Abstract
The emergence of multidrug-resistant bacteria, viruses and tumors is a serious threat to public health. Among natural peptides, indolicidin, a 13-residue peptide belonging to the cathelicidin family, deserves special attention. Indolicidin has a broad spectrum of biological activity and is active against a wide range of targets, such as bacteria (Gram+ and Gram−), fungi and viruses. Here, we review the most important features of the biological activity, potential applications and perspectives of indolicidin and its analogs. Although not yet approved for commercialization, this peptide has great potential to be applied in different areas, including the medical, biomedical, food industry and other unexplored areas. To achieve this goal, a multidisciplinary team of researchers must work together to fine tune peptides that overall lead to novel analogs and formulations to combat existing and possibly future diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Antimicrobial peptides
Antimicrobial peptides (AMPs) are part of the innate immune system constituting the body's first line of defense for pathogen inactivation (Pasupuleti et al. 2012). Among the many ways to classify AMPs, one takes into account their biosynthetic mechanism. Thus, there are ribosomal and nonribosomal AMPs. The first are gene-encoded, ribosomally synthesized peptides that are widely distributed in nature. On the other hand, nonribosomal AMPs are produced by nonribosomal peptide synthetases not limited to the 20 proteinogenic amino acids (Liu et al. 2019). To date, more than 3000 AMPs (naturals and synthetics) have been included in different databases (Kang et al. 2019; Wang et al. 2016). The antimicrobial activity of AMPs is regulated by chemical and physical properties, including peptide charge, hydrophobicity, amphipathicity, and self-assembly equilibria (Shagaghi et al. 2018). AMPs have wide-spectrum activity being active against pathogenic microbes, including bacteria, fungi, parasites and viruses (Huan et al. 2020). In addition to their antimicrobial activity, AMPs are candidates as antitumor agents (Deslouches and Peter Di 2017). A very interesting feature of AMPs is the low tendency to resistance by the targeted cells (Datta and Roy 2021; Huan et al. 2020; Magana et al. 2020). This is mainly because most AMPs exploit fundamental features of bacterial cells, such as the plasma membrane (Maróti Gergely et al. 2011). However, several studies have shown diverse mechanisms of bacterial resistance to AMPs, such as alteration of membrane charge or fluidity, degradation, and removal by efflux pumps (Abdi et al. 2019; Joo et al. 2016).
Despite the promising features, AMPs have some issues to resolve, such as selectivity, stability and cost of production (Chen and Lu 2020). Fortunately, AMPs can be chemically modified so their biological activity and stability can be improved (Li et al. 2021). Between the thousands of AMPs reported, we highlighted indolicidin. In this review, we summarized the main features of indolicidin and its analogs to push the further application of these peptides in various fields. This is the first review entirely dedicated to indolicidin, a peptide not yet fully explored.
Cathelicidins
The cathelicidin family, also named ‘myeloid antimicrobial peptides’, is a large group of AMPs found in a variety of mammalian species, such as humans and farm animals (bovine, porcine, caprine and chicken) (Kościuczuk et al. 2012; Tomasinsig et al. 2010). Cathelicidins are stored in the secretory granules of neutrophils and macrophages and are released upon leukocyte activation. These peptides are small, cationic, amphipathic and show a broad spectrum of antimicrobial activity. They are processed from precursors (prepropeptides) that are synthesized in myeloid bone marrow cells (Kastin 2013). The mature peptides vary markedly in sequence and length, ranging from 12 to 100 amino acid residues. Cathelicidin encoding genes contain four exons. The first exon encodes the conserved signal peptide (29–30 amino acids), whereas the second and third exons encode the major part of the cathelin-like domain (94–114 amino acids). Finally, the fourth exon encodes the last few amino acid residues of the cathelin domain and is hypervariable, encoding the heterogenous mature peptide, with the variable antimicrobial domain consisting of 12–100 amino acids, with very limited sequence similarity between species (Fig. 1).
C-terminal mature peptides could be activated by proteolytic cleavage and exert antimicrobial and immunomodulatory activities after being released from the N-terminal cathelin portion (Zanetti 2004). The exact mechanism of processing is still not understood, as the unprocessed propeptide can be found extracellularly as a variety of processed forms (Kastin 2013). Based on amino acid sequences, mature cathelicidin peptides after the protease cleaving steps are quite diverse and can be broadly categorized into cyclic dodecapeptides with one disulfide bond, porcine protegrins with two disulfide bonds, peptides with an α-helical structure, peptides containing a high number of tryptophan, proline and arginine residues, and short tandemly arranged peptides.
The mechanism of action of most cathelicidins, such as that of other AMPs, involves interactions with cell membranes. This action is based on the interaction of cationic residues and the negatively charged lipid membranes of microorganisms (Ramanathan et al. 2002). Cathelicidins can also act on other targets, such as protein synthesis and DNA replication. On the other hand, cathelicidin's immunomodulatory activity is mediated through its interaction with cells that are part of the innate immune system, such as monocytes, dendritic cells, T cells and epithelial cells (Bowdish et al. 2006). Other known biological functions of cathelicidins include wound repair, angiogenesis and chemotaxis (Kościuczuk et al. 2012).
The story of the cathelicidin family began when the first cathelicidin, cecropin, was isolated in 1980 from Hyalophora cecropia (Hultmark et al. 1980) and continue with magainin, isolated in 1987 by Zasloff from the skin of the Xenopus leavis frog. Later, Scocchi et al. in 1997, characterized the bovine cathelicidin gene family on the basis of molecular cloning and sequence analysis of cDNAs. According to Tomasinig et al. (2010), the different types of cathelicidins expressed in bovines, including indolicidin, may contribute to the integrated response to infection.
Indolicidin
Indolicidin, which was discovered by Selsted et al. (1992), is a natural peptide belonging to the cathelicidin family isolated from bovine neutrophils. The peptide has 13 amino acid residues, almost half of which are hydrophobic, including 5 residues of tryptophan. Tryptophan residues are responsible for insertion and partitioning in biological membranes, leading to the hemolytic activity of the peptide (Subbalakshmi et al. 1996). The sequence and physicochemical properties of indolicidin are presented in Table 1.
