Abstract
The growth of pathogens across the globe is developing at a very fast rate, thus turning into a worldwide health problem. Since, current treatment alternatives have failed to a large extent, novel antibiotics are highly in demand. Antimicrobial peptides (AMPs) have become a significant alternative in this scenario because of their wide-spectrum activity, rapid killing and often cell selectivity. They comprise diverse functional molecules with multifaceted properties, consisting of varied biological activity. Because of the different amino acids and elements in its structure, their action mechanism is specifically altered. Most of the AMPs have been derived from animals, plants and marine sources. They show therapeutic potential, yet, their use is limited because of their short plasma half-life. AMP production can be done at reasonable costs with the help of biotechnological methods. Thus, discovery of an efficient and long-lasting antimicrobial drugs is awaited as soon as these challenges are overcome. The market of antimicrobial peptides is growing at a fast rate. Bioactive peptides from natural sources open up new opportunities to discover lead molecules for management of various ailments. This systematic review centres around the antimicrobial activity, various properties, mechanism and sources of AMPs along with how these properties are exploited for the application of efficient and promising drug agents in pharmaceutical companies. Therefore, in this review information about antimicrobial activity, various properties, mechanism and sources of AMPs along with how these properties are exploited for the application of efficient and promising drug agents in pharmaceutical companies has been discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Peptides are class of pharmaceutical elements which lie between small molecules and proteins but differs from both of them in therapeutical and biochemical terms. Being an intrinsic signalling molecule, it plays a potential role in therapeutics similar to the natural pathway networks. Some of them are even entitled with ‘‘replacement therapies”, as they are supplemented when a particular peptide hormone is lacking. This can be further explained by the very first isolation and use of therapeutic insulin in the period of 1920s in case of diabetics, where the hormone was not released sufficiently (Banting et al. 1922). Moreover, these peptide molecules are selective and efficacious signalling molecules which bind with the specific cell receptors (on surface) like GPCRs (G protein-coupled receptors) or ion channels, where the intracellular effects are triggered. Peptides are the most excellent initial point for the new drug development. They have an attractive pharmacological profile, high specificity and low toxicity due to their tight bonding with targets. This specificity has proved to be safe for commercial use with respect to the efficacy of human profiles. Penetration of peptide drugs surpassing the biological barriers is usually achieved by addition of modules with respect to the active or passive transport (Teesaluetal et al. 2009). Amino acids which are positively charged, being added in the terminal positions of its structure, results in the improvement of peptide perforation (Li and Cho 2012). Peptides from natural derivatives, such as insulin and adrenocorticotropic hormone (ACTH), were the life-saving drugs in the early twentieth century. With the onset of sequence interpretation in the 1950s, chemical isolation and production of peptides became feasible along with synthetic oxytocin & vasopressin.
Peptides from various sources such as animal, plant and marine source have wide range of potential and they act as therapeutics for future drug development (Li et al. 2010; Rosa et al. 2012; Sánchez and Vázquez 2017; Sable et al. 2017). Peptides which prevents the human host from various microbial infection are termed as antimicrobial peptides. They are associated with the class of molecules which are principally manifested at the interfaces of host and environment such as oral, gastrointestinal and respiratory passages. These particles are predominantly present in these areas in order to inhibit invasion of microbes by performing antimicrobial function (Hirsch et al. 2008). AMPs are active compounds produced as an essential innate immunological reaction in numerous species. They possess defence mechanism in the host through exertion of cytotoxicity on the invading microbes which are pathogenic in nature. They also act as immune modulators in organisms of higher phyla (Zanetti et al. 2004). AMPs are even known for their promising drug candidate. This is because of their wide spectrum of activity, low toxicity and decreased resistance by the targeted cells (Hancock et al. 2002). This broad-spectrum activity is due to the specific properties such as charge, hydrophobicity, size, and structural stereo geometry. AMPs act as antitumor vehicles, drug delivery trajectories, agents triggering mitosis, signalling molecules and prophylactic factors in signal transmission pathways (Kamysz et al. 2003). Antimicrobial peptides are of two types, i.e. NRAMPs (non-ribosomally synthesized peptides) which synthesized in the cytosol of fungi and bacteria (Grünewald et al. 2006); and RAMPs (ribosomally synthesized peptides) which synthesized in the ribosomes of eukaryotic of cells (Papagianni et al. 2003). Species ranging from prokaryotes to eukaryotes are able to synthesize AMPs on their own, since they play natural defense of the host when exposed to thousands of pathogens (Hassan et al. 2012; Mishra et al. 2017) Even the higher classes of eukaryotes synthesize molecules against microbes in their defence. AMPs have the potential of killing pathogenic microbes, consisting of viruses, fungi, bacteria (both gram-positive and -negative) and protozoa. When compared to standard antibiotics, they have been proven to possess bactericidal activity instead of just inhibiting their growth (bacteriostatic) (Lohner et al. 2009). AMPs act as a safety regulator and enhance immunity which is the unique property of these peptides. Since they possess high biological activity and are low cost molecules, they are considered as a potential candidate for drug development. Therefore, in this review, mechanism of action of AMPs, structural characteristics, various source and multidimensional functions of AMPs has been discussed.
Antimicrobial Resistance
The discovery of antibiotics was a major breakthrough in modern medicine. But due to the antibiotic resistance of various microbes in the last few years resulted into development of few diseases that cannot be treated with the current antibiotics (Fleitas et al. 2016). Therefore, antimicrobial resistance (AMR) is termed as the resistance of microbes in response to the agent which they have encountered before (Andersson et al. 2016; Maria-Neto et al. 2015). AMR develops due to the various factors and one such factor is mutation. About 70% of disease-causing bacteria are resistant to at least one kind of antibiotic (Watkins et al. 2016). Majorly, pathogens are resistant to the classes of functional antibiotics at clinical levels, thus, decreasing the possibility of alternate therapies (Laxminarayan et al. 2013). Natural AMPs are still in primary phase for such manifestations; partially aimed at their physical and chemical properties along with the activity of membrane related characteristics, as amphiphilic nature and cationic charge. Globally, multi-drug resistance rates have been increasing in bacterial strains which because various diseases linked with healthcare. Keeping aside the health stress, effect of antimicrobial resistance, also influence the social and economic situation of a country. Environmental factors also pose resistance, due to its harboring a varied pool of resistance factors, consisting of resistance genes and genetic components (Berkner et al. 2014). Antimicrobial resistance comprises intrinsic or adaptive resistance. Intrinsic resistance is the efficiency of microbes to withstand antimicrobial therapy because of its innate functional characteristics, whereas adaptive resistance is the potential of microbes to survive in stress circumstances by quickly adapting their transcriptomes in the surrounding environment. Adaptive resistance is evolved through modification of genomic elements or as an outcome of a genetic mutation (Arzanlou et al. 2017). Staphylococci species secrete metalloproteases such as aureolysin and SepA, along with serine endopeptidases like V8 protease. These are known for degrading linear AMPs (human cathelicidin LL-37) (Sieprawska-Lupa et al. 2004). Some of these groups produce a protease named SpeB which is known to fragmentize several host AMPs consisting of LL-37 and beta-defensins (Nelson et al. 2011; Frick et al. 2011). Proteases derived from other gram-positive bacteria like Enterococcus faecalis, and gram-negative bacteria like Pseudomonas aeruginosa and Proteus mirabilis have also proven to degrade LL-37 (Schmidtchen et al. 2002). Pla in Yersinia pestis, OmpT present in Escherichia coli and PgtE in Salmonella enterica serotype Typhimurium (S. typhimurium) are known to cleave AMPs like protamine, LL- 37 and cathelicidin-related antimicrobial peptide (CRAMP) (Guina et al. 2000; Galvan et al. 2008). Some species of gram-negative bacteria even degrade AMPs after importing by particular transport proteins, amidst the intracellular environment, like S. typhimurium and Haemophilus influenzae which give rise to ABC transporter encoded by the sapABCDFZ operon, thus increasing the bacterial resistance against CAMPs (Mason et al. 2005).
Capsular polysaccharides (CPS) affect AMPs via capturing/repelling them. PIA a polysaccharide intercellular adhesin (poly-N-acetyl glucosamine) is produced by S. aureus, S. epidermidis, along with a variety of other bacteria consisting of other Staphylococci and E. coli is responsible for the resistance against cationic HBD-3 as well as anionic dermcidin (Wang et al. 2004; Vuong et al. 2004a, b). PIA deacetylation is IcaB-mediated, leads to the increase of positive net charge. Thus, results in increased repulsion against CAMPs and forming of a mechanical barrier (Vuong et al. 2004a, b). P. aeruginosa, Klebsiella pneumoniae or Streptococcus pneumoniae showed resistance against CAMPs like HBD-1, polymyxin and HNP-1 via structural hindrance and electrostatic trapping (Campos et al. 2004; Llobet et al. 2008). Even capsules produced by the phosphoglucomutase homologue of Streptococcus iniae serve an important role in giving resistance against AMP produced by fish, namely moronecidin (Buchanan et al. 2005). Various surface modifications are also adapted by gram positive and negative bacteria via cell envelope structures to resist AMPs. The primary molecules include anionic polymers attached in the outermost cell surface, teichoic acids (TA) in the gram-positive cell wall while lipopolysaccharide (LPS) in the gram-negative outer membrane. Primarily, teichoic acid is negatively charged due to the presence of disaccharide anchors and phosphodiester-linked polyglycerol phosphate in gram positive bacteria (Bera et al. 2007). Because of the D- alanylation of free hydroxyls of repeating sugar residues, it adds a positive charge on teichoic acid, thus lowering the attraction of CAMPs as shown in S. aureus against nisin, magainin, HNP-1,2,3 and gallidermin (Peschel et al. 1999). On the other hand, preventing binding of AMPs with gram negative cell surface is obtained via amino sugars, phosphoethanolamine (PEA) or glycine which increases the positive charge of the anionic LPS component of the other membrane (Moskowitz et al. 2004). Bordetella species, Acinetobacter baumannii and Francisella novicida tend to modify lipid A phosphate with galactosamine/glucosamine (Llewellyn et al. 2012; Shah et al. 2014; Pelletier et al. 2013) thus leading to decrease the anionic properties of LPS. Neisseria gonorrhoeae, A. baumannii and S. typhimurium attach PEA onto phosphates in lipid A (Pelletier et al. 2013; Lewis et al. 2013), along with Vibrio cholerae importing glycine into lipid A acyl chains for increasing the positive charge and thus diminishing AMP attraction (Hankins et al. 2012).
Structural Characteristics of AMPs
These are basically those peptides which are composed of hundred or less residues of amino acid, with net charge ranging from + 2 to + 9. It is also displayed by arginine and lysine (cationic amino acids) along with a considerable amount of residues which are hydrophobic. The physicochemical & structural characteristics of these peptides serve a crucial part in regulating their specificity with respect to the target cells.
Standard structural elements of AMPs provide crucial information on the phylogenetic importance of these peptides that play an important role with respect to the origin of AMPs (Yount et al. 2004). Antimicrobial PRP i.e. peptide with proline–arginine–proline motif comprises the arginine/proline-rich positively charged peptides (Yount et al. 2004; Li et al. 2014). These accommodate usually one or, sometimes more PRP motif, which thus, influence gram-negative and -positive bacteria. A glycine rich cationic AMP, Armadillidin, has a unique GGGFHR or GGGFHS fivefold repeated motifs, with addition of amide group in the C-terminal which represents formidable activity against Gram-positive bacteria (Herbiniere et al. 2005). Penaeidins are delusive peptides which are positively charged, comprising of PRP motifs at their N-terminal (PRP-domain) while the C-terminal end has the cysteine rich region (cysteine-rich domain) with a standard motif binding to chitin, owning activity against fungi and gram-positive bacteria (Tassanakajon et al. 2011).
Structural and physicochemical properties of AMPs play a significant role in targeted cell toxicity. An antimicrobial peptide namely, Tachystatin was isolated from blood cells of Tachypleus tridentatus, a horseshoe crab, has proven to display wide spectrum of antimicrobial range against gram-negative and -positive bacteria along with fungi (Osaki et al. 1999). Tenecin 1, an empirical peptide derived from the larval stage of Tenebrio molitor also possess antibacterial activity (Lee et al. 1998). It has both the fragments, α-helix and β-sheet at its C- and N-terminals which display the efficient antimicrobial activity especially with respect to gram-positive bacteria. A study reveals how the production of such peptides have displayed that the domain of β-sheet at C-terminal of tenecin 1 is majorly accountable for the antibacterial as well as antifungal activity. The entry of these peptides inside the protoplasm was via ATP independent or dependent mechanisms. Also, AMPs that comprise cationic amino acids usually incite endocytic pathways that are energy dependent, such as macropinocytosis. Whereas, other peptides such as maganin & marine metagenome-derived peptide1 enter into the cells via direct cell-penetration mechanism that is, energy independent (does not require ATP) (Guterstam et al. 2009; Pushpanathan et al. 2012; Park et al. 2000). Apart from this, AMPs, derived from natural sources are not reformed for potent activity and thus, should be enhanced using various strategies, before it can be used as commercial therapeutics. Some of them tested recently for efficient production of AMPs were adjusting the peptidal structures through cyclization, or hydrophobicity of the peptide by tagging or by elevating the charge. Methods involving random mutagenesis improve the quality of naturally derived AMPs by replacement or addition or deletion of single residues or more, N- or C-terminal truncation or by chimeric peptide generation via combining the above-mentioned methods (Pasupuleti et al. 2009). Also, in addition to this, cross links or covalent lactam bonds chemically induced in them provide methods to introduce constraints which are conformational and which converses newer properties in AMPs (Osapay et al. 2000). The activity of AMPs being antimicrobial could also be intensified via changes in their persistent structural manufacturing, by adjusting the flexibility or the hydrophobicity of secondary structures of peptides (Fig. 1).
