associated with the loss of forest resources (such as trophic resources) and conditions (Nichols et al. 2007; Hernández et al. 2014; Hewavithana et al. 2016; Gómez-Cifuentes et al. 2017). In our study, canopy cover was the main driver of dung beetle diversity; land uses preserving forest canopy cover (either exotic or native) preserved not only forest species richness but also species composition. In contrast, both richness and composition greatly decreased in the open habitats. Our results are similar to those found by Nichols et al. (2007) but differ from those of Gardner et al. (2008). This discrepancy is probably associated with the type of land uses considered in each study. Gardner et al. (2008) found microclimatic differences between forest and tree plantations, whereas Nichols et al. (2007) and us found that the microclimatic conditions of the forest and the land uses preserving canopy were similar. These differences could be explained by the physiological intolerance of forest dung beetles to high temperatures and low humidity (Sowig 1995; Lobo et al. 1998; Chown 2001).
In a recent study, Alvarado et al. (2018) also found that the canopy cover has a primary influence determining dung beetle communities in livestock areas at multiple scales and that the presence of livestock has a secondary effect. Canopy cover has probably an indirect influence on dung beetles through the regulation of several soil and understory microclimatic conditions such as radiant heat, light intensity, air temperature and humidity, and soil temperature and humidity (Halffter et al. 1992; Davis et al. 2002; Tuff et al. 2016). Given the narrow abiotic tolerances of many forest dung beetle species, disturbances that alter microclimatic factors directly affect dung beetle diversity (Davis et al. 2000; Duncan and Byrne 2000; Chown 2001; Nichols et al. 2007). In relation to the influence of livestock, although many species are primary coprophagous in the study area, cow dung is probably a low quality resource since the majority of species prefer dung for omnivorous species rather than herbivorous (Giménez Gómez et al. 2018); this low preference could probably explain the secondary role of livestock presence, explaining differences in species composition. Moreover, the diversity of dung types, and therefore the diversity of mammals, is more important than the abundance of only one dung type to maintain dung beetle communities in a specific habitat (Bogoni et al. 2016; Giménez Gómez et al. 2018). Additionally, the capacity of native species to exploit open habitats in the Atlantic forest is probably more related to physiological tolerance rather than resources availability (Giménez Gómez et al. 2018). As an example, Chalcocopris hesperus Olivier and Dichotomius sericeus are coprophagous species but the first species was captured only in the native forest and the second was present in all habitats. The main difference between both species is the activity pattern: Chalcocopris hesperus is a diurnal species while Dichotomius sericeus is nocturnal. The type of dung that a species uses can limit its capacity to use a specific habitat but its thermal tolerance is probably more important and is associated with canopy cover. Finally, the low influence of vegetation type (native or exotic) is not surprising because only very few species of dung beetles require plant material for feeding or nesting, such as species of the genera Paraphytus Harold (rotten wood), Pachysoma Macleay (vegetable detritus and dry dung), and Cephalodesmius Westwood (decomposed leaf pieces, flowers, seeds, and fruits) (Halffter and Matthews 1966; Monteith and Storey 1981; Scholtz et al. 2004; Davis et al. 2008; Halffter and Halffter 2009; Holter et al. 2009). In our study, only Dichotomius carbonarius Mannerheim has been found provisioning its brood chambers with a filling of small, comminuted, dry leaf pieces and an outer layer of more entire leaves (Dinghi et al. 2013).
Our results and previous studies suggest that the microclimatic conditions in the understory, rather than the type of vegetation or presence of livestock, determine patterns of dung beetles in land uses of tropical and subtropical regions. These results lead to a simple but powerful conclusion for the management of anthropogenic land uses: the conservation of canopy (either native or exotic) is determinant to preserve dung beetle communities and, highly probably, the ecological functions performed by this taxon. However, the ecophysiological mechanism behind the response of dung beetles to habitat disturbance is poorly understood. Thus, further studies in this direction should be performed to gather information to increase the long-term suitability of land uses.