The structure of indolicidin (in several environments) has been studied using different biophysical and theoretical techniques. Structural characterization using circular dichroism and 1H NMR spectroscopy supported the absence of any secondary structure for indolicidin (Podorieszach and Huttunen-Hennelly 2010). Furthermore, molecular dynamics simulations indicated that the peptide adopts a boat-shaped conformation in negatively charged lipid bilayers (Hsu and Yip 2007). The differentiated amino acid composition of indolicidin determines multiple spatial conformations of the molecule. This “structural plasticity” appears to be important in the biological activity of the peptide (Hsu et al. 2005). This plasticity also ensures the recognition of a broad spectrum of microorganisms (Nagpal et al. 2009).
Indolicidin’s targets
In terms of biological activity, indolicidin is also a unique peptide since it has a wide range of biological targets, including Gram-positive (Gram+) and Gram-negative (Gram−) bacteria (Vergis et al. 2020). Antibacterial activity is not confined to planktonic organisms, and there are also several studies showing antibiofilm activity (de Alteriis et al. 2018; Mataraci and Dosler 2012). Additionally, several researchers found antifungal and antiparasitic activities for indolicidin (de Alteriis et al. 2018; Haines et al. 2003). Furthermore, certain viruses are inactivated by indolicidin (Krajewski et al. 2004; Ron-Doitch et al. 2016). Indolicidin has also been evaluated in combination with conventional antibiotics with the aim of evaluating potential synergism (Ghaffar et al. 2015; Mataraci and Dosler 2012). Finally, Bacalum (2016) reported activity against neuroblastoma SH-SY-5Y.
Planktonic microorganisms
Most of the studies on AMP activity, including indolicidin, are conducted on planktonic microorganisms. Table 2 shows microorganisms, minimal inhibitory concentration (MIC) values and references of studies conducted with indolicidin. The wide spectrum of activity translates into a wide spectrum of applications, including human and animal health, the food industry and others.
Biofilms
Pathogens are able to adapt to different conditions and develop self-defense mechanisms, such as living in biofilms (Galdiero et al. 2016). Attachment to a living (mucosal and soft tissues) or nonliving surface (medical devices such as catheters) is common for several microorganisms. The clinical importance of biofilms is related to their structure, which grants more tolerance to antibiotics and disinfectants than the planktonic form. Antibiofilm activity is commonly addressed in two ways: the inhibition of biofilm formation and the destruction of the preformed biofilm. Interestingly, indolicidin has shown both activities. Alteriis et al. (2018), demonstrated that indolicidin causes 20–50% inhibition of biofilm formation and 10–60% destruction of preformed biofilms, both produced by fungi (Candida albicans and Candida tropicalis). Furthermore, indolicidin can also destroy and inhibit bacterial biofilms formed by resistant Escherichia coli and Staphylococcus aureus (Mataraci and Dosler 2012; Vergis et al. 2020).
There is a high demand for developing novel molecules to treat biofilm-associated microbial infection (Pervin and Hassan 2021). Evaluation of antibiofilm activity is underrated and constitutes a research field still less explored for indolicidin and its analogs.
Lipopolysaccharide interaction
Lipopolysaccharide (LPS) is considered an endotoxin and the major outer surface membrane component of almost all Gram-bacteria and acts as a powerful stimulant of the immune system (Cohen 2002). LPS activates inflammatory cells such as monocytes and macrophages, causing them to release cytokines (e.g., TNF-a, IL-1b and IL-6) and nitric oxide that trigger an inflammatory cascade (Nan et al. 2009a; Park et al. 2009). Interestingly, indolicidin has been shown to bind and neutralize LPS molecules with similar affinity than polymyxin B, defensins and cecropin–melittin hybrids (Falla et al. 1996; Nan et al. 2009a). It was demonstrated that the conformational plasticity of indolicidin contributes to binding. Hydrophobic interactions involving fatty acid chains of LPS and aromatic residues of indolicidin, plus salt bridges between arginine side chains of the peptide and phosphate groups of the LPS inner core, explain indolicidin–LPS binding (Nagpal et al. 2009). Because of the ability to bind and neutralize LPS, indolicidin is considered to be a potential anti-sepsis agent (Dwivedi et al. 2019). Additionally, this property is linked with the antibiofilm activity of indolicidin because LPS is one of the major virulence factors mostly involved in the production and adhesion of biofilms produced by Gram-bacteria such as Klebsiella pneumoniae and Pseudomonas aeruginosa.
Similar to LPS, lipoteichoic acid (LTA) present in Gram+ bacteria has been proven to be involved in the release of cytokines and biofilm production (Schröder et al. 2003). To the best of our knowledge, there are no studies evaluating the potential interaction of indolicidin with LTA, constituting an opportunity for research.
Mechanism of action
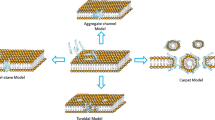
The elucidation of the mechanism of action of an AMP is important because it allows the understanding of biophysical processes that lead to microorganism death. This information is useful because it provides the basis for developing peptide analogs with improved features. Different strategies have been used to understand the mechanism of action of AMPs, such as spectroscopic techniques, nuclear magnetic resonance, isothermal titration calorimetry, molecular dynamics and others (Galdiero et al. 2013). In the case of indolicidin, studies related to its mechanism of action began with the work of Falla et al. (1996). The authors demonstrated that the peptide was capable of killing Gram-bacteria by crossing the outer membrane and causing disruption of the cytoplasmic membrane. Later, in 1998, Subbalakshmi and Sitaram demonstrated that the mechanism of action of indolicidin does not involve cell lysis. According to the authors, indolicidin permeabilizes both the outer and cytoplasmic membranes of E. coli but does not lead to lysis. Instead, once in the cytoplasm, indolicidin inhibits protein and DNA synthesis (Subbalakshmi et al. 2000). A few years later, Hsu et al. (2005), confirmed the DNA-binding ability of indolicidin using different experimental procedures, such as gel retardation, fluorescence measurement, and surface plasmon resonance. Similar to LPS, indolicidin–DNA interactions are mediated by the plasticity of the peptide. Finally, Rokitskaya et al. (2011), in a very interesting study conducted on uncharged lipid vesicles, concluded that indolicidin can operate as an organic anion carrier. The authors related this activity to the hemolytic action of indolicidin. The multiple mechanisms of action of indolicidin are shown in Fig. 2.