Mechanism of Action
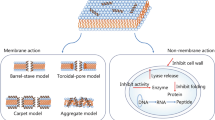
Antimicrobial activity of AMP is particularly linked with its corresponding amino acid composition and physicochemical characteristics. Variations in the sequence of peptide residue generally results in major impacts on the antimicrobial activity (Malanovic et al. 2016). Mechanisms of AMPs depends on the diversity of target microbes, especially gram bacteria (Malanovic et al. 2016). The composition of cell walls of different bacteria also affects the mechanism of AMPs. The initial step of any mechanism is the interaction at molecular level between the anionic molecules present on the cell membrane and peptides. The force responsible for this interconnection is the electrostatic force conferred by the peptide. They destroy bacteria by interfering with some of the major pathways present inside the cell which are crucial for survival such as protein synthesis and DNA replication (Brogden et al. 2005). There are two ways through which an AMP enters into the cell. The first is an uncertain translocation process through the membrane and second one is primarily due to the secondary structure of peptide which leads to formation of pore in the membrane. Therefore, AMPs destroy the target cells by membrane damage or by disruption of lipid bilayer (Lohner et al. 1999).
Source of AMPs
AMPs are synthesized by various sources such as animals, plants, bacteria, insects and marine organisms. Animal derived AMPs were initially discovered in invertebrates and later on found in vertebrates also. These peptides possess diverse sequences, structures and mechanisms (Li et al. 2012). Toxins from plants, animals, marine sources are natural and rich in biological molecules (Seyfi et al. 2019). In addition to natural sources, these AMPs can be produced synthetically (Fjell et al. 2012). AMPs that are produced by synthetic methods include culturing of industrial microorganisms, genetically modified organisms, enzymatic hydrolysis of proteins and separation from natural sources. With the advancement of protein engineering, modification in species is continuously improving.
Animal Sources of AMP
Numerous antimicrobial peptides are present in domesticated animals which show their expression in many tissues such as, mucosal epithelial cells, polymorphonuclear leukocytes and macrophages (Brogden et al. 2003). AMPs have a wide spectrum, with efficient antimicrobial properties against both veterinary and human pathogens. Domesticated animals play a valuable provenance of these peptides. They also act as a model for testing the potency, immunological properties and wound healing effects for AMPs.
Animals who exploit toxic proteins and peptides in order to predate are typically dependent on special structural modifications, like spines, fangs, or a stinger, for efficient intoxication. During an attack on a venomous or poisonous animal because of a predator, it generally has an instantaneous moment to release its toxins and thus, generate a reaction in its defence in order to protect itself. Therefore, animals secrete toxins carrying immediate impacts like distastefulness, or pain, or molecules having integral effects depending on rapid intrusion on the predator's body (Nelsen et al. 2014). Most of the frog species release comparatively bigger molecules along with integral targets, such as proteins (approximately 0.5–2 kDa) and even small proteins (ranging from 4 to 8 kDa) (Xu et al. 2015; König et al. 2015). Besides just secreting toxins, many species of frogs even produce antimicrobial peptides that are able to destroy a wide group of microbes via cell lysis (Conlon et al. 2011). It was considered that AMPs are a constituent of innate immunity of amphibians, and studied mainly in view of their efficiency against pathogens which are clinically important (Xu et al. 2015). A study explained a substitute role in the matter of antipredator defence. It was foretold that AMPs were able to permeabilize the epithelial tissue of predators by cytolytic activity, to ease toxin delivery (König et al. 2015). Evidence for this conjecture highlights a long-neglected consequence of AMPs derived from amphibians that apart from just innate immunity it gradually changes our opinion of their contribution for survival of an amphibian. In vitro experiments have also shown how frog AMPs elevate toxins’s transepithelial passage. Large proportion of the studies done on amphibian skin-secreted peptides conclude how they have predominant pharmacological scope, which aims to identify their physiological effects as well and not just their natural delivery procedures. On further researching the intoxication, it results in two major conclusions. First, evidence searches for the function of AMPs derived from amphibians, outside the immune system. Because of its expression and pore-forming ability on skin, AMPs are likely to play double roles, in innate immunity and antipredator defence. Its cytotoxic activity also causes pain on oral mucosa or irritation, thereby resulting in a quick effect on some of the predators (Xu et al. 2015; Conlon et al. 2014; Conlon et al. 2007). Second, toxin systems depend on epithelial absorption comprising various features that maximize the intoxication effect. The secretion of high levels of toxin further elevates the probability of toxin being effective, obtained in the predator’s body even when a minor percentage gets absorbed (Negri et al. 2006).
Scorpio venoms are also a potential source of AMPs. A study reported that venom of scorpion was a rich source of ion channel blockers i.e. biologically active particles. It has been rapidly identified as an ample supply of AMPs. They can also prevent infection in the gland storing the venom and further ease neurotoxic action (Hernandez-Aponte et al. 2011). The peptides developed by the scorpion venom are cationic amphipathic peptides. AMPs rich in cysteine are omnipresent in venoms of scorpion and also consist of three or four disulphide bridges. Such agents were recognized as those having connections with sodium, potassium, calcium and chloride ion channels. They comprise the largest peptide family of venoms as compared with the non-disulfide bridged peptides. Since peptides extracted from scorpions are seen to be a member of the non-disulfide bridged family, disulphide bridged peptides, on the other hand, have fascinating biological activities. The similarity to be noted is between the scorpion toxins and insect defensins (Bontems et al. 1991) resulting in extraction of the very first scorpion defensin, derived from haemolymph of Leiurus quinquestriatus. Thus, this AMP acts against M. luteus, but doesnt effective against E. coli (Cociancich et al. 1993). AMP discovered by scorpine (scorpion venom) was extracted through the venom of Pandinus imperator, (Conde et al. 2000). Scorpine has a unique configuration comprising similar N-terminals with few of the insect ceropins and similar C-terminals with certain scorpion defensins. Around six peptides have also been derived by the venom of Tityus discrepans containing four disulphide bridges, and known as bactridines (Diaz et al. 2009).
A long chain peptide, derived from Parabutoporin schlechteri, the South African scorpion venom was particularly regular in nature (Ortiz et al. 2015). Assays (antimicrobial) conducted on this peptide showed how it was responsible for inhibition of growth of most gram-negative bacteria and minimum inhibitory concentration varied from 1 to 6 mM. It also displayed low functioning in gram-positive bacteria with MIC being usually more than 25 mM. Also, it was recorded that a decrease in inhibition of growth was seen when magnesium ion was present (Moerman et al. 2002). A totally newly discovered class of AMPs derived from scorpion has been recorded from the venom gland of Heterometrus spinifer, (Nie et al. 2012). It has wide spectrum activity against both Gram-negative organisms (MIC approximately 25–50 mM) and Gram-positive (MIC ranging from 11 to 50 mM) without certain specificity of target-cell and also possess antifungal activity (MIC being 50 mM). The first short chain antimicrobial peptide was extracted from the venom gland of Opisthacanthus madagascariensis (Dai et al. 2006). The precursor of this peptide comprises a pro-sequence present at C-terminal with processing signals. It showed a wide spectrum against most both gram-negative and -positive (MIC approximately 0.6–16 mM). When analysing the short chain AMPs, derived from scorpion, through helical wheel projection, it suggested amphipathic nature with unclear “polar pocket” amongst the structures. But, on the other hand, it was proven that C-terminal amidation elevates its activity and increases cationic charge (Strandberg et al. 2007).
AMPs derived from insects possess short-lived humoral immunological reactions, for example, via high peptide amounts in the haemolymph, that lasts longer with respect to the initial cellular responses and that operate as a substitute against diseases (Makarova et al. 2016). Large reservoirs of AMPs are extracted from insects in comparison with any other taxonomic groups. The number for each of these differ from one another. Harmonia axyridis, meddlesome ladybird produces around 50 AMPs (Vilcinskas et al. 2013), Acyrthosiphon pisum, a pea aphid, on the other hand does not produce any known AMPs (Gerardo et al. 2010). These AMPs are further related with environmental threats faced during evolution, that is, those which are subjected to higher number of diverse pathogens are expected to have been adapted to a wider range of AMPs (Vilcinskas et al. 2013; Altincicek et al. 2007).These AMPs also show an extraordinary evolutionary flexibility in the coding genes with respect to the losses, operational shifts and gains. This encloses replication along with contrasting advancement of these peptides, which can thus proceed into contemporary operations of the final manifolds (Sackton et al. 2007). The very first AMP derived was cecropin, derived from an insect Hyalophora cecropia, the giant silk moth’s larvae. Being an α-helical AMP, it is linear and effective against gram-negative bacteria (Steiner et al. 1981). Discovering the evolutionary aspect of AMPs with respect to the insect, two prime drawbacks have been reported (Misof et al. 2014). First, they exist a considerable level of partiality in the interest of insect taxa with abundance of data sequencing and in opposition with the branches which are distinctly diminished. The second one being the identification of homology amidst genes of AMP. Their frequent gain and loss during evolution, short length as well as significant variation between species influence the recognition and distinction of AMP families (Vilcinskas et al. 2013; Sackton et al. 2007; Waterhouse et al. 2007). Thus, existing taxon- specific and widespread AMP families can be revealed by their mapping on the phylogenetic tree of insects. Further details for animal source AMPs mentioned in Table 1.
Plant Sources Used for AMP Production
AMPs derived from plants, are constituent of plant barrier defence systems. They could be extracted from various plant parts such as roots, seeds, flowers, leaves and stems and present in broad diversity of genus and species. They are also involved in phytopathogen activities and possess antibacterial responses against various microbes including those which are pathogenic to humans (Montesinos et al. 2007). Thus, these AMPs are potential antibiotic compounds with significant application in the field of biotechnology. They are categorized into various families, sharing basic characteristics such as presence of disulfide bonds, positive charge, and target mechanism of action in the external membrane structures. Plant AMPs are tissue-specific and susceptible to evolution since they have sequences which are hypervariable, enclosed in a specific scaffold characteristic of the corresponding AMP family (Tam et al. 2015). Plant AMPs are classified based on their similar genetic sequences, cysteine rich motifs and disulfide linkages which provide information about their folding of tertiary structure. These include families like thionins, plant defensins, snakins, knottin-type and hevein-like proteins, α-hairpinin families, etc. Further details for plant source AMPs mentioned in Table 2.
Thionins
This is an AMP family rich in lysine, arginine and cysteine components having less molecular weight. A single double-stranded β-sheet and two α- helices, both being antiparallel, together builds the structure along with disulfide linkages (three to four). Initially, they were termed as toxins isolated from plants due to their lethality towards fungi (Ebrahimnesbat et al. 1989), bacterial species (Fernandez de Caleya et al. 1972), plant and animal cells (Evans et al. 1989), along with larvae of insects (Kramer et al. 1979). The compound responsible for antimicrobial activity of thionin was α-purothionin which was extracted from the wheat endosperm (Fernandez de Caleya et al. 1972). They are hydrophobic in nature and induce their toxicity in fungi, bacteria, as well as animal and plant cells through interconnections of membranes along with their corresponding constituents which were hydrophobic and carry positive surface charge (Stec et al. 2006; Mander et al. 2010). Thionin is able to directly come in contact with membrane lipids, keeping aside the protein receptors (Richard et al. 2005). Pyrularia, an example of thionin, extracted from Pyrularia pubera, carries out calcium ion inflow during some of the cellular reactions, meanwhile, iodination of tyrosine lowers down the cytotoxicity along with the process of hemolysis (Evans et al. 1989). Their toxicity was assumed to elevate from membrane lysis of the cells to be attached with. Although, the proper procedure responsible for the virulence still is not known as for now.
Defensins
Defensins are well known with respect to their abundance in area of membranolytic functions, among all plant AMPs. These are additionally produced in the biotic stress reaction along with plant growth and evolution. They can also attach with the membranes of microbes via interacting with receptors (Thevissen et al. 2000, 2004). Binding of these with the cell membrane ends up in the efflux and influx of calcium and potassium ions (Thevissen et al. 1996, 1999). The initial plant defensin was extracted from Triticum aestivum along with Hordeum vulgare and also grouped in the class γ- thionins. Defensins have two classes, the first being, precursor protein, comprises amino signal peptide which targets the extracellular space; and second having C- terminal prodomains. Other features include characters related with growth regulation, development and fertilization (Oomen et al. 2011).
Snakins
These peptides are extracted from potato tubers and include snakin-1 and snakin-2 which contains 63 amino acid residues and it’s a cell wall-associated peptide which shows antimicrobial activity. They show 38% sequence similarity and possess almost similar antimicrobial properties against fungus and bacteria of vivid plant species. All snakins contain twelve reserved residues of cysteine along with six disulfide bonds (Segura et al. 1999). Snakin mode of action is still unknown, although they do avoid interaction with artificial membranes of lipids. Snakin-1 gene derived from Solanum tuberosum is essentially displayed in various tissue development and doesn’t react to biotic or abiotic stresses. Snakin-2 gene induces characteristic response to pathogenic infection. These peptides are fundamentally cysteine rich, forming six disulphide bridges, thus stabilizing its structure (Berrocal-Lobo et al. 2002). Snakins showed antimicrobial activity against both Gram-negative and -positive bacteria, hence, are identified as plant defence constituents of inducible barriers. Their functional aspects are not specifically explained and very little is known about its mode of action. Most of these genes are operated by phytohormones and their participation was seen in hormonal signalling pathways (Nahirñak et al. 2012).