References
Alvarado F, Escobar F, Williams DR, Arroyo-Rodriguez V, Escobar-Hernández F (2018) The role of livestock intensification and landscape structure in maintaining tropical biodiversity. J Appl Ecol 55:185–194
Andresen E, Feer F (2005) The role of dung beetles as secondary seed dispersers and their effect on plant regeneration in tropical rainforests. In: Forget PM, Lambert JE, Hulme PE, Vander Wall SB (eds) Predation, dispersal and seedling establishment. CABI International, Wallingford, pp 331–349
Arellano L, León-Cortés J, Halffter G (2008) Response of dung beetle assemblages to landscape structure in remnant natural and modified habitats in southern Mexico. Insect Conserv Diver 1:253–262
Audino I, Louzada J, Comita L (2014) Dung beetles as indicators of tropical forest restoration success: is it possible to recover species and functional diversity? Biol Conserv 169:248–257
Bogoni JA, Graipel ME, Volkmer de Castilho P, Moreli Fantacini F, Villanova Kuhnen V, Ribeiro Luiz M, Bernardes Maccarini T, Marcon CB, Pimentel Teixeira CS, Tortato MA, Vaz-de-Mello FZ, Hernández MIM (2016) Contributions of the mammal community, habitat structure, and spatial distance to dung beetle community structure. Biodivers Conserv 25:1661–1675
Campanello PI, Montti L, Goldstein G, Mac Donagh P (2009) Reduced impact logging and post-harvesting forest management in the Atlantic Forest: alternative approaches to enhance canopy tree growth and regeneration and to reduce the impact of invasive species. In: Grossberg SP (ed) forest management. Nova Science, New York, pp 39–59
Chao A, Ma KH, Hsieh TC (2016) iNEXT (iNterpolation and EXTrapolation) Online: software for interpolation and extrapolation of species diversity. Program and user’s guide. http://chao.stat.nthu.edu.tw/wordpress/software_download/
Chown SL (2001) Physiological variation in insects: hierarchical levels and implications. J Insect Physiol 47:649–660
Clarke KR (1993) Non-parametric multivariate analyses of changes in community structure. Aust J Ecol 18:117–143
Clarke KR, Gorley RN (2006) PRIMER v6. User manual/tutorial. PRIMER-E, Plymouth
Culot L, Bovy E, Vaz-de-Mello FZ, Guevara R, Galetti M (2013) Selective defaunation affects dung beetle communities in continuous Atlantic rainforest. Biol Conserv 163:79–89
Da Silva PG, Hernández MIM (2016) Spatial variation of dung beetle assemblages associated with forest structure in remnants of southern Brazilian Atlantic Forest. Rev Bras Entomol 60:73–81
Davies KF, Margules CR (1998) Effects of habitat fragmentation on carabid beetles: experimental evidence. J Anim Ecol 67:460–471
Davis ALV, Chown SL, McGeoch MA, Scholtz CH (2000) A comparative analysis of metabolic rate in six Scarabaeus species (Coleoptera: Scarabaeidae) from southern Africa: further caveats when inferring adaptation. J Insect Physiol 46:553–562
Davis ALV, Van Aarde RJ, Scholtz CH, Delport JH (2002) Increasing representation of localized dung beetles across a chronosequence of regenerating vegetation and natural dune forest in South Africa. Glob Ecol Biogeogr 11:191–209
Davis ALV, Frolov AV, Scholtz CH (2008) The African dung beetle genera. Protea Book Publishers, Pretoria
Dinghi PA, Sánchez MV, Cantil LF, Sarzetti LC, Genise JF (2013) Leaf-litter brood chambers in dichotomius (luederwaldtinia) carbonarius (mannerheim, 1829) (coleoptera: scarabaeidae): a novel behavior for dung beetles. Coleopt Bull 67:388–396
Dinno A (2017) Conover.test: conover-iman test of multiple comparisons using rank sums. R package version 1.1.4. https://CRAN.R-project.org/package=conover.test. Accessed 2nd Dec 2017