Representation of indolicidin’s mechanism of action after transient membrane perturbation, intracellular potential targets are proteins and nucleic acids (a), indolicidin–LPS interaction related to anti-sepsis and anti-biofilm activity (b) and the indolicidin anion carrier mechanism is thought to explain hemolytic activity (c)
Even with the data described above, the mechanism of action of indolicidin is not yet fully understood. The complete elucidation of the mechanism of action of any AMP depends on several factors, such as the model used to mimic the real target and the peptide concentration (Gregory et al. 2009; Wang et al. 2011). In addition, the multiple targets of indolicidin contribute to the difficulty of completely elucidating the molecular basis of indolicidin’s mechanism of action. Furthermore, the antiviral activity of indolicidin remains unclear.
Combination with conventional antibiotics
Several research groups have evaluated the potential of AMPs to work as adjuvants to conventional antibiotics (Li et al. 2020). The mechanisms underlying the synergistic behavior depend on the specific kind of antibiotic evaluated. For instance, the synergism between membrane-active AMPs combined with conventional antibiotics is related to the membrane-disruptive ability of the first to assist the second to reach their molecular targets more rapidly and efficiently (Shang et al. 2019). Studies conducted by Mataraci and Dosler have demonstrated that indolicidin in combination with daptomycin, linezolid, ciprofloxacin and azithromycin presents an additive interaction against MRSA stains. Additionally, indolicidin combined with teicoplanin showed a synergistic interaction (Dosler and Mataraci 2013; Mataraci and Dosler 2012). Furthermore, Ghaffar et al. (2015) showed that the combination of indolicidin with levofloxacin improves the antimicrobial activity against Gram+ and Gram− bacteria by decreasing the MIC values in relation to both separately. The valuation of indolicidin synergism with conventional antibiotics also constitutes an opportunity that researchers can explore further.
Indolicidin’s inspired peptides
Natural peptides are usually models for the design of AMP analogs to obtain new molecules with improved activity. Its biological features plus its small size encouraged several research groups in the design of indolicidin analogs. In addition to an increase in antimicrobial activity, the researchers' goal is to decrease the hemolytic activity of indolicidin. A comparison between indolicidin and selected analogs is presented in Table 3.
The race for analogs started immediately after the discovery of indolicidin. It began with the study of Falla et al. (1997). Researchers designed an indolicidin analog with an increased number of positively charged residues by the use of lysine and arginine. The molecule, called CP-11, has higher activity against Gram+ and Gram− bacteria and yeast than its parent peptide. Furthermore, the peptide has less toxicity than indolicidin. The increased charge and amphipathicity could explain the improved activity.
A few years later, inspired by CP-11, Rozek et al. (2003), designed cycloCP-11 (ICLKKWPWWPWRRCK), a cyclic disulfide-bonded peptide. The major goal was to increase protease resistance. CycloCP-11 proved to be 4.5 times more stable than CP-11 against trypsin, mainly because cycloCP-11 had a more compact packing of the lysine and tryptophan side chains. Cyclic and linear CP-11 were similar in terms of microbiological and hemolytic activity, both better than indolicidin. In 2004, Sader et al. published a study in which they designed and evaluated omiganan (ILRWPWWPWRRK) against 1437 clinical bacterial and 214 clinical yeast isolates. Omiganan was very effective against all Gram+ and Gram− bacteria tested, including the antibiotic-resistant strains. Additionally, omiganan demonstrated excellent antifungal activity against all Candida species. Furthermore, a study showed that omiganan has a rapid time kill for both bacterial and yeast strains. To date, omiganan has been tested in a total of sixteen clinical studies in the United States and Europe. Almost all of them have been completed. Omiganan was studied to evaluate its efficacy as a topical gel formulation to treat Rosacea for the treatment of catheter colonization and the prevention of bloodstream infections, genital warts and acne vulgaris (for more details, see the corresponding clinical trial identifiers: NCT00231153, NCT03091426, NCT02849262, NCT02571998 and NCT02576847).
Later, in 2009, Kim et al. conducted a study in which amide bonds at various positions in indolicidin were replaced with reduced amide bonds ψ[CH2NH]. The pseudopeptide containing two reduced amide bonds was less hemolytic without a decrease in its antimicrobial activity, probably related to the decreased hydrophobicity and enhanced conformational flexibility compared to indolicidin. Furthermore, the analog gained stability, an important feature for AMPs..
In 2013, Shin et al. tested a lys-linked dimeric analog of indolicidin (Di-Ind-6). This peptide displayed a two- to fourfold increase in antimicrobial activity against Gram+ and Gram− bacteria. The authors did not report information about the mechanism of action of the dimeric peptide. The factors that lead a monomeric peptide to become a more active dimeric molecule are not well established (Lorenzon et al. 2019). Additionally, Di-Ind-6 proves to be more hemolytic than Ind-6 but less hemolytic than native indolicidin. Furthermore, Di-Ind-6 inhibited nitric oxide production similar to the parent peptide.
Later, in a very complete set of experiments, Jindal et al. (2015) studied indolicidin–ranalexin hybrid peptides. Between 13 promising hybrids, RN7-IN10, RN7-IN9, RN7-IN8, and RN7-IN6 showed the strongest antibacterial activity against Streptococcus pneumoniae. Interestingly, all the hybrid peptides showed very low toxicity against human red blood cells. Later, in 2017, the authors conducted experiments that suggested that the cell wall/membrane or the inhibition of DNA synthesis are the most likely targets for hybrid peptides against S. pneumoniae (Jindal et al. 2017).