Hevein-like Plant Proteins
These peptides are isolated from lutoid bodies of Hevea brasiliensis latex that originated from rubber trees (Van Parijs et al. 1991). It binds to chitin and inhibits the hyphal growth of fungus. Other such peptides having antimicrobial properties have been recognized in many plant species (Koo et al. 1998; Kiba et al. 2003; Huang et al. 2004; Porto et al. 2012). These AMPs have variable disulfide bonds, out of which, most of them contain eight residues of cysteine, establishing four disulfide bonds; hevein homolog extracted from Pharabitis nil and Avena sativa seeds (Li et al. 2003). Apart from this, they contain six residues, derived from seeds of Ginkgo biloba or Amaranthus caudatus (Huang et al. 2000). Few hevein-like AMPs have ten cysteine residues and extracted from the bark of Euonymus europaeus, Eucommia ulmoides and seeds of Triticum kiharae (Van den Berg et al. 2002; Huang et al. 2002; Odintsova et al. 2009). Hevein is the perfect prototype for researching the carbohydrate- peptide linkage, because it's a chitin binding motif. It was also reported that its mediation takes place with the help of hydrogen bonds and van der Waals forces (Asensio et al. 2000; Chavez et al. 2010). The interaction of π-electrons of aromatic amino acids of hevein-like peptides with the C-H groups (hydrophobic) of carbohydrates results into binding of chitin. Apart from this it has an alternative function, where it serves a role of plant defence against infections caused by fungus (Slavokhotova et al. 2014).
Knottin-like Proteins
They belong to the member of a superfamily, which consist of inhibitors of trypsin, α-amylase, carboxypeptidase and cyclotides. Generally, their sizes are small but possess diverse functional among the plants. These types of antifungal peptides were extracted from Phytolacca americana and Mirabilis jalapa (Cammue et al. 1992; Gao et al. 2001). A characteristic feature of such family is that they show a wide diversity of bioactive operations such as enzyme-inhibitory, insecticidal, antimicrobial, cytotoxic, and anti-HIV activities as well as hormone-like functions (Pallaghy et al. 1994). Previously, discovery of these peptides was done as protease inhibitors with a cysteine motif. Therefore, termed as cystine-knot inhibitor peptides. This prototype was first noted in 1982, in the PCI (potato carboxypeptidase inhibitor) (Rees et al. 1982). The disulfide bonds present in such scaffolds are significant in order of maintaining the enzymatic & chemical consistency. Such AMPs are also amphipathic in nature, along with membranolytic functions. This is the characteristic required for the interaction of membranes, which leads to the antimicrobial action. It is also a perfect alternative for peptide-based pharmaceutical engineering application because of various features such as (1) excellent thermal, chemical and proteolytic stability; (2) chemical feasibility because of the small size; and (3) proper maintenance of sequence because of the variation of loop areas (Wong et al. 2012).
Marine Sources Used For AMP Production
Marine organisms produce naturally derived antibacterial compounds because of its initial response against foreign organisms for survival. Peptides derived from marine sources are a rich source of new antimicrobials. Structurally, marine AMPs vary from their substitutes, originating from terrestrial species (Cheung et al. 2015). Also, their antimicrobial activity depends on their interaction (electrostatic) with bacterial surface carrying negatively-charged particles. Free ions generated due to the heavy saline concentrations in the medium surrounding them, in the cases of certain diseases, can potently reduce the interaction as well as the antimicrobial activity (Falanga et al. 2016). These AMPs have evolved over time and can sustain in these high salt concentrations of sea water with the help of replacement, by lysines in the place of arginines. Thus, these species are reservoirs of biologically active compounds for betterment of therapeutic options. Such AMPs are different from each other on the basis of pI (isoelectric point), length, hydrophobicity, amphipathicity, secondary structure, and antimicrobial properties. Apart from the variability of marine AMPs, existence of certain key features is prevalent, which are common in most of them, thus leading to a wide classification based on structural and biochemical properties (Otero-González et al. 2010), such as (1) linear & helical peptides; (2) linear-helical peptides containing specific kind of amino acid (proline, tryptophan, or glycine rich peptides); (3) proteins forming hairpin-like structures/sheets and regulated via intramolecular disulfide bonding; and (4) cyclic peptides. AMPs derived from marine sources have been adapted to survive in presence of high saline conditions and protease activity. These peptides exploit the post-translational modifications in order to AMPs having high maintenance and efficiency, for medical treatments in humans. These modifications also help marine species; by inducing structural changes such as folding in scaffolds which are required for the interconnection between targeted bacterial cells and peptidal membranes, and also maintain proper stability (Wang et al. 2012). These changes include: bromination, sulphidation, chlorination, modification of selected amino acids, amidation at C-terminal, etc. Further details for marine source AMPs mentioned in Table 3.
Aurelin
Aurelin is isolated from Aurelia aurita which contains three disulfide bonds (Ovchinnikova et al. 2006; Shenkarev et al. 2012). Another class of such AMPs are arenicins which possess antibacterial activity against P. aeruginosa and S. aureus (Lee et al. 2007; Ovchinnikova et al. 2004, 2007). Another class of marine AMPs, Mylitin, loses its properties against microbes when its structure containing cysteine is destroyed since that counts for structural stability (Roch et al. 2008). These are more active in response to antibacterial activity against gram-positive bacteria and less active against Gram-negative bacteria and fungus. Aurelin display wide diversity against bacteria, which can due to two mode of action i.e. (a) peptide toxin which blocks potassium ion channels, (b) membrane operative antimicrobial peptide (Shenkarev et al. 2012). They have shown no homology sequences with any previously recognized AMP, but exhibited certain resemblance with respect to defensins along with toxins blocking K- channels of sea anemones. They are also identified for inhibition of T-lymphocyte membrane through potassium channels which are voltage- gated, owned by the ShK toxic domain family (Ovchinnikova et al. 2006).
Hepcidins
These are peptides rich in cysteine, extracted from Oreochromis mossambicus and possess 50–100 μg/mL range of MICs against S. aureus, Enterococcus faecium and L. monocytogenes (Huang et al. 2007). Its basic structure contains four to eight residues, rich in cysteine and disulfide bonds (two to four). These contain antimicrobial attributes which serve significant purposes in providing resistance against any pathogenic infection. Fish hepcidins show antimicrobial activity against a wide variety of bacteria and display mainly potent activity against fish pathogens.
Damicornin
It is an AMP containing 40 residues, derived from Pocillora damicornis (Vidal-Dupio et al. 2011). It is positively charged, amidated at C-terminal and recognized by six residues rich in cysteine, linked with three intramolecular disulfide bridges. Such an array of cysteine residues is common with another class of AMPs and toxins extracted from cnidarians (Vidal-Dupiol et al. 2011). It shows reaction against gram-positive bacteria (B. megaterium, M. luteus, Brevibacterium stationis, S. aureus and Microbacterium maritypicum; MIC = 1.25–20 μM) along with Fusarium oxysporum fungus (MIC = 20 μM), whereas negligible response against Gram-negative species. Its genetic expression was controlled with a concomitant ectodermal cell of host by Vibrio coralliilyticus (Vidal-Dupiol et al. 2011).
Mytimacin
It is a peptide isolated from marine molluscs, specifically from Achatina fulica snail. Such peptides are distinguished by richness of cysteine composition and eighty amino acid residues, ten out of which are cysteine residues. Mytimacin-AF is capable of fighting against both Gram-negative and -positive bacteria, although it is more efficient against Staphylococcus aureus with MIC value around 1.9 g/ml. Furthermore, its activity against Klebseilla pneumonia is also promising (Zhong et al. 2013).
Myticusin
Another kind of cysteine-rich peptide, Myticusin-1 was extracted from mussels, and characterized from the hemolymph of Mytilus coruscus. Its contribution regarding the host immunological reaction was proved, hence it serves as an important antimicrobial agent. This peptide was more active against Gram-positive bacteria as compared with Gram-negative bacteria, as it showed MIC value lesser than 5 mM in response to the diversity of Gram-positive tested strains, such as S. aureus, when compared with MIC value greater than 10 mM in response to -negative bacteria, such as E. coli (Liao et al. 2013).
AMPs from Marine Invertebrates
The class of invertebrates originating through marine sources defend themselves against pathogens mainly through innate immunity. Vital elements such as immunological memory, antibodies, lymphocytes and identification of self and non-self-cells are absent in such organisms. But they are able to combat surrounding pathogens. Its humoral immunity is based on the amount of antimicrobial agents present in haemocytes and blood plasma. Their cellular immunity depends on their reactions with respect to cell defence, including phagocytosis, encapsulation and nodule formation (Mydlarz et al. 2006). The cellular constituent of immunity of marine invertebrates is regulated by mobile cells that causes cell lysis of microbes and hemocytes via secretion of cytotoxic substances as well as soluble antimicrobial substances in the hemolymph (Mitta et al. 2000). Marine derived AMPs have characteristics which carry biomedical significance, thus, making them interesting alternatives for rational drug designs and pharmaceuticals with a large potential in biotechnological and pharmaceutical areas.
Multidimensional Features of AMPs
Their interaction with various bacterial cell membranes make them capable of playing the role of flexible effector molecules. AMPs have a variety of properties which make them useful. Greater inclination of tumour cells with the cationic membrane activates AMPs because of their inflated amount of negatively charged phosphatidylserine elements, present on their cell membranes. Thus, it is fascinating to make normal cells a preferable candidate for AMP as antitumor agents (Utsugi et al. 1991). A report suggested that exhibition of tumoricidal activity against carcinoma and melanoma cells has been seen, both under in vivo and in vitro conditions. Generally, it is seen that higher levels of these peptides are required in order to reach the favourable tumoricidal activity as studies show that cytotoxicity of tumour cells is detected only at those levels mainly because of the incapability of magnesium ion binding in the membrane along with its further entry. Also, they are slightly more prone to modification via proteases present in the matrix (extracellular) of tumour cells, thus leading in dropping of the tumoricidal activity (Muller et al. 2002). Further, this issue was dealt by genes encoded by AMPs, right into the tumour cells or with replacing of their D-amino acids and improvisation via amidation of peptide terminal, in the place of peptidal amino acids.
Another role of AMPs is that it acts as Host defence peptides (HDPs), which are small positively charged AMPs assembled from the immunological systems of those species who serve an important role in providing innate immunity (Steinstraesser et al. 2011). Most of these HDPs help in the inflection of host immune response and play the role of modulators with signal transduction cycles via adjusting intracellular activity of signalling targets as protein kinases. Apart from their antimicrobial and immune regulating mechanisms, these peptides play a vital role in immune neuroendocrine interactions. They take part in the pathogenesis of corticostatic reactions (stress action) as well as act as administrative peptides (Kokriakov et al. 2006). At their subinhibitory concentrations, AMPs are able to activate various genes which take part in signal transduction networks. In this matter, sigma factors of RNA polymerases are crucial components for the determination of promoter selectivity. The substitution of sigma factor in place of another one can deflect the cell’s RNA polymerases to generate gene transcription. The extra cytoplasmic function, that is ECF sigma factors are defined as the short regulatory proteins, varying in sequence and correlated to other factors which operate as anti-sigma elements, attaching and inhibiting cognate sigma factors after receiving a stimulus from the surrounding (Heimann et al. 2002) (Fig. 2).
Therapeutic Studies of AMPs
AMPs tend to show a wide spectrum of antibacterial activity comprising MDR pathogens. This leads to the lower risks of development of resistance which is required for them to be qualified as drug candidates. In the past few decades, natural AMPs have been used for commercial use after various attempts and none of them including linear peptides. At present, even AMPs, synthetically derived, have not been finalized by the FDA. Many of the peptide antibiotics produced from clinical use are mostly isolated from Bacillus species. Some of such peptides are polymyxin, bacitracin, tyrothricin and gramicidin (Sumi et al. 2015). The first AMP used for clinical purposes was Tyrothricin, consisting of tyrocidine (main component) and gramicidin. Another naturally obtained AMP, Nisin was isolated from Lactococcus lactis, which showed efficient activity in response to gram-positive bacteria, specifically mastitis pathogens. Because of the minimal toxicity, it has also been approved for food preservative use in 48 countries, particularly as dairy products (Deegan et al. 2002). Bacitracin (cyclic polypeptides), executed commercially as a combination drug, comprises polymyxin along with neomycin (Neosporin ™) for the purpose of topical treatment of skin & eye illnesses. Moreover, bacitracin has also shown nephrotoxicity (Awais et al. 2007). Another AMP daptomycin, a lipopeptide (cyclic), is used for treating the infections associated with MDR Gram-positive pathogens (Humphries et al. 2013). Polymyxin B and E (cyclic lipopeptides), termed as colistin, have shown their antimicrobial activity against gram-negative bacteria, and used for therapeutic purposes. They are also considered as the last-line or final therapy associated with MDR gram-negative infections (Rabanal et al. 2017). Most of the AMPs fail in the preclinical tests during the last phase, specifically because of their high probability of degradation as well as unexpected toxicity. Hence, pharmaceutical companies only used them for therapeuctic application. Majorly, AMPs in the current scenario, target skin infections associated by gram-positive bacteria.
AMPs in clinical trials
AMPs in Preclinical Trials
MU1140 is a lantibiotic (major source being Streptococcus mutans), targeted against gram positive bacteria, especially C. difficile with its administration still unspecified (Ghobrial et al. 2009). Another lipopeptide, HB1345 targets broad-spectrum antibiotics and its administration is topical (https://helixbiomedix.com/antiinfective.html). A synthetic antimicrobial namely, Novarifyn (NP432) mainly targets E. coli, P. aeruginosa, A. baumannii and C. difficile along with topical administration (https://www.novabiotics.co.uk/pipeline/novarifyn-np432). Arenicin (AP139) is another antimicrobial peptide with source being Lugworm Arenicol marina and targets gram negative bacteria with unspecified administration (Andra et al. 2008). Arenicin analog, AP138 targets MRSA implant infections and has also an unspecified administration (https://adeniumbiotech.com/pipeline/). Avidocin and purocin are modified R-type bacteriocins who target both gram positive and negative bacteria and both of them involve oral administration (Gebhart et al. 2015).