Dirzo R, Raven PH (2003) Global state of biodiversity and loss. Annu Rev Environ Resour 28:137–167
Duncan FD, Byrne MJ (2000) Discontinuous gas exchange in dung beetles: patterns and ecological implications. Oecologia 122:452–458
Filgueiras B, Tabarelli M, Leal I, Vaz-De-Mello FZ, Iannuzzi L (2015) Dung beetle persistence in human-modified landscapes: combining indicator species with anthropogenic land uses and fragmentation- related effects. Ecol Indic 55:65–73
Filloy J, Zurita GA, Corbelli J, Bellocq MI (2010) On the similarity among bird communities: testing the influence of distance and land use. Acta Oecol 36:333–338
Gardner TA, Hernández MIM, Barlow J, Peres CA (2008) Understanding the biodiversity consequences of habitat change: the value of secondary and plantation forests for neotropical dung beetles. J Appl Ecol 45:883–893
Giménez Gómez VC, Verdú JR, Gómez-Cifuentes A, Vaz-de-Mello FZ, Zurita GA (2018) Influence of land use on the trophic niche overlap of dung beetles in the semideciduous Atlantic forest of Argentina. Insect Conserv Divers. https://doi.org/10.1111/icad.12299
Gómez-Cifuentes A, Munevar A, Gimenez VC, Gatti MG, Zurita GA (2017) Influence of land use on the taxonomic and functional diversity of dung beetles (Coleoptera: Scarabaeinae) in the southern Atlantic forest of Argentina. J Insect Conserv 21:147–156
Halffter G, Arellano L (2002) Response of dung beetle diversity to human-induced changes in a tropical landscape. Biotropica 34:144–154
Halffter G, Halffter V (2009) Why and where coprophagous beetles (Coleoptera: Scarabaeinae) eat seed, fruits or vegetable detritus. Bol SEA 45:1–22
Halffter G, Matthews EG (1966) The natural history of dung beetles of the subfamily Scarabaeinae (Coleoptera, Scarabaeidae). Folia Entomol Mex 14:1–312
Halffter G, Favila ME, Halffter V (1992) A comparative study of the structure of the scarab guild in Mexican tropical rain forests and derived ecosystems. Folia Entomol Mex 84:131–156
Hanski I, Cambefort Y (1991) Dung beetle ecology. Princeton University Press, Princeton
Hernández MIM, Vaz-de-Mello FZ (2009) Seasonal and spatial species richness variation of dung beetle (Coleoptera, Scarabaeidae s. str.) in the Atlantic Forest of southeastern Brazil. Rev Bras Entomol 153:607–613
Hernández MIM, Barreto PSCS, Costa VH, Creao-Duarte J, Favila ME (2014) Response of a dung beetle assemblage along a reforestation gradient in Restinga forest. J Insect Conserv 18:539–546
Hewavithana DK, Wijesinghe MR, Dangalle CD, Dharmarathna HAS (2016) Habitat and dung preferences of scarab beetles of the subfamily Scarabaeinae: a case study in a tropical monsoon forest in Sri Lanka. Int J Trop Insect Sci 36:97–105
Holter P, Scholtz CH, Stenseng L (2009) Desert detritivory: nutritional ecology of a dung beetle (Pachysoma glentoni) subsisting on plant litter in arid South African sand dunes. J Arid Environ 73:1090–1094
Izquierdo AE, De Angelo CD, Aide TM (2008) Thirty years of human demography and land use change in the Atlantic Forest of Misiones, Argentina: an evaluation of the forest transition model. Ecol Soc 13:3. http://www.ecologyandsociety.org/vol13/iss2/art3/
Larsen TH, Forsyth A (2005) Trap spacing and transect design for dung beetles biodiversity studies. Biotropica 37:322–325
Lobo JM, Lumaret JP, Jay-Robert P (1998) Sampling dung beetles in the French Mediterranean area: effects of abiotic factors and farm practices. Pedobiologia 42:252–266