In a recent study, Smirnova et al. (2020) designed two analogs of indolicidin that have increased antimicrobial activity and decreased hemolytic activity. One of them is the peptide H-Ile-Leu-Pro-(2-Me)Phe-Lys-(2-Me)Phe-Pro-(2-Me)Phe-(2-Me)Phe-Pro-(2-Me)Phe-Arg-Arg-NH2, an analog containing methylated phenylalanine residues instead of tryptophan. The other analog, HN2-(CH2)10-Ile-Leu-Pro-D-Phe-Lys-D-Phe-Pro-D-Phe-D-Phe-Pro-DPhe-Arg-Arg-NH2, has D-enantiomer phenylalanine residues (also instead of tryptophan) and ten carbon fatty acids at the N-terminus. The modifications in these analogs made them more hydrophobic than the parent peptide indolicidin, suggesting that their mechanism of action is based on the disruption of cell membranes, which is mostly dependent on hydrophobicity.
As described, several strategies can be applied to design more efficient indolicidin analogs. However, other promising strategies of peptide modifications and novel formulations have not yet been explored. It is evident that many new analogs can still be designed using indolicidin as a template. Li et al. (2021) have reported a complete set of peptide chemical modifications to enhance antimicrobial activity, including lipidation, glycosylation and multimerization.
Perspectives
The studies of indolicidin began in 1992 as described above and continue to the present day (Hamed and Seleem 2021; Selsted et al. 1992). More than a hundred scientific papers were published between 1992 and 2020. Furthermore, a considerable number of patents can also be found, evidencing the interest of industry. However, similar to other AMPs, indolicidin and its analogs must overcome some limitations, such as instability, selectivity and production cost. Biological instability is considered the main issue for most peptides, specifically when systemic application is considered. However, it is important to note that many peptides, including indolicidin, act relatively quickly on their targets, and the natural elimination of the peptide by the body can be considered an advantage, avoiding the adverse effects of tissue accumulation (common for many conventional antibiotics). Indolicidin, like other AMPs, has hemolytic activity. Thus, topical administration of indolicidin is more likely than systemic application. In this regard, indolicidin analogs could be designed to increase selectivity toward desired targets. Alternatively, nanosystems for the delivery of indolicidin could be a promising alternative. A good peptide delivery platform could increase the peptide half-life time and targeting capability, improving its therapeutic applicability and reducing side effects.
Solid-phase peptide synthesis (SPPS) is still the standard method for producing synthetic peptides. However, several authors consider the high cost of production one of the reasons that keeps the industry away from interest in synthetic peptides. However, indolicidin, unlike other peptides (long and difficult sequences to synthesize), can be synthesized in high yields by SPPS. Conventional protocols for the use of 9-fluorenylmethoxycarbonyl amino-protecting group and standard coupling methods (Jaradat 2018) can be used to obtain indolicidin in high yield. Recently, we obtained indolicidin by manual SPPS with high yield and purity (Supplementary Data, not published). Furthermore, recombinant synthesis could be used to reduce the cost, obtain peptides on a large scale and reduce the use of hazardous materials.
In summary, there are still many strategies that researchers can take advantage of to improve the performance of indolicidin and, finally, be approved for commercialization. Despite several potential applications described in the literature, there are still some others that could be better addressed by the utilization of indolicidin and its analogs, such as antitumoral activity and gene delivery. There are few studies evaluating the potential antitumoral activity of the peptide. Cationic AMPs, such as indolicidin, are able to interact with negatively charged phosphatidylserine (PS) moieties that are selectively exposed on the outer surface of cancer cell plasma membranes (Tornesello et al. 2020). The negative charge of the cancer cell membrane is a characteristic also shared by bacterial cells. This fact constitutes the basis of the hypothesis of a close similarity between the selectivity and activity of AMPs and anticancer peptides (Gaspar et al. 2013). To date, only Bacalum (2016) has tested the antitumor activity of indolicidin. The author suggested that the peptide can inhibit cell growth of neuroblastoma SH-SY-5Y cells by apoptosis. The molecular basis of the mechanisms of action of indolicidin is still under debate but apparently is related to membrane penetration. Taking this into account, indolicidin and its analogs have the potential to act as carriers to deliver small molecules (Tsai et al. 2015). In this context, Hu et al. (2018) grafted indolicidin with polyethylenimine to investigate the potential of these conjugates as a gene delivery system. Researchers have shown that this peptide-conjugated carrier is an effective gene vehicle.
The potential use of indolicidin is not limited to biomedical applications but could also be used in different areas, such as the food industry (food conservation), veterinary (animal infection control, sperm/oocyte conservation, etc.) and other unexplored areas.
Conclusions
Indolicidin is a unique peptide with the potential to fight against the emergence of multidrug-resistant microorganisms, being active against bacteria, fungi, parasites and certain viruses. The multiple targets of indolicidin reinforce its potential therapeutic applications and could contribute to low resistance induction. Added to its broad spectrum of activity, ease of synthesis and good solubility position, indolicidin (and certain analogs) is an excellent candidate in the growing market for bioactive peptides. Like almost all AMPs, indolicidin has to overcome its limitations, mainly instability and hemolytic activity. In this regard, several strategies for peptide tuning (such as punctual modifications and multimerization) have not yet been fully explored. On the other hand, new formulations including nanosystem delivery could be developed. Indolicidin and its analogs could be used in different areas, including medical (infectious disease control), biomedical (such as medical device contamination control), food industry (food conservation) and other unexplored areas. To achieve this goal, a multidisciplinary team of researchers must work together to fully understand the chemistry and biological properties of indolicidin.