AMPs in Phase I Clinical Trials
NVB-302 an antibiotic targets C. difficile and involves in oral administration (Crowther et al. 2013). BAL30072 is an antimicrobial peptide with monobactam being its lead compound and it targets both gram positive and negative bacteria with penicillin-binding protein being its mechanism of action (Sutcliffe et al. 2011). Another peptide NVB302 contains deoxyactagardine B (that is, Type B lantibiotic) and targets gram positive bacteria via lipid II binding as its mode of action (Hochlowski et al. 1987). LCB01-0371 is a peptide comprising oxazolidinone and targets gram positive bacteria by inhibiting the protein synthesis (Theriault et al. 1987). Aztreonam contains Monobactam and it targets gram positive bacteria by Penicillin-binding protein (Silver et al. 2008). Avibactam contains diazabicyclooctane and it acts on gram negative bacteria by inhibiting β-lactamase (Boucher et al. 2009).
AMPs in Phase II Clinical Trials
C16G2 a synthetic peptide targets to prevent tooth decay caused by Streptococcus mutans and its administration involves mouthwash (Kaplan et al. 2011). An indolicidin analog, IMX942 (SGX942) aims at the prevention of immunomodulation at the time of oral mucositis by oral administration (oral rinse)( https://www.soligenix.com/wp-content/uploads/sgx94_executive_summary_032216.pdf). DPK-060 is a kininogen for human protein targets acute external otitis prevention via ear drops as its administration (https://clinicaltrials.gov/ct2/show/NCT01447017?term=DPK-060&rank=1). A human lactoferrin analog, PXL01 targets prevention of surgical adhesion by topical administration (https://clinicaltrials.gov/ct2/show/NCT01022242?term=PXL01&rank=1). Defending analog namely Brilacidin aims at radiation-induced oral mucositis in those patients who are suffering from head and neck cancer, via oral administration (https://clinicaltrials.gov/ct2/show/NCT02324335?term=Brilacidin&rank=1). Histatin analog known as PAC113 aims at oral candidiasis in patients being HIV seropositive, using mouth rinse as its form of administration (https://clinicaltrials.gov/ct2/show/NCT00659971?term=PAC113&rank=1). An antimicrobial peptide, LFF-57 has GE2270 A as its lead compound which targets gram positive bacteria via the elongation factor Tu as its mode of action (Silver et al. 2011). Another peptide Auriclosene, containing N-Chlorotaurine and targets both gram positive and negative bacteria by adapting oxidation as its mechanism of action (Boucher et al. 2013). Sarecycline, a tetracycline containing compound which targets gram positive bacteria by inhibiting protein synthesis (https://www.astrazeneca.com/Media/Press-releases/Article/20130321–astrazeneca-outlines-strategy-return-to-growth-scientific-leadership). BC-3781 is an antimicrobial peptide consisting of Pleuromutilin which targets both gram positive and negative bacteria and acts upon them by inhibiting protein synthesis (Shlaes et al. 2011). Plazomicin is an aminoglycoside containing peptide which aims against both gram negative and positive bacteria via inhibiting protein synthesis (Payne et al. 2007). LTX-109 is a cationic peptide which targets both gram negative and positive bacteria via disrupting the membrane (https://www.merckmanuals.com/professional/lexicomp/quinupristin%20and%20dalfopristin.html). PA-824 is a Nitroimidazole containing peptide which aims against gram positive bacteria by DNA as well as cellular damage (Bhavnani et al. 2009). AFN-1252 a synthetic peptide aiming against gram positive bacteria by inhibiting FabI (https://investor.cempra.com/releasedetail.cfm?ReleaseID=767526). Another peptide WCK-771 contains fluoroquinolone and targets both gram positive and negative bacteria by DNA gyrase and topoIV as its mode of action (Shlaes et al. 2012).
AMPs in Phase III Clinical Trials
p2TA (AB 103), a synthetic peptide targeting infections involving necrotizing soft tissue via intravenous administration (https://clinicaltrials.gov/ct2/show/NCT02469857?term=p2TA&rank=1). Dalbavancin is a glycopeptide which targets gram positive bacteria via inhibiting the cell wall (Berdy et al. 2012). Solithromycin is an antimicrobial peptide which contains Erythromycin and targets both gram positive and negative bacteria via inhibiting the protein synthesis (Wellington et al. 2013). Surotomycin is a lipopeptide targeting gram positive bacteria via depolarizing the membrane (Rodrı´guez-Rojas et al. 2013). Delamanid contains nitroimidazole as its lead compound and targets gram positive bacteria by DNA and cellular damage as its mechanism of action (Hawser et al. 2011). Finafloxacin is a Fluoroquinolone containing peptide which targets both gram positive and negative bacteria by DNA gyrase and topoIV as its mode of action (https://www.nytimes.com/2013/03/06/health/deadly-drug-resistant-infections-rise-inhospitals-report-warns.html). Nemonoxacin is a quinolone comprising peptide which aims against both gram positive and negative bacteria via using DNA gyrase and topoIV as its mechanism of action (Laxminarayan et al. 2011) (Fig. 3).
Challenges
Despite the fact that AMPs are viable alternatives for conventional antibiotics because of their potential antimicrobial activity, they still do have some drawbacks. Due to their proteinaceous nature, these are recognized via very low bioavailability & thus undergo poor proteolytic maintenance. These features have critically affected its progress, clinically till now. Numerous adaptations and changes have been attempted to overcome such disadvantages, and to create new synthetic analogues that have the same properties as of AMPs (Giuliani et al. 2011; Scorciapino et al. 2012). Those AMPs which become the principal model for such changes are the ones found in nature. They are usually confined to the physico-chemical changes along with activity in membrane-related features, such as cationic charge and amphiphilic nature. Applications of AMPs at clinical levels have not been remarkable in the last decade and thus requires further efforts (Hancock et al. 2006; Lipsky et al. 2008; Vaara et al. 2009). The reasons for this failure are multidimensional, yet the unstable peptides in vivo are due to the following three reasons. First, in the present scenario, AMPs are potent agents. On the other hand, in traditional times, AMPs were isolated and purified right from the tissues, in order to obtain their antimicrobial properties. Presently, a wide number of AMPs are usually predicted by using Bioinformatics. Sometimes, they are produced in the form of peptides also, to test for the activity against microbes. Regardless of this, such synthetic AMPs have displayed quite potential activity against the microorganisms. Second, AMPs have highly diversified structure and it's difficult to verify the antimicrobial traits of these agents, mainly because of the long period of time required and financial crunch involved. Although, to overcome this obstacle, selection of specific AMPs is of utmost importance along with the estimation of its antibacterial properties. Four predominant AMPa are: beta-sheet formed with 2–3 disulfide bridges, helix, linear peptides rich in special amino acids and loop peptides formed by one disulfide bridge (Vaara et al. 2009). Third, the screening process in the primary laboratory of AMPs generally comprise of testing their ability to fight against bacteria, on several types of culture strains or various clinical spots, randomly selected. Being intuitive at times, these results rarely pose apt results. Thus, there is a need for development of newly designed AMPs.
Future Prospects
Traditionally, there was an approach for discovering new bioactive products from natural sources by working on the required sample or metabolite and bioguiding the characterization of extracts (Farha et al. 2016). However, these techniques have many drawbacks. The commercialisation of these compounds at reasonable prices can be done with the help of various biotechnological techniques. On detection of peptides in microbes, scaling up the process or culture is feasible. However, for execution of gene-encoded AMPs, various other techniques are preferable such as cDNA encoding as the biosynthetic precursor of Dermaseptin-PH was recognized from Pithecopus hypochondrialis skin (Huang et al. 2017). The cDNA (complementary DNA) rapid amplification ends by polymerase chain reaction allows the identification of cDNA, further encoding for the Dermaseptin-PH’s biosynthetic precursor. The primary compounds which was secreted via skin was detected by reverse phase high performance liquid chromatography) and then its chemical synthesis was processed. This peptide leads to the decrease in bacterial growth of both gram-positive and -negative bacteria along with Candida albicans. A significant drawback associated with the search for naturally obtained products is that various interesting compounds are not displayed by the organism. Their genetics stay in a state termed as ‘cryptic’, till the time any change occurs in the environment, which leads to the promotion of their transcription. For instance, some of the actinomycetes species don’t generate antibiotics till they are relatively cultured with bacteria or till the time the culture medium consists of some activators such as goadsporin (Onaka et al. 2017). Therefore, there is a need to find a method that can tackle these ‘silent’ or ‘cryptic’ natural products (Zarins-Tutt et al. 2016). Bioinformatic procedures have been in use for predicting such silent products, coded in the known genome of various species, from microorganisms to animals (Vila-Farres et al. 2017).
Conclusion
Antimicrobial peptides are termed as host-defence peptides due to their immunomodulatory characteristics. They have potential to become the future generation of antimicrobial “superdrugs” if the drawbacks associated with its stability, reasonable production costs and bioavailability are resolved. Along with this, their efficiency against pathogens, currently not responding to the prevalent treatments is also remarkable with their multiple mechanisms of action. The significant assets for pharmaceutical companies consist of their efficiency against microbes, mode of action with low resistance of pathogens, antimicrobial properties and ability to modify immune responses. Although, only a few antimicrobial peptides are present in current markets; most of them of which are used for topical use. Development of new biotechnological techniques has preferably reduced their costs for industrial production. Few of them, interestingly, are focused to fight standard diseases or infections resulted by pathogen threats. It is expected that a better and certainly new generation of efficient, long-lasting and versatile drugs with antimicrobial qualities, will soon be feasible and available in the markets.