Mittermeier RA, Myers N, Thomsen JB, Da Fonseca GAB, Olivieri S (1998) Biodiversity hotspots and major tropical wilderness areas: approaches to setting conservation priorities. Conserv Biol 12:516–520
Monteith GB, Storey RI (1981) The biology of Cephalodesmius, a genus of dung beetles which synthesizes “dung” from plant material (Coleoptera: Scarabaeidae: Scarabaeinae). Mem Queensl Mus 20:253–277
Myers N, Knoll AH (2001) The biotic crisis and the future of evolution. Proc Natl Acad Sci USA 98:5389–5392
Myers N, Mittermeier RA, Mittermeier CG, Da Fonseca GAB, Kent YJ (2000) Biodiversity hotspots for conservation priorities. Nature 403:853–858
Neita JC, Escobar F (2012) The potential value of agroforestry to dung beetle diversity in the wet tropical forests of the Pacific lowlands of Colombia. Agrofor Syst 85:121–131
Nichols E, Larsen T, Spector S, Davis AL, Escobar F, Favila M, Vulinec K (2007) Global dung beetle response to tropical forest modification and fragmentation: a quantitative literature review and meta-analysis. Biol Conserv 137:1–19
Nichols E, Spector S, Louzada J, Larsen T, Amezquita S, Favila ME (2008) Ecological functions and ecosystem services provided by Scarabaeinae dung beetles. Biol Conserv 141:1461–1474
Nichols E, Uriarte M, Bunker DE, Favila ME, Slade EM, Vulinec K (2013) Trait-dependent response of dung beetle populations to tropical forest conversion at local and regional scales. Ecology 94:180–189
Novacek MJ, Cleland EE (2001) The current biodiversity extinction event: scenarios for mitigation and recovery. Proc Natl Acad Sci USA 98:5466–5470
Ocampo FC, Hawks DC (2006) Molecular phylogenetics and evolution of the food relocation behaviour of the dung beetle tribe Euraniini (Coleoptera: Scarabaeidae: Scarabaeinae). Invertebr Syst 20:557–570
Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens MHM, Szoecs E, Wagner H (2017) vegan: Community Ecology Package. R package version 2.4-3. https://CRAN.R-project.org/package=vegan2012. Accessed 6 Nov 2017
Oliveira-Filho AT, Fontes IAM (2000) Patterns of floristic differentiation among Atlantic forests in Southeastern Brazil and the influence of climate. Biotropica 32:793–810
Osberg DC, Doube BM, Hanrahan SA (1993) Habitat specificity in African dung beetles: the effect of soil type on dung burial by two species of ball-rolling dung beetles (Coleoptera: Scarabaeidae). Trop Zool 6:243–251
Peyras M, Vespa N, Bellocq M, Zurita G (2012) Quantifying edge effects: the role of habitat contrast and species specialization. J Insect Conserv 17:807–820
Philips K, Pretorius E, Scholtz C (2004) A phylogenetic analysis of dung beetles (Scarabaeinae: Scarabaeidae): unrolling an evolutionary history. Invertebr Taxon 18:53–88
Pineda E, Moreno C, Escobar F, Halffter G (2005) Frog, bat and dung beetle diversity in the cloud forest and coffee agroecosystems of Veracruz, Mexico. Conserv Biol 19:400–410
Quintero I, Roslin T (2005) Rapid recovery of dung beetle communities following habitat fragmentation in central Amazonia. Ecology 12:3303–3311
R Development Core Team (2017) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. ISBN 3-900051-07-0. http://www.R-project.org
Sánchez-de-Jesús H, Arroyo-Rodríguez V, Andresen E, Escobar F (2016) Forest loss and matrix composition are the major drivers shaping dung beetle assemblages in a fragmented rainforest. Landsc Ecol 31:843–854
Scheffler PY (2005) Dung beetle (Coleoptera: Scarabaeidae) diversity and community structure across three disturbance regimes in eastern Amazonia. J Trop Ecol 21:9–19