References
Abdi M, Mirkalantari S, Amirmozafari N (2019) Bacterial resistance to antimicrobial peptides. J Pept Sci 25:e3210. https://doi.org/10.1002/psc3210
Albiol Matanic VC, Castilla V (2004) Antiviral activity of antimicrobial cationic peptides against Junin virus and herpes simplex virus. Int J Antimicrob Agents 23:382–389. https://doi.org/10.1016/jijantimicag200307022
Bacalum M (2016) Antimicrobial peptides show antitumor activity against sh-sy-5y human neuroblastoma cells. In: Florescu M (ed) Biophysics for biomedical and environmental sciences. Transilvania University of Brasov Press, Braşov, pp 39–48
Benincasa M, Scocchi M, Pacor S, Tossi A, Nobili D, Basaglia G, Busetti M, Gennaro R (2006) Fungicidal activity of five cathelicidin peptides against clinically isolated yeasts. J Antimicrob Chemother 58:950–959. https://doi.org/10.1093/jac/dkl382
Bera S, Ghosh A, Sharma S, Debnath T, Giri B, Bhunia A (2015) Probing the role of proline in the antimicrobial activity and lipopolysaccharide binding of indolicidin. J Colloid Interface Sci 452:148–159. https://doi.org/10.1016/jjcis201504031
Bowdish DME, Davidson DJ, Hancock REW (2006) Immunomodulatory properties of defensins and cathelicidins. Curr Top Microbiol Immunol 306:27–66. https://doi.org/10.1007/3-540-29916-5_2
Chen CH, Lu TK (2020) Development and challenges of antimicrobial peptides for therapeutic applications. Antibiotics 13:24. https://doi.org/10.3390/antibiotics9010024
Cirioni O, Giacometti A, Barchiesi F, Scalise G (1998) In vitro activity of lytic peptides alone and in combination with macrolides and inhibitors of dihydrofolate reductase against Pneumocystis carinii. J Antimicrob Chemother 42:445–451. https://doi.org/10.1093/jac/424445
Cohen J (2002) The immunopathogenesis of sepsis. Nature 420:885–891. https://doi.org/10.1038/nature01326
Datta S, Roy A (2021) Antimicrobial peptides as potential therapeutic agents: a review. Int J Pept Res Ther 27:555–577. https://doi.org/10.1007/s10989-020-10110-x
de Alteriis E, Maselli V, Falanga A, Galdiero S, Di Lella FM, Gesuele R, Guida M, Galdiero E (2018) Efficiency of gold nanoparticles coated with the antimicrobial peptide indolicidin against biofilm formation and development of Candida spp. clinical isolates. Infect Drug Resist 11:915–925. https://doi.org/10.2147/IDRS164262
Deslouches B, Di Peter Y (2017) Antimicrobial peptides with selective antitumor mechanisms: prospect for anticancer applications. Oncotarget 8:46635–46651. https://doi.org/10.18632/oncotarget16743
Dosler S, Mataraci E (2013) In vitro pharmacokinetics of antimicrobial cationic peptides alone and in combination with antibiotics against methicillin resistant Staphylococcus aureus biofilms. Peptides 49:53–58. https://doi.org/10.1016/jpeptides201308008
Dwivedi R, Aggarwal P, Bhavesh NS, Kaur KJ (2019) Design of therapeutically improved analogue of the antimicrobial peptide, indolicidin, using a glycosylation strategy. Amino Acids 51:1443–1460. https://doi.org/10.1007/s00726-019-02779-2
Edward Robinson W, McDougall B, Tran D, Selsted ME (1998) Anti-HIV-1 activity of indolicidin, an antimicrobial peptide from neutrophils. J Leukoc Biol 63:94–100. https://doi.org/10.1002/jlb63194
Falla TJ, Hancock REW (1997) Improved activity of a synthetic indolicidin analog. Antimicrob Agents Chemother 41:771–775. https://doi.org/10.1128/aac414771
Falla TJ, Nedra Karunaratne D, Hancock REW (1996) Mode of action of the antimicrobial peptide indolicidin. J Biol Chem 271:19298–19303. https://doi.org/10.1074/jbc2713219298
Friedrich CL, Moyles D, Beveridge TJ, Hancock REW (2000) Antibacterial action of structurally diverse cationic peptides on Gram-positive bacteria. Antimicrob Agents Chemother 44:2086–2092. https://doi.org/10.1128/AAC4482086-20922000
Galdiero E, Siciliano A, Maselli V, Gesuele R, Guida M, Fulgione D, Galdiero S, Lombardi L, Falanga A (2016) An integrated study on antimicrobial activity and ecotoxicity of quantum dots and quantum dots coated with the antimicrobial peptide indolicidin. Int J Nanomed 11:4199–4211. https://doi.org/10.2147/IJNS107752
Galdiero S, Falanga A, Cantisani M, Vitiello M, Morelli G, Galdiero M (2013) Peptide–lipid interactions: experiments and applications. Int J Mol Sci 41:18758–18789. https://doi.org/10.3390/ijms140918758
Gaspar D, Salomé Veiga A, Castanho MARB (2013) From antimicrobial to anticancer peptides: a review. Front Microbiol 4:294. https://doi.org/10.3389/fmicb201300294
Ghaffar K, Hussein W, Khalil Z, Capon R, Skwarczynski M, Toth I (2015) Levofloxacin and indolicidin for combination antimicrobial therapy. Curr Drug Deliv 12:108–114. https://doi.org/10.2174/1567201811666140910094050
Giacometti A, Cirioni O, Barchiesi F, Caselli F, Scalise G (1999) In vitro activity of polycationic peptides against Cryptosporidium parvum, Pneumocystis carinii and yeast clinical isolates. J Antimicrob Chemother 44:403–406. https://doi.org/10.1093/jac/443403
Gregory SM, Pokorny A, Almeida PFF (2009) Magainin 2 revisited: a test of the quantitative model for the all-or-none permeabilization of phospholipid vesicles. Biophys J 96:116–131. https://doi.org/10.1016/jbpj200809017