References
A study of DPK-060 to investigate clinical safety and efficacy in patients with acute external otitis. https://clinicaltrials.gov/ct2/show/NCT01447017?term=DPK-060&rank=1. Accessed on 18 Aug 2017
Adenium biotech pipeline. https://adeniumbiotech.com/pipeline/. Accessed on 18 Aug 2017
Altincicek B, Vilcinskas A (2007) Analysis of the immune-inducible transcriptome from microbial stress resistant, rat-tailed maggots of the drone fly Eristalis tenax. Bmc Genomics 8(1):326
Andersson DI, Hughes D, Kubicek-Sutherland JZ (2016) Mechanisms and consequences of bacterial resistance to antimicrobial peptides. Drug Resist Updates 1(26):43–57
Andra J, Jakovkin I, Grotzinger J, Hecht O, Krasnosdembskaya AD, Goldmann T, Gutsmann T, Leippe M (2008) Structure and mode of action of the antimicrobial peptide arenicin. Biochem J 410:113–122
Anti-infective. https://helixbiomedix.com/antiinfective.html. Accessed on 18 Aug 2017
Arenecin. https://adeniumbiotech.com/arencin/. Accessed on 18 Aug 2017
Arzanlou M, Chai WC, Venter H (2017) Intrinsic, adaptive and acquired antimicrobial resistance in Gram-negative bacteria. Essays Biochem 61(1):49–59
Asensio JL, Siebert HC, von der Lieth CW, Laynez J, Bruix M, Soedjanaamadja UM, Beintema JJ, Cañada FJ, Gabius HJ, Jiménez-Barbero J (2000) NMR investigations of protein‐carbohydrate interactions: Studies on the relevance of Trp/Tyr variations in lectin binding sites as deduced from titration microcalorimetry and NMR studies on hevein domains. Determination of the NMR structure of the complex between pseudohevein and N,N′,N″‐triacetylchitotriose. Proteins 40(2):218–236
AstraZeneca outlines strategy to return to growth and achieve scientific leadership Press release 21 March 2013. https://www.astrazeneca.com/Media/Press-releases/Article/20130321–astrazeneca-outlines-strategy-return-to-growth-scientific-leadership. Accessed on 27 May 2013
AvidocinTM & PurocinTM proteins. https://avidbiotics.com/technology/avidocinproteins/. Accessed on 18 Aug 2017
Awais M, Shah AA, Hameed A, Hasan F (2007) Isolation, identification and optimization of bacitracin produced by Bacillus sp. Pak J Bot 39(4):1303
Bacitracin. https://www.drugbank.ca/drugs/DB00626. Accessed on 18 Aug 2017
Banting FG, Best CH, Collip JB, Campbell WR, Fletcher AA (1922) Pancreatic extracts in the treatment of diabetes mellitus. Can Med Assoc J 12(3):141
Bayer AS, Mishra NN, Sakoulas G, Nonejuie P, Nast CC, Pogliano J, Chen KT, Ellison SN, Yeaman MR, Yang SJ (2014) Heterogeneity of mprF sequences in methicillin-resistant Staphylococcus aureus clinical isolates: role in cross-resistance between daptomycin and host defense antimicrobial peptides. Antimicrob Agents Chemother 58(12):7462–7467
Bera A, Biswas R, Herbert S, Kulauzovic E, Weidenmaier C, Peschel A, Gotz F (2007) Influence of wall teichoic acid on lysozyme resistance in Staphylococcus aureus. J Bacteriol 189:280–283. https://doi.org/10.1128/JB.01221-06
Berdy J (2012) Thoughts and facts about antibiotics: where we are now and where we are heading. J Antibiot 65:385–395
Berkner S, Konradi S, Schönfeld J (2014) Antibiotic resistance and the environment: there and back again. EMBO Rep 15(7):740–744
Berrocal-Lobo M, Segura A, Moreno M, López G, Garcıa-Olmedo F, Molina A (2002) Snakin-2, an antimicrobial peptide from potato whose gene is locally induced by wounding and responds to pathogen infection. Plant Physiol 128(3):951–961
Bhavnani SM, Prakhya A, Hammel JP, Ambrose PG (2009) Cost-effectiveness of daptomycin versus vancomycin and gentamicin for patients with methicillin-resistant Staphylococcus aureus bacteremia and/or endocarditis. Clin Infect Dis 49:691–698
Bontems F, Roumestand C, Gilquin B, Menez A, Toma F (1991) Refined structure of charybdotoxin: common motifs in scorpion toxins and insect defensins. Science 254(5037):1521–1523
Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, Scheld M, Spellberg B, Bartlett J (2009) Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis 48:1–12
Boucher HW, Talbot GH, Benjamin DK, Bradley J, Guidos RJ, Jones RN, Murray BE, Bonomo RA, Gilbert D (2013) 10x‘20 Progress: development of new drugs active against Gram-negative bacilli: an update from the Infectious Diseases Society of America. Clin Infect Dis 56:1685–1694
Brogden KA (2005) Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol 3(3):238–250
Brogden KA, Ackermann M, McCray PB Jr, Tack BF (2003) Antimicrobial peptides in animals and their role in host defences. Int J Antimicrob Agents 22(5):465–478
Buchanan JT, Stannard JA, Lauth X, Ostland VE, Powell HC, Westerman ME, Nizet V (2005) Streptococcus iniae phosphoglucomutase is a virulence factor and a target for vaccine development. Infect Immun 73:6935–6944. https://doi.org/10.1128/IAI.73.10.6935-6944.2005
Büssing A, Stein GM, Wagner M, Barbara W, Schaller G, Pfüller U, Schietzel M (1999) Accidental cell death and generation of reactive oxygen intermediates in human lymphocytes induced by thionins from Viscum album L. Eur J Biochem 262(1):79–87. https://doi.org/10.1046/j.1432-1327.1999.00356.x
C16G2 STAMP Program. https://www.c3jtherapeutics.com/technologies/c16g2/. Accessed on 18 Aug 2017
Cammue BP, De Bolle MF, Terras FR, Proost P, Van Damme J, Rees SB, Vanderleyden J, Broekaert WF (1992) Isolation and characterization of a novel class of plant antimicrobial peptides form Mirabilis jalapa L. seeds. J Biol Chem 267(4):2228–2233
Campos MA, Vargas MA, Regueiro V, Llompart CM, Alberti S, Bengoechea JA (2004) Capsule polysaccharide mediates bacterial resistance to antimicrobial peptides. Infect Immun 72:7107–7114. https://doi.org/10.1128/Iai.72.12.7107-7114.2004
Carlsson AN, Engström P, Palva ET, Bennich HA (1991) Attacin, an antibacterial protein from Hyalophora cecropia, inhibits synthesis of outer membrane proteins in Escherichia coli by interfering with omp gene transcription. Infect Immun 59(9):3040–3045
Cempra awarded $58 million contract to develop antibiotic for pediatric use and biodefense by Biomedical Advanced Research and Development Authority (BARDA) (Press release 28 May 2013). https://investor.cempra.com/releasedetail.cfm?ReleaseID=767526. Accessed on 30 May 2013
Chávez MI, Vila-Perelló M, Cañada FJ, Andreu D, Jiménez-Barbero J (2010) Effect of a serine-to-aspartate replacement on the recognition of chitin oligosaccharides by truncated hevein. A 3D view by using NMR. Carbohydr Res 345(10):1461–1468
Cheung RC, Ng TB, Wong JH (2015) Marine peptides: bioactivities and applications. Marine drugs 13(7):4006–4043
Cociancich S, Goyffon M, Bontems F, Bulet P, Bouet F, Menez A, Hoffmann J (1993) Purification and characterization of a scorpion defensin, a 4kDa antibacterial peptide presenting structural similarities with insect defensins and scorpion toxins. Biochem Biophys Res Commun 194(1):17–22
Conde R, Zamudio FZ, Rodriguez MH, Possani LD (2000) Scorpine an anti-malaria and anti-bacterial agent purified from scorpion venom. FEBS Lett 471(2–3):165–168
Conlon JM (2011) Structural diversity and species distribution of host-defense peptides in frog skin secretions. Cell Mol Life Sci 68(13):2303–2315
Conlon JM, Al-Ghaferi N, Abraham B, Leprince J (2007) Strategies for transformation of naturally-occurring amphibian antimicrobial peptides into therapeutically valuable anti-infective agents. Methods 42(4):349–357
Conlon JM, Mechkarska M (2014) Host-defense peptides with therapeutic potential from skin secretions of frogs from the family Pipidae. Pharmaceuticals 7(1):58–77
Corzo G, Escoubas P, Villegas E, Barnhjam KJ, He W, Norton RS, Nakajima T (2001) Characterization of unique amphipathic antimicrobial peptides from venom of the scorpion Pandinus imperator. Biochem J 359(1):35–45
Crowther GS, Baines SD, Todhunter S, Freeman J, Chilton CH, Wilcox MH (2013) Evaluation of NVB302 versus vancomycin activity in an in vitro human gut model of Clostridium difficile infection. J Antimicrob Chemother 68:168–176
Dai L, Corzo G, Naoki H, Andriantsiferana M, Nakajima T (2006) Purification, structure–function analysis, and extension. Int Dairy J 16(9):1058–1071
Deegan LH, Cotter PD, Hill C, Ross P (2002) Bacteriocins: biological tools for bio-preservation and shelf-life molecular characterization of novel linear peptides from scorpion Opisthacanthus madagascariensis. Biochem Biophys Res Commun 293(5):1514–1522
De Caleya RF, Gonzalez-Pascual B, Garcia-Olmedo F, Carbonero P (1972) Susceptibility of phytopathogenic bacteria to wheat purothionins in vitro. Appl Environ Microbiol 23(5):998–1000
Dennison SR, Whittaker M, Harris F, Phoenix DA (2006) Anticancer α-helical peptides and structure/function relationships underpinning their interactions with tumour cell membranes. Curr Protein Pept Sci 7(6):487–499
Destoumieux D, Munoz M, Bulet P, Bachere E (2000) Penaeidins, a family of antimicrobial peptides from penaeid shrimp (Crustacea, Decapoda). Cell Mol Life Sci 57(8–9):1260–1271
Diamond G, Zasloff M, Eck H, Brasseur M, Maloy WL, Bevins CL (1991) Tracheal antimicrobial peptide, a cysteine-rich peptide from mammalian tracheal mucosa: peptide isolation and cloning of a cDNA. Proc Natl Acad Sci USA 88(9):3952–3956. https://doi.org/10.1073/pnas.88.9.3952.PMID:2023943;PMCID:PMC51571
Díaz P, D'Suze G, Salazar V, Sevcik C, Shannon JD, Sherman NE, Fox JW (2009) Antibacterial activity of six novel peptides from Tityus discrepans scorpion venom. A fluorescent probe study of microbial membrane Na+ permeability changes. Toxicon 54(6):802–817
Ebrahim-Nesbat F, Behnke S, Kleinhofs A, Apel K (1989) Cultivar-related differences in the distribution of cell-wall-bound thionins in compatible and incompatible interactions between barley and powdery mildew. Planta 179(2):203–210
Evans J, Wang YD, Shaw KP, Vernon LP (1989) Cellular responses to Pyrularia thionin are mediated by Ca2+ influx and phospholipase A2 activation and are inhibited by thionin tyrosine iodination. Proc Natl Acad Sci USA 86(15):5849–5853
Falanga A, Lombardi L, Franci G, Vitiello M, Iovene MR, Morelli G, Galdiero M, Galdiero S (2016) Marine antimicrobial peptides: nature provides templates for the design of novel compounds against pathogenic bacteria. Int J Mol Sci 17(5):785
Farha MA, Brown ED (2016) Strategies for target identification of antimicrobial natural products. Nat Product Rep 33(5):668–680
Fasano A (1998) Innovative strategies for theory delivery of drugs and peptides. Trends Biotechnol 16:152–157
Fjell CD, Hiss JA, Hancock RE, Schneider G (2012) Designing antimicrobial peptides: form follows function. Nat Rev Drug Discovery 11(1):37–51
Fleitas O, Franco OL (2016) Induced bacterial cross-resistance toward host antimicrobial peptides: a worrying phenomenon. Front Microbiol 24(7):381
Frick IM, Nordin SL, Baumgarten M, Morgelin M, Sorensen OE, Olin AI, Egesten A (2011) Constitutive and inflammation-dependent antimicrobial peptides produced by epithelium are differentially processed and inactivated by the commensal Finegoldia magna and the pathogen Streptococcus pyogenes. J Immunol 187:4300–4309. https://doi.org/10.4049/jimmunol.1004179
Galvan EM, Lasaro MA, Schifferli DM (2008) Capsular antigen fraction 1 and Pla modulate the susceptibility of Yersinia pestis to pulmonary antimicrobial peptides such as cathelicidin. Infect Immun 76:1456–1464. https://doi.org/10.1128/IAI.01197-07
Gao GH, Liu W, Dai JX, Wang JF, Hu Z, Zhang Y, Wang DC (2001) Solution structure of PAFP-S: a new knottin-type antifungal peptide from the seeds of Phytolacca americana. Biochemistry 40(37):10973–10978
Gao Y, Wu D, Xi X, Wu Y, Ma C, Zhou M, Wang L, Yang M, Chen T, Shaw C (2016) Identification and characterisation of the antimicrobial peptide, phylloseptin-PT, from the skin secretion of Phyllomedusa tarsius, and comparison of activity with designed, cationicity-enhanced analogues and diastereomers. Molecules 21(12):1667
Gebhart D, Lok S, Clare S, Tomas M, Stares M, Scholl D, Donskey CJ, Lawley TD, Govoni GR (2015) A modified r-type bacteriocin specifically targeting Clostridium difficile prevents colonization of mice without affecting gut microbiota diversity. mBio 6:e02368–e2414
Gerardo NM, Altincicek B, Anselme C, Atamian H, Barribeau SM, De Vos M, Duncan EJ, Evans JD, Gabaldón T, Ghanim M, Heddi A (2010) Immunity and other defenses in pea aphids, Acyrthosiphon pisum. Genome Biol 11(2):R21
Ghobrial OG, Derendorf H, Hillman JD (2009) Pharmacodynamic activity of the lantibiotic MU1140. Int J Antimicrob Agents 33:70–74
Giuliani A, Rinaldi AC (2011) Beyond natural antimicrobial peptides: multimeric peptides and other peptidomimetic approaches. Cell Mol Life Sci 68(13):2255–2266