Scholtz CH, Harrison JG, Grebennikov VV (2004) Dung beetle (Scarabaeus (Pachysoma)) biology and immature stages: reversal to ancestral states under desert conditions (Coleoptera: Scarabaeidae). Biol J Linnean Soc 83:453–460
Sowig P (1995) Habitat selection and offspring survival rate in three paracoprid dung beetles: the influence of soil type and soil moisture. Ecography 18:147–154
Spector S (2006) Scarabaeine dung beetles (Coleopteran: Scarabaeidae: Scarabaeinae): an invertebrate focal taxon for biodiversity research and conservation. Coleopt Bull 60:71–83
Tonelli M, Verdú JR, Zunino M (2017) Effects of grazing intensity and the use of veterinary medical products on dung beetle biodiversity in the sub-mountainous landscape of Central Italy. PeerJ Preprints 4:e2358v1. https://doi.org/10.7287/peerj.preprints.2358v1
Tshikae BP, Davis ALV, Scholtz CH (2013) Species richness e energy relationships and dung beetle diversity across an aridity and trophic resource gradient. Acta Oecol 49:71–82
Tuff KT, Tuff T, Davies KF (2016) A framework for integrating thermal biology into fragmentation research. Ecol Lett 19:361–374
Verdú JR, Moreno CE, Sánchez-Rojas G, Numa C, Galante E, Halffter G (2007) Grazing promotes dung beetle diversity in the xeric landscape of a Mexican biosphere reserve. Biol Conserv 140:308–317
Verdú JR, Lobo MJ, Sánchez-Piñero F, Gallego B, Numa C, Lumaret JP, Cortez V, Ortiz A, Tonelli M, García-Teba Rey A, Rodriguez A, Durán J (2017) Ivermectin residues disrupt dung beetle diversity, soil properties and ecosystem functioning: an interdisciplinary field study. Sci Total Environ 618:219–228
Vulinec K (2002) Dung beetle communities and seed dispersal in primary forest and disturbed land in Amazonia. Biotropica 34:297–309
Zaninovich SC, Fontana JL, Gatti MG (2016) Atlantic forest replacement by non-native tree plantations: comparing aboveground necromass between native forest and pine plantation ecosystems. For Ecol Manag 363:39–46
Zurita GA, Bellocq MI (2012) Bird assemblages in anthropogenic habitats: identifying a suitability gradient for native species in the Atlantic forest. Biotropica 44:412–419
Acknowledgements
We wish to thank Fernando Foletto, Andrés Gómez-Cifuentes, Gisele Jaime, Natalia Vespa and Soledad Soto for their help in the fieldwork. Also, we wish to thank farmers for their hospitality, Fernando Vaz-de-Mello for helping us to examine the taxonomic component in the identification of dung beetles, Santiago José Velazco for the assistance in statistical analysis and Juan Ariel Insaurralde for helping us with the map. Finally, we wish to thank anonymous reviewers who have helped to improve the manuscript. The Centro de Investigaciones del Bosque Atlántico (CeIBA) Misiones, Argentina, provided logistical support. Financial support was provided by CONICET (Project UE IBS # 22920160100130CO to M. Di Bitteti), UCAR-MAGyP (BIO 23, PIA 10105-14057 to G. Zurita) and ANPCyT (PICT-PRH 2702 to G. Zurita). National Park Administration, the Misiones Ministry of Ecology and Arauco Argentina S.A. provided the necessary permissions to collect dung beetles.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by David Hawksworth.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Giménez Gómez, V.C., Verdú, J.R., Guerra Alonso, C.B. et al. Relationship between land uses and diversity of dung beetles (Coleoptera: Scarabaeinae) in the southern Atlantic forest of Argentina: which are the key factors?. Biodivers Conserv 27, 3201–3213 (2018). https://doi.org/10.1007/s10531-018-1597-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-018-1597-8