Haines LR, Hancock REW, Pearson TW (2003) Cationic antimicrobial peptide killing of African trypanosomes and Sodalis glossinidius, a bacterial symbiont of the insect vector of sleeping sickness. Vector Borne Zoonotic Dis 3:175–186. https://doi.org/10.1089/153036603322662165
Hamed MI, Seleem MN (2021) Evaluation of short synthetic antimicrobial peptides against Staphylococcus pseudintermedius. J Adv Vet Res 11:69–72
Hsu CH, Chen C, Jou ML, Lee AYL, Lin YC, Yu YP, Huang WT, Wu SH (2005) Structural and DNA-binding studies on the bovine antimicrobial peptide, indolicidin: evidence for multiple conformations involved in binding to membranes and DNA. Nucleic Acids Res 33:4053–4064. https://doi.org/10.1093/nar/gki725
Hsu JCY, Yip CM (2007) Molecular dynamics simulations of indolicidin association with model lipid bilayers. Biophys J 92:L100–L102. https://doi.org/10.1529/biophysj107108050
Hu WW, Yeh CC, Tsai CW (2018) The conjugation of indolicidin to polyethylenimine for enhanced gene delivery with reduced cytotoxicity. J Mater Chem B 6:5781–5794. https://doi.org/10.1039/c8tb01408f
Huan Y, Kong Q, Mou H, Yi H (2020) Antimicrobial peptides: classification, design, application and research progress in multiple fields. Front Microbiol 11:582779. https://doi.org/10.3389/fmicb2020582779
Hultmark D, Steiner H, Rasmuson T, Boman HG (1980) Insect immunity purification and properties of three inducible bactericidal proteins from hemolymph of immunized pupae of Hyalophora cecropia. Eur J Biochem 106:104258. https://doi.org/10.1111/j1432-10331980tb05991x
Jaradat DMM (2018) Thirteen decades of peptide synthesis: key developments in solid phase peptide synthesis and amide bond formation utilized in peptide ligation. Amino Acids 50:39–68. https://doi.org/10.1007/s00726-017-2516-0
Jindal HM, Le CF, Yusof MYM, Velayuthan RD, Lee VS, Zain SM, Isa DM, Sekaran SD (2015) Antimicrobial activity of novel synthetic peptides derived from indolicidin and ranalexin against Streptococcus pneumoniae. PLoS ONE 10:e0128532. https://doi.org/10.1371/journalpone0128532
Jindal HM, Zandi K, Ong KC, Velayuthan RD, Rasid SM, Raju CS, Sekaran SD (2017) Mechanisms of action and in vivo antibacterial efficacy assessment of five novel hybrid peptides derived from indolicidin and ranalexin against Streptococcus pneumoniae. PeerJ 5:e3887. https://doi.org/10.7717/peerj3887
Joo HS, Fu CI, Otto M (2016) Bacterial strategies of resistance to antimicrobial peptides. Philos Trans R Soc B 371:20150292. https://doi.org/10.1098/rstb20150292
Joshi S, Bisht GS, Rawat DS, Kumar A, Kumar R, Maiti S, Pasha S (2010) Interaction studies of novel cell selective antimicrobial peptides with model membranes and E. coli ATCC 11775. Biochim Biophys Acta Biomembr 1798:1864–1875. https://doi.org/10.1016/jbbamem201006016
Kang X, Dong F, Shi C, Liu S, Sun J, Chen J, Li H, Xu H, Lao X, Zheng H (2019) DRAMP 20, an updated data repository of antimicrobial peptides. Sci Data 6:148. https://doi.org/10.1038/s41597-019-0154-y
Kastin A (2013) Handbook of biologically active peptides. Elsevier, Amsterdam. https://doi.org/10.1016/C2010-0-66490-X
Kim SM, Kim JM, Joshi BP, Cho H, Lee KH (2009) Indolicidin-derived antimicrobial peptide analogs with greater bacterial selectivity and requirements for antibacterial and hemolytic activities. Biochim Biophys Acta Proteins Proteomics 1794:185–192. https://doi.org/10.1016/jbbapap200810009
Kościuczuk EM, Lisowski P, Jarczak J, Strzałkowska N, Jóźwik A, Horbańczuk J, Krzyżewski J, Zwierzchowski L, Bagnicka E (2012) Cathelicidins: family of antimicrobial peptides: a review. Mol Biol Rep 39:10957–10970. https://doi.org/10.1007/s11033-012-1997-x
Krajewski K, Marchand C, Long YQ, Pommier Y, Roller PP (2004) Synthesis and HIV-1 integrase inhibitory activity of dimeric and tetrameric analogs of indolicidin. Bioorg Med Chem Lett 14:5595–5598. https://doi.org/10.1016/jbmcl200408061
Li J, Fernández-Millán P, Boix E (2020) Synergism between host defence peptides and antibiotics against bacterial infections. Curr Top Med Chem 20:1238–1263. https://doi.org/10.2174/1568026620666200303122626
Li W, Separovic F, O’Brien-Simpson NM, Wade JD (2021) Chemically modified and conjugated antimicrobial peptides against superbugs. Chem Soc Rev 50:4932–4973. https://doi.org/10.1039/d0cs01026j
Liu Y, Ding S, Shen J, Zhu K (2019) Nonribosomal antibacterial peptides that target multidrug-resistant bacteria. Nat Prod Rep 36:573–592. https://doi.org/10.1039/c8np00031j
Lorenzon EN, Piccoli JP, Santos-Filho NA, Cilli EM (2019) Dimerization of antimicrobial peptides: a promising strategy to enhance antimicrobial peptide activity. Protein Pept Lett 26:98–107. https://doi.org/10.2174/0929866526666190102125304
Magana M, Pushpanathan M, Santos AL, Leanse L, Fernandez M, Ioannidis A, Giulianotti MA, Apidianakis Y, Bradfute S, Ferguson AL, Cherkasov A, Seleem MN, Pinilla C, de la Fuente-Nunez C, Lazaridis T, Dai T, Houghten RA, Hancock REW, Tegos GP (2020) The value of antimicrobial peptides in the age of resistance. Lancet Infect Dis 20:1216–1230. https://doi.org/10.1016/S1473-3099(20)30327-3
Maróti Gergely G, Kereszt A, Kondorosi É, Mergaert P (2011) Natural roles of antimicrobial peptides in microbes, plants and animals. Res Microbiol 162:363–374. https://doi.org/10.1016/jresmic201102005