Glukhov E, Stark M, Burrows LL, Deber CM (2005) Basis for selectivity of cationic antimicrobial peptides for bacterial versus mammalian membranes. J Biol Chem 280(40):33960–33967
Grady D (2013) Deadly bacteria that resist strongest drugs are Spreading (5 March 2013). https://www.nytimes.com/2013/03/06/health/deadly-drug-resistant-infections-rise-inhospitals-report-warns.html. Accessed on 11 Jun 2013
Grünewald J, Marahiel MA (2006) Chemoenzymatic and template-directed synthesis of bioactive macrocyclic peptides. Microbiol Mol Biol Rev 70(1):121–146
Guina T, Yi EC, Wang H, Hackett M, Miller SI (2000) A PhoP-regulated outer membrane protease of Salmonella enterica serovar typhimurium promotes resistance to alpha-helical antimicrobial peptides. J Bacteriol 182:4077–4086. https://doi.org/10.1128/JB.182.14.4077-4086.2000
Guterstam P, Madani F, Hirose H, Takeuchi T, Futaki S, Andaloussi SE, Gräslund A, Langel Ü (2009) Elucidating cell-penetrating peptide mechanisms of action for membrane interaction, cellular uptake, and translocation utilizing the hydrophobic counter-anion pyrene butyrate. Biochim Biophys Acta 1788(12):2509–2517
Hancock RE, Sahl HG (2006) Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat Biotechnol 24(12):1551–1557
Hancock RE, Patrzykat A (2002) Clinical development of cationic antimicrobial peptides: from natural to novel antibiotics. Curr Drug Targets Infect Disord 2(1):79–83
Hankins JV, Madsen JA, Giles DK, Brodbelt JS, Trent MS (2012) Amino acid addition to Vibrio cholerae LPS establishes a link between surface remodeling in Gram-positive and Gram-negative bacteria. Proc Natl Acad Sci USA 109:8722–8727. https://doi.org/10.1073/pnas.1201313109
Hassan M, Kjos M, Nes IF, Diep DB, Lotfipour F (2012) Natural antimicrobial peptides from bacteria: characteristics and potential applications to fight against antibiotic resistance. J Appl Microbiol 113(4):723–736
Hawser S (2012) Antibiotic resistance. In: Coates ARM (ed) Handbook of experimental pharmacology. Springer, Berlin, pp 31–43
Henderson JC, Fage CD, Cannon JR, Brodbelt JS, Keatinge-Clay AT, Trent MS (2014) Antimicrobial peptide resistance of Vibrio cholerae results from an LPS modification pathway related to nonribosomal peptide synthetases. ACS Chem Biol 9(10):2382–2392
Hernández-Aponte CA, Silva-Sanchez J, Quintero-Hernández V, Rodríguez-Romero A, Balderas C, Possani LD, Gurrola GB (2011) Vejovine, a new antibiotic from the scorpion venom of Vaejovis mexicanus. Toxicon 57(1):84–92
Heimann JD (2002) The extracytoplasmic function (ECF) sigma factors. Adv Microb Physiol 46:47–110
Herbinière J, Braquart-Varnier C, Grève P, Strub JM, Frère J, Van Dorsselaer A, Martin G (2005) Armadillidin: a novel glycine-rich antibacterial peptide directed against gram-positive bacteria in the woodlouse Armadillidium vulgare (Terrestrial Isopod, Crustacean). Dev Comp Immunol 29(6):489–499
Hirsch T, Jacobsen F, Steinau HU, Steinstraesser L (2008 Mar 1) Host defense peptides and the new line of defence against multiresistant infections. Protein Pept Lett 15(3):238–243
Hochlowski JE, Swanson SJ, Ranfranz LM, Whittern DN, Buko AM, McAlpine JB (1987) Tiacumicins, a novel complex of 18-membered macrolides. II. Isolation and structure determination. J Antibiot 40:575–588
Hoskin DW, Ramamoorthy A (2008) Studies on anticancer activities of antimicrobial peptides. Biochem Biophys Acta 1778(2):357–375
Huang RH, Xiang Y, Liu XZ, Zhang Y, Hu Z, Wang DC (2002) Two novel antifungal peptides distinct with a five-disulfide motif from the bark of Eucommia ulmoides Oliv. FEBS Lett 521(1–3):87–90
Huang X, Xie WJ, Gong ZZ (2000) Characteristics and antifungal activity of a chitin binding protein from Ginkgo biloba. FEBS Lett 478(1–2):123–126
Huang PH, Chen JY, Kuo CM (2007) Three different hepcidins from tilapia, Oreochromis mossambicus: analysis of their expressions and biological functions. Mol Immunol 44(8):1922–1934
Huang L, Chen D, Wang L, Lin C, Ma C, Xi X, Chen T, Shaw C, Zhou M (2017) Dermaseptin-ph: a novel peptide with antimicrobial and anticancer activities from the skin secretion of the south American orange-legged leaf frog, pithecopus (phyllomedusa) hypochondrialis. Molecules 22(10):1805
Hughes P, Dennis E, Whitecross M, Llewellyn D, Gage P (2000) The cytotoxic plant protein, β-purothionin, forms ion channels in lipid membranes. J Biol Chem 275(2):823–827
Humphries RM, Pollett S, Sakoulas G (2013) A current perspective on daptomycin for the clinical microbiologist. Clin Microbiol Rev 26(4):759–780
Imjongjirak C, Amparyup P, Tassanakajon A, Sittipraneed S (2009) Molecular cloning and characterization of crustin from mud crab Scylla paramamosain. Mol Biol Rep 36(5):841–850
Jensen RT, Battey JF, Spindel ER, Benya RV (2008) International Union of Pharmacology. LVIII. Mammalian bombesin receptors: nomenclature, distribution, pharmacology, signaling and functions in normal and disease states. Pharmacol Rev 60:1–42
Jiravanichpaisal P, Lee SY, Kim YA, Andrén T, Söderhäll I (2007) Antibacterial peptides in hemocytes and hematopoietic tissue from freshwater crayfish Pacifastacus leniusculus: characterization and expression pattern. Dev Comp Immunol 31(5):441–455
Kaplan CW, Sim JH, Shah KR, Kolesnikova-Kaplan A, Shi WY, Eckert R (2011) Selective membrane disruption: mode of action of C16G2, a specifically targeted antimicrobial peptide. Antimicrob Agents Chemother 55:3446–3452
Kawano K, Yoneya T, Miyata T, Yoshikawa K, Tokunaga F, Terada Y, Iwanaga S (1990) Antimicrobial peptide, tachyplesin I, isolated from hemocytes of the horseshoe crab (Tachypleus tridentatus) NMR determination of the beta-sheet structure. J Biol Chem 265(26):15365–15367
Khoo L, Robinette DW, Noga EJ (1999) Callinectin, an antibacterial peptide from blue crab, Callinectes sapidus, hemocytes. Mar Biotechnol 1(1):44–51
Kiba A, Saitoh H, Nishihara M, Omiya K, Yamamura S (2003) C-terminal domain of a hevein-like protein from Wasabia japonica has potent antimicrobial activity. Plant Cell Physiol 44(3):296–303
König E, Bininda-Emonds OR, Shaw C (2015) The diversity and evolution of anuran skin peptides. Peptides 1(63):96–117
Koo JC, Lee SY, Chun HJ, Cheong YH, Choi JS, Kawabata SI, Miyagi M, Tsunasawa S, Ha KS, Bae DW, Han CD (1998) Two hevein homologs isolated from the seed of Pharbitis nil L. exhibit potent antifungal activity. Biochim Biophys Acta 1382(1):80–90
Kourie JI, Shorthouse AA (2000) Properties of cytotoxic peptide-formed ion channels. Am J Physiol Cell Physiol 278(6):C1063–C1087
Kramer KJ, Klassen LW, Jones BL, Speirs RD, Kammer AE (1979) Toxicity of purothionin and its homologues to the tobacco hornworm, Manduca sexta (L.)(Lepidoptera: Sphingidae). Toxicol Appl Pharmacol 48(1):179–183
Kokriakov VN, Koval'chuk LV, Aleshina GM, Shamova OV (2006) Cationic antimicrobial peptides as molecular immunity factors: multi-functionality. Zh Mikrobiol Epidemiol Immunobiol 2:98–105
Kamysz W, Okrój M, Łukasiak J (2003) Novel properties of antimicrobial peptides. Acta Biochim Pol 50(2):461–469
Kristian SA, Datta V, Weidenmaier C, Kansal R, Fedtke I, Peschel A, Gallo RL, Nizet V (2005) d-Alanylation of teichoic acids promotes group a streptococcus antimicrobial peptide resistance, neutrophil survival, and epithelial cell invasion. J Bacteriol 187(19):6719–6725
Kuzmin DV, Emelianova AA, Kalashnikova MB, Panteleev PV, Balandin SV, Serebrovskaya EO (2017) Belogurova-Ovchinnikova OYu, Ovchinnikova TV. Comparative in vitro study on cytotoxicity of recombinant b-hairpin peptides. Chem Biol Drug Des. https://doi.org/10.1111/cbdd.13081
Lohner K, Prenner EJ (1999) Differential scanning calorimetry and X-ray diffraction studies of the specificity of the interaction of antimicrobial peptides with membrane-mimetic systems. Biochim Biophys Acta 1462(1–2):141–156
Lambert J, Keppi E, Dimarcq JL, Wicker C, Reichhart JM, Dunbar B, Lepage P, Van Dorsselaer A, Hoffmann J, Fothergill J (1989) Insect immunity: isolation from immune blood of the dipteran Phormia terranovae of two insect antibacterial peptides with sequence homology to rabbit lung macrophage bactericidal peptides. Proc Natl Acad Sci USA 86(1):262–266
Laxminarayan R, Duse A, Wattal C, Zaidi AK, Wertheim HF, Sumpradit N, Vlieghe E, Hara GL, Gould IM, Goossens H, Greko C (2013) Antibiotic resistance—the need for global solutions. Lancet Infect Dis 13(12):1057–1098
Laxminarayan R, Powers JH (2011) Antibacterial R&D incentives. Nat Rev Drug Discov 10:727–728
Lee JU, Kang DI, Zhu WL, Shin SY, Hahm KS, Kim Y (2007) Solution structures and biological functions of the antimicrobial peptide, arenicin-1, and its linear derivative. Peptide Sci 88(2):208–216
Lee KH, Hong SY, Oh JE (1998) Synthesis and structure function study about tenecin 1, an antibacterial protein from larvae of Tenebrio molitor. FEBS Lett 439(1–2):41–45
Lewis LA, Shafer WM, Dutta Ray T, Ram S, Rice PA (2013) Phosphoethanolamine residues on the lipid A moiety of Neisseria gonorrhoeae lipooligosaccharide modulate binding of complement inhibitors and resistance to complement killing. Infect Immun 81:33–42. https://doi.org/10.1128/IAI.00751-12
Li C, Haug T, Stensvag K (2010) Antimicrobial peptides in echinoderms. Invertebr Surv J 7:132–140
Li C, Haug T, Styrvold OB, Jørgensen TØ, Stensvåg K (2008) Strongylocins, novel antimicrobial peptides from the green sea urchin, Strongylocentrotus droebachiensis. Dev Comp Immunol 32(12):1430–1440
Li SS, Claeson P (2003 Jun 1) Cys/Gly-rich proteins with a putative single chitin-binding domain from oat (Avena sativa) seeds. Phytochemistry 63(3):249–255
Li W, Tailhades J, O’Brien-Simpson NM, Separovic F, Otvos L, Hossain MA, Wade JD (2014) Proline-rich antimicrobial peptides: potential therapeutics against antibiotic-resistant bacteria. Amino Acids 46(10):2287–2294
Li Y, Xiang Q, Zhang Q, Huang Y, Su Z (2012) Overview on the recent study of antimicrobial peptides: origins, functions, relative mechanisms and application. Peptides 37(2):207–215
Li ZJ, Cho CH (2012) Peptides as targeting probes against tumor vasculature for diagnosis and drug delivery. J Transl Med 10:S1
Liao Z, Wang XC, Liu HH, Fan MH, Sun JJ, Shen W (2013) Molecular characterization of a novel antimicrobial peptide from Mytilus coruscus. Fish Shellfish Immunol 34(2):610–616
Lichtenstein A (1991) Mechanism of mammalian cell lysis mediated by peptide defensins. Evidence for an initial alteration of the plasma membrane. J Clin Investig 88:93–100
Lipsky BA, Holroyd KJ, Zasloff M (2008) Topical versus systemic antimicrobial therapy for treating mildly infected diabetic foot ulcers: a randomized, controlled, double-blinded, multicenter trial of pexiganan cream. Clin Infect Dis 47(12):1537–1545
Lohner K (2009) New strategies for novel antibiotics: peptides targeting bacterial cell. Gen Physiol Biophys 28:105–116
Llewellyn AC, Zhao J, Song F, Parvathareddy J, Xu Q, Napier BA, Laroui H, Merlin D, Bina JE, Cotter PA, Miller MA, Raetz CRH, Weiss DS (2012) NaxD is a deacetylase required for lipid A modification and Francisella pathogenesis. Mol Microbiol 86:611–627. https://doi.org/10.1111/mmi.12004
Llobet E, Tomas JM, Bengoechea JA (2008) Capsule polysaccharide is a bacterial decoy for antimicrobial peptides. Microbiology 154:3877–3886. https://doi.org/10.1099/mic.0.2008/022301-0
Makarova O, Rodriguez-Rojas A, Eravci M, Weise C, Dobson A, Johnston P, Rolff J (2016) Antimicrobial defence and persistent infection in insects revisited. Philos Trans R Soc B 371(1695):20150296
Malanovic N, Lohner K (2016) Antimicrobial peptides targeting gram-positive bacteria. Pharmaceuticals 9(3):59
Malanovic N, Lohner K (2016) Gram-positive bacterial cell envelopes: the impact on the activity of antimicrobial peptides. Biochim Biophys Acta 1858(5):936–946
Mander L, Liu HW (2010) Comprehensive natural products II: chemistry and biology. Elsevier, Amsterdam
Maria-Neto S, de Almeida KC, Macedo ML, Franco OL (2015) Understanding bacterial resistance to antimicrobial peptides: from the surface to deep inside. Biochim Biophys Acta 1848(11):3078–3088
Mason KM, Munson RS Jr, Bakaletz LO (2005) A mutation in the sap operon attenuates survival of nontypeable Haemophilus influenzae in a chinchilla model of otitis media. Infect Immun 73:599–608. https://doi.org/10.1128/IAI.73.1.599-608.2005
Meng M, Ning J, Yu J, Chen D, Meng X, Xu J, Zhang J (2014) Antitumor activity of recombinant antimicrobial peptide penaeidin-2 against kidney cancer cells. J Huazhong Univ Sci Technol 34(4):529–534
Mishra B, Reiling S, Zarena D, Wang G (2017) Host defense antimicrobial peptides as antibiotics: design and application strategies. Curr Opin Chem Biol 1(38):87–96
Mishra NN, Bayer AS, Tran TT, Shamoo Y, Mileykovskaya E, Dowhan W, Guan Z, Arias CA (2012) Daptomycin resistance in enterococci is associated with distinct alterations of cell membrane phospholipid content. PLoS ONE 7:8
Mishra NN, Bayer AS (2013) Correlation of cell membrane lipid profiles with daptomycin resistance in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 57(2):1082–1085
Misof B, Liu S, Meusemann K, Peters RS, Donath A, Mayer C et al (2014) Phylogenomics resolves the timing and pattern of insect evolution. Science 346:763–767. https://doi.org/10.1126/science.1257570
Mitta G, Vandenbulcke F, Noël T, Romestand B, Beauvillain JC, Salzet M, Roch P (2000) Differential distribution and defence involvement of antimicrobial peptides in mussel. J Cell Sci 113(15):2759–2769
Moerman L, Bosteels S, Noppe W, Willems J, Clynen E, Schoofs L, Thevissen K, Tytgat J, Van Eldere J, Van Der Walt J, Verdonck F (2002) Antibacterial and antifungal properties of α-helical, cationic peptides in the venom of scorpions from southern Africa. Eur J Biochem 269(19):4799–4810
Montesinos E (2007) Antimicrobial peptides and plant disease control. FEMS Microbiol Lett 270(1):1–1
Moody TW, Chan D, Fahrenkrug J, Jensen RT (2003) Neuropeptides as autocrine growth factors in cancer cells. Curr Pharm Des 9:495–509
Moody TW, Moreno P, Jensen RT (2015) Neuropeptides as lung cancer growth factors. Peptides 72:106–111. Recent review of ability of neuropeptides including Bn related peptides to function as growth factors in lung cancer
Moskowitz SM, Ernst RK, Miller SI (2004) PmrAB, a two-component regulatory system of Pseudomonas aeruginosa that modulates resistance to cationic antimicrobial peptides and addition of aminoarabinose to lipid A. J Bacteriol 186:575–579. https://doi.org/10.1128/JB.186.2.575-579.2004
Muller CA, Markovic-Lipkovski J, Klatt T, Gamper J, Schwarz G, Beck H, Deeg M, Kalbacher H, Widmann S, Wessels JT, Volker Becker V, Müller GA, Flad T (2002) Human α-defensins HNPs-1, -2, and -3 in renal cell carcinoma: influences on tumor cell proliferation. Am J Pathol 160(4):1311–1324
Mydlarz LD, Jones LE, Harvell CD (2006) Innate immunity, environmental drivers, and disease ecology of marine and freshwater invertebrates. Annu Rev Ecol Evol Syst 1(37):251–288
Nahirñak V, Almasia NI, Hopp HE, Vazquez-Rovere C (2012) Snakin/GASA proteins: involvement in hormone crosstalk and redox homeostasis. Plant Signal Behav 7(8):1004–1008
Nawrot R, Barylski J, Nowicki G, Broniarczyk J, Buchwald W, Goździcka-Józefiak A (2014) Plant antimicrobial peptides. Folia Microbiol 59(3):181–196
Negri L, Melchiorri P (2006) Handbook of biologically active peptides. Academic Press, California, pp 269–275
Nelsen DR, Nisani Z, Cooper AM, Fox GA, Gren EC, Corbit AG, Hayes WK (2014) Poisons, toxungens, and venoms: redefining and classifying toxic biological secretions and the organisms that employ them. Biol Rev 89(2):450–465
Nelson DC, Garbe J, Collin M (2011) Cysteine proteinase SpeB from Streptococcus pyogenes: a potent modifier of immunologically important host and bacterial proteins. Biol Chem 392:1077–1088. https://doi.org/10.1515/BC.2011.208
Nguyen GK, Zhang S, Nguyen NT, Nguyen PQ, Chiu MS, Hardjojo A, Tam JP (2011) Discovery and characterization of novel cyclotides originated from chimeric precursors consisting of albumin-1 chain a and cyclotide domains in the fabaceae family. J Biol Chem 286:24275–24287. https://doi.org/10.1074/jbc.M111.229922
Nie Y, Zeng XC, Yang Y, Luo F, Luo X, Wu S, Zhang L, Zhou J (2012) A novel class of antimicrobial peptides from the scorpion Heterometrus spinifer. Peptides 38(2):389–394
Novarifyn® (NP432) is antibacterial peptide with a number of key benefits over conventional antibiotic therapies and the clear potential to succeed where existing treatments for a number of bacterial infections, including those caused by MRSA, P. aeruginosa, and C. difficile, are failing. https://www.novabiotics.co.uk/pipeline/novarifyn-np432. Accessed on 18 Aug 2017
Odintsova TI, Vassilevski AA, Slavokhotova AA, Musolyamov AK, Finkina EI, Khadeeva NV, Rogozhin EA, Korostyleva TV, Pukhalsky VA, Grishin EV, Egorov TA (2009) A novel antifungal hevein-type peptide from Triticum kiharae seeds with a unique 10-cysteine motif. FEBS J 276(15):4266–4275
Onaka H (2017) Novel antibiotic screening methods to awaken silent or cryptic secondary metabolic pathways in actinomycetes. J Antibiot 70(8):865–870
Oomen RJ, Séveno-Carpentier E, Ricodeau N, Bournaud C, Conéjéro G, Paris N, Berthomieu P, Marquès L (2011) Plant defensin AhPDF1. 1 is not secreted in leaves but it accumulates in intracellular compartments. New Phytol 192(1):140–150
Opar A (2007) Bad bugs need more drugs. Nat Rev Drug Discov 6:943–944
Oragenics-intrexon collaboration announces significant progress towards commercial production of lead lantibiotic MU1140. https://www.oragenics.com/news-media/press-releases/detail/13/oragenics-intrexon-collaboration-announces-significant
Ortiz E, Gurrola GB, Schwartz EF, Possani LD (2015) Scorpion venom components as potential candidates for drug development. Toxicon 93:125–135. https://doi.org/10.1016/j.toxicon.2014.11.233
Osaki T, Omotezako M, Nagayama R, Hirata M, Iwanaga S, Kasahara J, Hattori J, Ito I, Sugiyama H, Kawabata SI (1999) Horseshoe crab hemocyte-derived antimicrobial polypeptides, tachystatins, with sequence similarity to spider neurotoxins. J Biol Chem 274(37):26172–26178
Osapay K, Tran D, Ladokhin AS, White SH, Henschen AH, Selsted ME (2000) Formation and characterization of a single Trp-Trp cross-link in indolicidin that confers protease stability without altering antimicrobial activity. J Biol Chem 275(16):12017–12022
Otero-González AJ, Magalhaes BS, Garcia-Villarino M, López-Abarrategui C, Sousa DA, Dias SC, Franco OL (2010) Antimicrobial peptides from marine invertebrates as a new frontier for microbial infection control. FASEB J 24(5):1320–1334
Ovchinnikova TV, Aleshina GM, Balandin SV, Krasnosdembskaya AD, Markelov ML, Frolova EI, Leonova YF, Tagaev AA, Krasnodembsky EG, Kokryakov VN (2004) Purification and primary structure of two isoforms of arenicin, a novel antimicrobial peptide from marine polychaeta Arenicola marina. FEBS Lett 577(1–2):209–214
Ovchinnikova TV, Balandin SV, Aleshina GM, Tagaev AA, Leonova YF, Krasnodembsky ED, Menshenin AV, Kokryakov VN (2006) Aurelin, a novel antimicrobial peptide from jellyfish Aurelia aurita with structural features of defensins and channel-blocking toxins. Biochem Biophys Res Commun 348(2):514–523
Ovchinnikova TV, Shenkarev ZO, Nadezhdin KD, Balandin SV, Zhmak MN, Kudelina IA, Finkina EI, Kokryakov VN, Arseniev AS (2007) Recombinant expression, synthesis, purification, and solution structure of arenicin. Biochem Biophys Res Commun 360(1):156–162
Pallaghy PK, Norton RS, Nielsen KJ, Craik DJ (1994) A common structural motif incorporating a cystine knot and a triple-stranded β-sheet in toxic and inhibitory polypeptides. Protein Sci 3(10):1833–1839
Papagianni M (2003) Ribosomally synthesized peptides with antimicrobial properties: biosynthesis, structure, function, and applications. Biotechnol Adv 21(6):465–499
Parachin NS, Franco OL (2014) New edge of antibiotic development: antimicrobial peptides and corresponding resistance. Front Microbiol 8(5):147
Paredes-Gamero EJ, Martins MN, Cappabianco FA, Ide JS, Miranda A (2012) Characterization of dual effects induced by antimicrobial peptides: regulated cell death or membrane disruption. Biochim Biophys Acta 1820(7):1062–1072
Park IY, Park CB, Kim MS, Kim SC (1998) Parasin I, an antimicrobial peptide derived from histone H2A in the catfish Parasilurus asotus. FEBS Lett 437(3):258–262
Park CB, Yi KS, Matsuzaki K, Kim MS, Kim SC (2000) Structure–activity analysis of buforin II, a histone H2A-derived antimicrobial peptide: the proline hinge is responsible for the cell-penetrating ability of buforin II. Proc Natl Acad Sci USA 97(15):8245–8250
Payne DJ, Gwynn MN, Holmes DJ, Pompliano DL (2007) Drugs for bad bugs: confronting the challenges of antibacterial discovery. Nat Rev Drug Discov 6:29–40
Pelegrini PB, Franco OL (2005) Plant γ-thionins: novel insights on the mechanism of action of a multi-functional class of defense proteins. Int J Biochem Cell Biol 37(11):2239–2253
Pelegrini PB, Quirino BF, Franco OL (2007) Plant cyclotides: an unusual class of defense compounds. Peptides 28(7):1475–1481
Pelletier MR, Casella LG, Jones JW, Adams MD, Zurawski DV, Hazlett KR, Doi Y, Ernst RK (2013) Unique structural modifications are present in the lipopolysaccharide from colistin-resistant strains of Acinetobacter baumannii. Antimicrob Agents Chemother 57:4831–4840. https://doi.org/10.1128/AAC.00865-13
Peschel A, Otto M, Jack RW, Kalbacher H, Jung G, Gotz F (1999) Inactivation of the dlt operon in Staphylococcus aureus confers sensitivity to defensins, protegrins, and other antimicrobial peptides. J Biol Chem 274:8405–8410. https://doi.org/10.1074/jbc.274.13.8405
Phase 2B Dose-Ranging Study of PAC113 Mouthrinse in HIV Seropositive IndividualsWith Oral Candidiasis. https://clinicaltrials.gov/ct2/show/NCT00659971?term=PAC113&rank=1. Accessed on 18 Aug 2017
Phase III Efficacy and Safety Study of AB103 in the treatment of patients with necrotizing soft tissue infections (ACCUTE). https://clinicaltrials.gov/ct2/show/NCT02469857?term=p2TA&rank=1. Accessed on 18 Aug 2017
Pöppel AK, Vogel H, Wiesner J, Vilcinskas A (2015) Antimicrobial peptides expressed in medicinal maggots of the blow fly Lucilia sericata show combinatorial activity against bacteria. Antimicrob Agents Chemother 59(5):2508–2514
Porto WF, Souza VA, Nolasco DO, Franco OL (2012) In silico identification of novel hevein-like peptide precursors. Peptides 38(1):127–136
Pasupuleti M, Schmidtchen A, Chalupka A, Ringstad L, Malmsten M (2009) End-tagging of ultra-short antimicrobial peptides by W/F stretches to facilitate bacterial killing. PLoS ONE 4:4
Pushpanathan M, Gunasekaran P, Rajendhran J (2013) Antimicrobial peptides: versatile biological properties. Int J Peptides. https://doi.org/10.1155/2013/675391
Pushpanathan M, Rajendhran J, Jayashree S, Sundarakrishnan B, Jayachandran S, Gunasekaran P (2012) Direct cell penetration of the antifungal peptide, MMGP1, Candida albicans. J Pept Sci 18(11):657–660
Quinupristin and Dalfopristin, The Merck Manual, https://www.merckmanuals.com/professional/lexicomp/quinupristin%2520and%2520dalfopristin.html. Accessed on 15 July 2013
Rabanal F, Cajal Y (2017) Recent advances and perspectives in the design and development of polymyxins. Nat Product Rep 34(7):886–908
Raetz CR, Whitfield C (2002) Lipopolysaccharide endotoxins. Annu Rev Biochem 71(1):635–700
Rees DC, Lipscomb WN (1982) Refined crystal structure of the potato inhibitor complex of carboxypeptidase A at 2.5 Å resolution. J Mol Biol 160(3):475–498
Reubi JC, Wenger S, Schumuckli-Maurer J et al (2002) Bombesin receptor subtypes in human cancers: detection with the universal radoligand (125)I-[D-TYR(6), beta-ALA(11), PHE(13), NLE(14)] bombesin(6–14). Clin Cancer Res. 8:1139–1146
Richard JA, Kelly I, Marion D, Auger M, Pézolet M (2005) Structure of β-purothionin in membranes: a two-dimensional infrared correlation spectroscopy study. Biochemistry 44(1):52–61
Roch P, Yang Y, Toubiana M, Aumelas A (2008) NMR structure of mussel mytilin, and antiviral–antibacterial activities of derived synthetic peptides. Dev Comp Immunol 32(3):227–238
Rodrıguez A, Villegas E, Montoya-Rosales A, Rivas-Santiago B, Corzo G (2014) Characterization of antibacterial and hemolytic activity of synthetic pandinin 2 variants and their inhibition against Mycobacterium tuberculosis. PLoS ONE 9:e101742
Rodrıguez-Rojas A, Rodrıguez-Beltran J, Couce A, Blazquez J (2013) Antibiotics and antibiotic resistance: a bitter fight against evolution. Int J Med Microbiol 303:293–297
Rosa RD, Barraco MA (2010) Antimicrobial peptides in crustaceans. Invertebr Surv J 7:262–284
Sable R, Parajuli P, Jois S (2017) Peptides, peptidomimetics, and polypeptides from marine sources: a wealth of natural sources for pharmaceutical applications. Mar Drugs 15(4):124. https://doi.org/10.3390/md15040124.PMID:28441741;PMCID:PMC5408270
Sackton TB, Lazzaro BP, Schlenke TA, Evans JD, Hultmark D, Clark AG (2007) Dynamic evolution of the innate immune system in Drosophila. Nat Genet 39(12):1461
Sánchez A, Vázquez A (2017) Bioactive peptides: a review. Food Qual Saf 1(1):29–46