Mataraci E, Dosler S (2012) In vitro activities of antibiotics and antimicrobial cationic peptides alone and in combination against methicillin-resistant Staphylococcus aureus biofilms. Antimicrob Agents Chemother 56:6366–6371. https://doi.org/10.1128/AAC01180-12
Nagpal S, Kaur KJ, Jain D, Salunke DM (2009) Plasticity in structure and interactions is critical for the action of indolicidin, an antibacterial peptide of innate immune origin. Protein Sci 11:2158–2167. https://doi.org/10.1110/ps0211602
Nan YH, Bang JK, Shin SY (2009a) Design of novel indolicidin-derived antimicrobial peptides with enhanced cell specificity and potent anti-inflammatory activity. Peptides 30:832–838. https://doi.org/10.1016/jpeptides200901015
Nan YH, Park KH, Park Y, Jeon YJ, Kim Y, Park IS, Hahm KS, Shin SY (2009b) Investigating the effects of positive charge and hydrophobicity on the cell selectivity, mechanism of action and anti-inflammatory activity of a Trp-rich antimicrobial peptide indolicidin. FEMS Microbiol Lett 292:134–140. https://doi.org/10.1111/j1574-6968200801484x
Park KH, Nan YH, Park Y, Kim JI, Park IS, Hahm KS, Shin SY (2009) Cell specificity, anti-inflammatory activity, and plausible bactericidal mechanism of designed Trp-rich model antimicrobial peptides. Biochim Biophys Acta Biomembr 1788:1193–1203. https://doi.org/10.1016/jbbamem200902020
Pasupuleti M, Schmidtchen A, Malmsten M (2012) Antimicrobial peptides: key components of the innate immune system. Crit Rev Biotechnol 32:143–171. https://doi.org/10.3109/073885512011594423
Pervin Z, Hassan MM (2021) Synergistic therapeutic actions of antimicrobial peptides to treat multidrug-resistant bacterial infection. Rev Med Microbiol. https://doi.org/10.1097/mrm0000000000000239
Podorieszach AP, Huttunen-Hennelly HEK (2010) The effects of tryptophan and hydrophobicity on the structure and bioactivity of novel indolicidin derivatives with promising pharmaceutical potential. Org Biomol Chem 8:1679–1687. https://doi.org/10.1039/b921248e
Pompilio A, Scocchi M, Pomponio S, Guida F, Di Primio A, Fiscarelli E, Gennaro R, Di Bonaventura G (2011) Antibacterial and anti-biofilm effects of cathelicidin peptides against pathogens isolated from cystic fibrosis patients. Peptides 32:1807–1814. https://doi.org/10.1016/jpeptides201108002
Portell-Buj E, Vergara A, Alejo I, López-Gavín A, Rosa Montè M, San Nicolàs L, Gonzàlez-Martín J, Tudó G (2019) In vitro activity of 12 antimicrobial peptides against Mycobacterium tuberculosis and Mycobacterium avium clinical isolates. J Med Microbiol 68:211–215. https://doi.org/10.1099/jmm0000912
Ramanathan B, Davis EG, Ross CR, Blecha F (2002) Cathelicidins: microbicidal activity, mechanisms of action, and roles in innate immunity. Microbes Infect 4:361–372. https://doi.org/10.1016/S1286-4579(02)01549-6
Ron-Doitch S, Sawodny B, Kühbacher A, David MMN, Samanta A, Phopase J, Burger-Kentischer A, Griffith M, Golomb G, Rupp S (2016) Reduced cytotoxicity and enhanced bioactivity of cationic antimicrobial peptides liposomes in cell cultures and 3D epidermis model against HSV. J Control Release 229:163–171. https://doi.org/10.1016/jjconrel201603025
Rozek A, Powers JPS, Friedrich CL, Hancock REW (2003) Structure-based design of an indolicidin peptide analogue with increased protease stability. Biochemistry 42:14130–14138. https://doi.org/10.1021/bi035643g
Ryge TS, Doisy X, Ifrah D, Olsen JE, Hansen PR (2004) New indolicidin analogues with potent antibacterial activity. J Pept Res 64:171–185. https://doi.org/10.1111/j1399-3011200400177x
Sader HS, Fedler KA, Rennie RP, Stevens S, Jones RN (2004) Omiganan pentahydrochloride (MBI 226), a topical 12-amino-acid cationic peptide: spectrum of antimicrobial activity and measurements of bactericidal activity. Antimicrob Agents Chemother 48:3112–3118. https://doi.org/10.1128/AAC4883112-31182004
Schröder NWJ, Morath S, Alexander C, Hamann L, Hartung T, Zähringer U, Göbel UB, Weber JR, Schumann RR (2003) Lipoteichoic acid (LTA) of Streptococcus pneumoniae and Staphylococcus aureus activates immune cells via Toll-like receptor (TLR)-2, lipopolysaccharide-binding protein (LBP), and CD14, whereas TLR-4 and MD-2 are not involved. J Biol Chem 278:15587–15594. https://doi.org/10.1074/jbcM212829200
Scocchi M, Wang S, Zanetti M (1997) Structural organization of the bovine cathelicidin gene family and identification of a novel number. FEBS Lett 417:311–315. https://doi.org/10.1016/S0014-5793(97)01310-0
Selsted ME, Novotny MJ, Morris WL, Tang YQ, Smith W, Cullor JS (1992) Indolicidin, a novel bactericidal tridecapeptide amide from neutrophils. J Biol Chem 267:4292–4295. https://doi.org/10.1016/s0021-9258(18)42830-x
Shagaghi N, Palombo EA, Clayton AHA, Bhave M (2018) Antimicrobial peptides: biochemical determinants of activity and biophysical techniques of elucidating their functionality. World J Microbiol Biotechnol 34:62. https://doi.org/10.1007/s11274-018-2444-5
Shang D, Liu Y, Jiang F, Ji F, Wang H, Han X (2019) Synergistic antibacterial activity of designed Trp-containing antibacterial peptides in combination with antibiotics against Multidrug-Resistant Staphylococcus epidermidis. Front Microbiol 10:2719. https://doi.org/10.3389/fmicb201902719