Scherlach K, Hertweck C (2009) Triggering cryptic natural product biosynthesis in microorganisms. Org Biomol Chem 7(9):1753–1760
Scorciapino MA, Rinaldi AC (2012) Antimicrobial peptidomimetics: reinterpreting nature to deliver innovative therapeutics. Front Immunol 28(3):171
Schmidtchen A, Frick IM, Andersson E, Tapper H, Bjorck L (2002) Proteinases of common pathogenic bacteria degrade and inactivate the antibacterial peptide LL-37. Mol Microbiol 46:157–168. https://doi.org/10.1046/j.1365-2958.2002.03146.x
Segura A, Moreno M, Madueño F, Molina A, García-Olmedo F (1999) Snakin-1, a peptide from potato that is active against plant pathogens. Mol Plant Microbe Interact 12(1):16–23
Seyfi R, Kahaki FA, Ebrahimi T, Montazersaheb S, Eyvazi S, Babaeipour V, Tarhriz V (2019) Antimicrobial peptides (AMPs): roles, functions and mechanism of action. Int J Peptide Res Therapeut. https://doi.org/10.1007/s10989-019-09946-9
Shah NR, Hancock RE, Fernandez RC (2014) Bordetella pertussis lipid A glucosamine modification confers resistance to cationic antimicrobial peptides and increases resistance to outer membrane perturbation. Antimicrob Agents Chemother 58:4931–4934. https://doi.org/10.1128/AAC.02590-14
Shenkarev ZO, Panteleev PV, Balandin SV, Gizatullina AK, Altukhov DA, Finkina EI, Kokryakov VN, Arseniev AS, Ovchinnikova TV (2012) Recombinant expression and solution structure of antimicrobial peptide aurelin from jellyfish Aurelia aurita. Biochem Biophys Res Commun 429(1–2):63–69
Shlaes D (2011) Pfizer Abandons Antibiotic Research! (3 February 2011) https://antibioticstheperfectstorm.blogspot.com.au/2011/02/pfizer-abandons-antibiotic-research.html. Accessed on 27 May 2013
Shlaes DM, Spellberg B (2012) Overcoming the challenges to developing new antibiotics. Curr Opin Pharmacol 12:522–526
Sieprawska-Lupa M, Mydel P, Krawczyk K, Wójcik K, Puklo M, Lupa B, Suder P, Silberring J, Reed M, Pohl J, Shafer W, McAleese F, Foster T, Travis J, Potempa J (2004) Degradation of human antimicrobial peptide LL-37 by Staphylococcus aureus-derived proteinases. Antimicrob Agents Chemother 48:4673–4679. https://doi.org/10.1128/AAC.48.12.4673-4679.2004
Silver LL (2008) Are natural products still the best source for antibacterial discovery? The bacterial entry factor. Expert Opin Drug Discov 3:487–500
Silver LL (2011) Challenges of antibacterial discovery. Clin Microbiol Rev 24:71–109
Simmaco M, Mignogna G, Barra D (1998) Antimicrobial peptides from amphibian skin: what do they tell us? Pept Sci 47(6):435–450
Slavokhotova AA, Naumann TA, Price NP, Rogozhin EA, Andreev YA, Vassilevski AA, Odintsova TI (2014) Novel mode of action of plant defense peptides–hevein-like antimicrobial peptides from wheat inhibit fungal metalloproteases. FEBS J 281(20):4754–4764
Soligenix Inc. SGX9 executive summary. https://www.soligenix.com/wp-content/uploads/sgx94_executive_summary_032216.pdf. Accessed on 18 Aug 2017
Stec B (2006) Plant thionins: the structural perspective. Cell Mol Life Sci 63(12):1370–1385
Stec B, Markman O, Rao U, Heffron G, Henderson S, Vernon LP, Brumfeld V, Teeter MM (2004) Proposal for molecular mechanism of thionins deduced from physico-chemical studies of plant toxins. J Peptide Res 64(6):210–224
Steiner H, Hultmark D, Engström Å, Bennich H, Boman HG (1981) Sequence and specificity of two antibacterial proteins involved in insect immunity. Nature 292(5820):246–248
Steinstraesser L, Kraneburg U, Jacobsen F, Al-Benna S (2011) Host defense peptides and their antimicrobial-immunomodulatory duality. Immunobiology 216(3):322–333
Stotz HU, Thomson J, Wang Y (2009) Plant defensins: defense, development and application. Plant Signal Behav 4(11):1010–1012
Strandberg E, Tiltak D, Ieronimo M, Kanithasen N, Wadhwani P, Ulrich AS (2007) Influence of C-terminal amidation on the antimicrobial and hemolytic activities of cationic α-helical peptides. Pure Appl Chem 79(4):717–728
Study of PXL01 versus placebo to inhibit adhesion formation after flexor tendon surgery (PHSU02). https://clinicaltrials.gov/ct2/show/NCT01022242?term=PXL01&rank=1. Accessed on 18 Aug 2017
Study of the effects of brilacidin oral rinse on radiation-induced oral mucositis in patients with head and neck cancer (Brilacidin). https://clinicaltrials.gov/ct2/show/NCT02324335?term=Brilacidin&rank=1. Accessed on 18 Aug 2017
Sumi CD, Yang BW, Yeo IC, Hahm YT (2015) Antimicrobial peptides of the genus Bacillus: a new era for antibiotics. Can J Microbiol 61(2):93–103
Sutcliffe JA (2011) Antibiotics in development targeting protein synthesis. Ann N Y Acad Sci 1241:122–152
Tam JP, Wang S, Wong KH, Tan WL (2015) Antimicrobial peptides from plants. Pharmaceuticals (Basel) 8(4):711–757. https://doi.org/10.3390/ph8040711.PMID:26580629;PMCID:PMC4695807
Tassanakajon A, Amparyup P, Somboonwiwat K, Supungul P (2011) Cationic antimicrobial peptides in penaeid shrimp. Mar Biotechnol 13(4):639–657
Tasiemski A, Schikorski D, Le Marrec-Croq F, Pontoire-Van Camp C, Boidin-Wichlacz C, Sautière PE (2007) Hedistin: a novel antimicrobial peptide containing bromotryptophan constitutively expressed in the NK cells-like of the marine annelid. Ner Divers Develop Comp Immunol 31(8):749–762
Teesalu T, Sugahara KN, Kotamraju VR, Ruoslahti E (2009) C-end rule peptides mediate neuropilin-1-dependent cell, vascular, and tissue penetration. Proc Natl Acad Sci USA 106(38):16157–16162
Theriault RJ, Karwowski JP, Jackson M, Girolami RL, Sunga GN, Vojtko CM, Coen LJ (1987) Tiacumicins, a novel complex of 18-membered macrolide antibiotics. I. Taxonomy, fermentation and antibacterial activity. J Antibiot 40:567–574
Thevissen K, Ghazi A, De Samblanx GW, Brownlee C, Osborn RW, Broekaert WF (1996) Fungal membrane responses induced by plant defensins and thionins. J Biol Chem 271(25):15018–15025
Thevissen K, Terras FR, Broekaert WF (1999) Permeabilization of fungal membranes by plant defensins inhibits fungal growth. Appl Environ Microbiol 65(12):5451–5458
Thevissen K, Osborn RW, Acland DP, Broekaert WF (2000) Specific binding sites for an antifungal plant defensin from Dahlia (Dahlia merckii) on fungal cells are required for antifungal activity. Mol Plant Microbe Interact 13(1):54–61
Thevissen K, Warnecke DC, François IE, Leipelt M, Heinz E, Ott C, Zähringer U, Thomma BP, Ferket KK, Cammue BP (2004) Defensins from insects and plants interact with fungal glucosylceramides. J Biol Chem 279(6):3900–3905
Torres-Larios A, Gurrola GB, Zamudio FZ, Possani LD (2000) Hadrurin, a new antimicrobial peptide from the venom of the scorpion Hadrurus aztecus. Eur J Biochem 267(16):5023–5031
Utsugi T, Schroit AJ, Connor J, Bucana CD, Fidler IJ (1991) Elevated expression of phosphatidylserine in the outer membrane leaflet of human tumor cells and recognition by activated human blood monocytes. Can Res 51(11):3062–3066
Vaara M (2009) New approaches in peptide antibiotics. Curr Opin Pharmacol 9(5):571–576
Van den Bergh KP, Proost P, Van Damme J, Coosemans J, Van Damme EJ, Peumans WJ (2002) Five disulfide bridges stabilize a hevein-type antimicrobial peptide from the bark of spindle tree (Euonymus europaeus L.). FEBS Lett 530(1–3):181–185
Van Parijs J, Broekaert WF, Goldstein IJ, Peumans WJ (1991) Hevein: an antifungal protein from rubber-tree (Hevea brasiliensis) latex. Planta 183(2):258–264
Vidal-Dupiol J, Ladrière O, Destoumieux-Garzón D, Sautière PE, Meistertzheim AL, Tambutté E, Tambutté S, Duval D, Fouré L, Adjeroud M, Mitta G (2011) Innate immune responses of a scleractinian coral to vibriosis. J Biol Chem 286(25):22688–22698
Vila-Farres X, Chu J, Inoyama D, Ternei MA, Lemetre C, Cohen LJ, Cho W, Reddy BV, Zebroski HA, Freundlich JS, Perlin DS, Brady SF (2017) Antimicrobials inspired by nonribosomal peptide synthetase gene clusters. J Am Chem Soc 139:1404–1407
Vilcinskas A (2013) Evolutionary plasticity of insect immunity. J Insect Physiol 59(2):123–129
Vilcinskas A, Mukherjee K, Vogel H (2013) Expansion of the antimicrobial peptide repertoire in the invasive ladybird Harmonia axyridis. Proc R Soc B 280(1750):20122113
Vizioli J, Bulet P, Charlet M, Lowenberger C, Blass C, Muller HM, Dimopoulos G, Hoffmann J, Kafatos FC, Richman A (2000) Cloning and analysis of a cecropin gene from the malaria vector mosquito Anopheles gambiae. Insect Mol Biol 9(1):75–84. https://doi.org/10.1046/j.1365-2583.2000.00164.x
Wang G (2012) Post-translational modifications of natural antimicrobial peptides and strategies for peptide engineering. Curr Biotechnol 1(1):72–79
van Watering S, Sterk PJ, Rabe KF, Hiemstra PS (1999) Defensins: key players or bystanders in infection, injury, and repair in the lung? J Allergy Clin Immunol 104:1131–1138
Vuong C, Voyich JM, Fischer ER, Braughton KR, Whitney AR, DeLeo FR, Otto M (2004) Polysaccharide intercellular adhesin (PIA) protects Staphylococcus epidermidis against major components of the human innate immune system. Cell Microbiol 6:269–275. https://doi.org/10.1046/j.1462-5822.2004.00367.x
Vuong C, Kocianova S, Voyich JM, Yao Y, Fischer ER, DeLeo FR, Otto M (2004) A crucial role for exopolysaccharide modification in bacterial biofilm formation, immune evasion, and virulence. J Biol Chem 279(881–54):886. https://doi.org/10.1074/jbc.M411374200
Wang X, Preston JF III, Romeo T (2004) The pgaABCD locus of Escherichia coli promotes the synthesis of a polysaccharide adhesin required for biofilm formation. J Bacteriol 186:2724–2734. https://doi.org/10.1128/JB.186.9.2724-2734.2004
Waterhouse RM, Kriventseva EV, Meister S, Xi Z, Alvarez KS, Bartholomay LC, Barillas-Mury C, Bian G, Blandin S, Christensen BM, Dong Y (2007) Evolutionary dynamics of immune-related genes and pathways in disease-vector mosquitoes. Science 316(5832):1738–1743
Watkins RR, Bonomo RA (2016) Overview: global and local impact of antibiotic resistance. Infect Dis Clin 30(2):313–322
Wei L, Dong L, Zhao T, You D, Liu R, Liu H, Yang H, Lai R (2011) Analgesic and anti-inflammatory effects of the amphibian neurotoxin, anntoxin. Biochimie 93(6):995–1000
Wellington EMH et al (2013) The role of the natural environment in the emergence of antibiotic resistance in Gram-negative bacteria. Lancet Infect Dis 13:155–165
Willems J, Moerman L, Bosteels S, Bruyneel E, Ryniers F, Verdonck F (2004) Parabutoporin-an antibiotic peptide from scorpion venom-can both induce activation and inhibition of granulocyte cell functions. Peptides 25(7):1079–1084
Wong CT, Rowlands DK, Wong CH, Lo TW, Nguyen GK, Li HY, Tam JP (2012) Orally active peptidic bradykinin B1 receptor antagonists engineered from a cyclotide scaffold for inflammatory pain treatment. Angew Chem Int Ed 51(23):5620–5624
Wu D, Gao Y, Wang L, Xi X, Wu Y, Zhou M, Zhang Y, Ma C, Chen T, Shaw C (2016) A combined molecular cloning and mass spectrometric method to identify, characterize, and design frenatin peptides from the skin secretion of Litoria infrafrenata. Molecules 21(11):1429
Wu X, Pan J, Wu Y, Xi X, Ma C, Wang L, Zhou M, Chen T (2017) PSN-PC: a novel antimicrobial and anti-biofilm peptide from the skin secretion of Phyllomedusa camba with cytotoxicity on human lung cancer cell. Molecules 22(11):1896
Xu X, Lai R (2015) The chemistry and biological activities of peptides from amphibian skin secretions. Chem Rev 115(4):1760–1846
Yount NY, Yeaman MR (2004) Multidimensional signatures in antimicrobial peptides. Proc Natl Acad Sci USA 101(19):7363–7368
Zarins-Tutt JS, Barberi TT, Gao H, Mearns-Spragg A, Zhang L, Newman DJ, Goss RJ (2016) Prospecting for new bacterial metabolites: a glossary of approaches for inducing, activating and upregulating the biosynthesis of bacterial cryptic or silent natural products. Nat Product Rep 33(1):54–72
Zasloff M (1987) Magainins, a class of antimicrobial peptides from Xenopus skin: isolation, characterization of two active forms, and partial cDNA sequence of a precursor. Proc Natl Acad Sci USA 84(15):5449–5453
Zeng XC, Corzo G, Hahin R (2005) Scorpion venom peptides without disulfide bridges. IUBMB Life 57(1):13–21
Zhong J, Wang W, Yang X, Yan X, Liu R (2013) A novel cysteine-rich antimicrobial peptide from the mucus of the snail of Achatina fulica. Peptides 1(39):1–5
Zanetti M (2004) Cathelicidins, multifunctional peptides of the innate immunity. J Leukoc Biol 75(1):39–48
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Datta, S., Roy, A. Antimicrobial Peptides as Potential Therapeutic Agents: A Review. Int J Pept Res Ther 27, 555–577 (2021). https://doi.org/10.1007/s10989-020-10110-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10989-020-10110-x