Shin SY (2013) Prokaryotic selectivity, bactericidal mechanism and LPS-neutralizing activity of lys-linked dimeric peptide of indolicidin C-terminal hexapeptide. Bull Korean Chem Soc 34:2187–2190. https://doi.org/10.5012/bkcs20133472187
Shin YP, Park HJ, Shin SH, Lee YS, Park S, Jo S, Lee YH, Lee IH (2010) Antimicrobial activity of a halocidin-derived peptide resistant to attacks by proteases. Antimicrob Agents Chemother 54:2855–2866. https://doi.org/10.1128/AAC01790-09
Smirnova MP, Kolodkin NI, Kolobov AA, Afonin VG, Afonina IV, Stefanenko LI, Shpen VM, Shamova OV (2020) Indolicidin analogs with broad-spectrum antimicrobial activity and low hemolytic activity. Peptides 132:170356. https://doi.org/10.1016/jpeptides2020170356
Subbalakshmi C, Bikshapathy E, Sitaram N, Nagaraj R (2000) Antibacterial and hemolytic activities of single tryptophan analogs of indolicidin. Biochem Biophys Res Commun 274:714–716. https://doi.org/10.1006/bbrc20003214
Subbalakshmi C, Krishnakumari V, Nagaraj R, Sitaram N (1996) Requirements for antibacterial and hemolytic activities in the bovine neutrophil derived 13-residue peptide indolicidin. FEBS Lett 395:48–52. https://doi.org/10.1016/0014-5793(96)00996-9
Tomasinsig L, Benincasa M, Scocchi M, Skerlavaj B, Tossi A, Zanetti M, Gennaro R (2010) Role of cathelicidin peptides in bovine host defense and healing. Probiotics Antimicrob Proteins 2:12–20. https://doi.org/10.1007/s12602-010-9035-6
Tornesello AL, Borrelli A, Buonaguro L, Buonaguro FM, Tornesello ML (2020) Antimicrobial peptides as anticancer agents: functional properties and biological activities. Molecules 25:2850. https://doi.org/10.3390/molecules25122850
Tsai CW, Hu WW, Liu CI, Ruaan RC, Tsai BC, Jin SLC, Chang Y, Chen WY (2015) The consideration of indolicidin modification to balance its hemocompatibility and delivery efficiency. Int J Pharm 494:498–505. https://doi.org/10.1016/jijpharm201508037
Vasilchenko AS, Vasilchenko AV, Pashkova TM, Smirnova MP, Kolodkin NI, Manukhov IV, Zavilgelsky GB, Sizova EA, Kartashova OL, Simbirtsev AS, Rogozhin EA, Duskaev GK, Sycheva MV (2017) Antimicrobial activity of the indolicidin-derived novel synthetic peptide In-58. J Pept Sci 23:855–863. https://doi.org/10.1002/psc3049
Vergis J, Malik SVS, Pathak R, Kumar M, Sunitha R, Barbuddhe SB, Rawool DB (2020) Efficacy of indolicidin, cecropin A (1–7)-melittin (CAMA) and their combination against biofilm-forming multidrug-resistant enteroaggregative Escherichia coli. Probiotics Antimicrob Proteins 12:705–715. https://doi.org/10.1007/s12602-019-09589-8
Wang G, Li X, Wang Z (2016) APD3: the antimicrobial peptide database as a tool for research and education. Nucleic Acids Res 44:1087–1093. https://doi.org/10.1093/nar/gkv1278
Wang Y, Wang M, Shan A, Feng X (2020) Avian host defense cathelicidins: structure, expression, biological functions, and potential therapeutic applications. Poult Sci 99:6434–6445. https://doi.org/10.1016/j.psj.2020.09.030
Wang KF, Nagarajan R, Mello CM, Camesano TA (2011) Characterization of supported lipid bilayer disruption by chrysophsin-3 using QCM-D. J Phys Chem B 115:15228–15235. https://doi.org/10.1021/jp209658y
Yan H, Hancock REW (2001) Synergistic interactions between mammalian antimicrobial defense peptides. Antimicrob Agents Chemother 45:1558–1560. https://doi.org/10.1128/AAC4551558-15602001
Yang ST, Yub Shin S, Kim YC, Kim Y, Hahm KS, Kim JI (2002) Conformation-dependent antibiotic activity of tritrpticin, a cathelicidin-derived antimicrobial peptide. Biochem Biophys Res Commun 296:1044–1050. https://doi.org/10.1016/S0006-291X(02)02048-X
Yasin B, Pang M, Turner JS, Cho Y, Dinh NN, Waring AJ, Lehrer RI, Wagar EA (2000) Evaluation of the inactivation of infectious herpes simplex virus by host-defense peptides. Eur J Clin Microbiol Infect Dis 19:187–194. https://doi.org/10.1007/s100960050457
Zanetti M (2004) Cathelicidins, multifunctional peptides of the innate immunity. J Leukoc Biol 75:39–48. https://doi.org/10.1189/jlb0403147
Zasloff M (1987) Magainins, a class of antimicrobial peptides from Xenopus skin: isolation, characterization of two active forms, and partial cDNA sequence of a precursor. Proc Natl Acad Sci USA 84:5449–5453. https://doi.org/10.1073/pnas84155449
Acknowledgements
The authors are grateful to Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for financial support (427961/2018-1) and John Oluwafemi Teibo for English revision.
Author information
Authors and Affiliations
Contributions
ENL devised the review and the main conceptual ideas and wrote the manuscript with help from JBA and GSS.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
Not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Batista Araujo, J., Sastre de Souza, G. & Lorenzon, E.N. Indolicidin revisited: biological activity, potential applications and perspectives of an antimicrobial peptide not yet fully explored. World J Microbiol Biotechnol 38, 39 (2022). https://doi.org/10.1007/s11274-022-03227-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-022-03227-2