Abstract
Lactoferrin chimera (LFchimera), a heterodimeric peptide containing lactoferrampin (LFampin265–284) and a part of lactoferricin (LFcin17–30), possesses a broad spectrum of antimicrobial activity. However, there is no report on the inhibitory effects of LFchimera against multispecies oral biofilms. This study aimed to determine the effects of LFchimera in comparison to chlorhexidine digluconate (CHX) and minocycline hydrochloride (MH), on in vitro multispecies biofilms derived from subgingival plaque of periodontitis patients harboring Aggregatibacter actinomycetemcomitans. First the effects of LFchimera against planktonic and an 1-day old biofilm of the periodontopathic bacteria, A. actinomycetemcomitans ATCC 43718 were established. Then, the effects on biofilm formation and bacterial viability in the multispecies biofilm were determined by crystal violet staining and LIVE/DEAD BacLight Bacterial Viability kit, respectively. The results revealed that a significant reduction (P < 0.05) in biofilm formation occurred after 15 min exposure to 20 µM of LFchimera or CHX compared to control. In contrast, MH at concentration up to 100 µM did not inhibit biofilm formation. The ratio of live/dead bacteria in biofilm was also significantly lower after 15 min exposure to 20 µM of LFchimera compared to control and 20–50 µM of CHX and MH. Altogether, the results obtained indicate that LFchimera is able to inhibit in vitro subgingival biofilm formation and reduce viability of multispecies bacteria in biofilm better than CHX and MH.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Periodontitis is a major public health problem worldwide (Petersen et al. 2005). It is a polymicrobial infection caused by a group of specific microorganisms such as Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis, Treponema denticola, Prevotella intermedia and Tannerella forsythia (Socransky et al. 1998; Wara-aswapati et al. 2009; Pradhan-Palikhe et al. 2013) that are cohabitating in subgingival plaque. A recent study in a Thai population reported that the presence of P. gingivalis, and high colonization by A. actinomycetemcomitans, T. denticola, and P. intermedia play an important role in severe periodontitis (Torrungruang et al. 2015). A combination of A. actinomycetemcomitans, P. gingivalis, and P. intermedia also showed the strongest association with the disease in a Finnish population (Hyvarinen et al. 2009). Another Finnish study reported that the presence of A. actinomycetemcomitans with P. gingivalis and T. denticola in saliva has been shown to contribute to deepened pockets (Paju et al. 2009). A treatment for periodontitis that is associated with success is removal of bacterial biofilm, calculus, and toxins by scaling and root planing (SRP) (Greenstein 2000; Aimetti 2014). However, a limitation of SRP is the inability to access deeper areas of the gingival sulcus wherein remaining plaque could lead to microbial re-colonization and recurrence of disease. It is well accepted that bacteria growing in a biofilm, as is the case in periodontitis, are more recalcitrant to the action of antibiotics than bacteria growing in a planktonic state (Hoiby et al. 2010). This has led researchers to search for novel antimicrobial agents to overcome the problem of antibiotic resistance of bacterial biofilms and to investigate whether local delivery of antimicrobial agents can be effective on inaccessible areas of the gingival sulcus.
Chlorhexidine (CHX) in oral care products has been used as an antiseptic for many decades to help control dental plaque. Different CHX preparations applied subgingivally as an adjunct to SRP in the treatment of patients with chronic periodontitis have been reported (Da Rocha et al. 2015; Lecic et al. 2016). In addition, there are several options of antimicrobials, such as minocycline, metronidazole, doxycycline and tetracycline, which can be locally delivered into periodontal pockets as an adjunct to SRP in the treatment of periodontitis patients (Goodson et al. 2007; Da Rocha et al. 2015). However, long term use of antimicrobial oral care products may contribute to the development of multidrug resistance (Webber et al. 2015; Saleem et al. 2016).
Antimicrobial peptides (AMPs) are considered as potential agents to overcome the problem of the increasing resistance of bacterial strains to conventional antibiotics (Brogden 2005). Lactoferrin (LF) is a multifunctional iron-binding protein, which has broad-spectrum antimicrobial activity against bacteria, fungi, protozoa and viruses (Farnaud and Evans 2003), and is recognized as a source for antimicrobial peptides (Bruni et al. 2016). Two antimicrobial moieties have been discovered: lactoferricin (LFcin), which was discovered after pepsin digestion (Bellamy et al. 1992), and lactoferrampin (LFampin) identified based on screening of the LF domains exhibiting typical features of antimicrobial peptides, like amphipathicity, cationicity (van der Kraan et al. 2004). A heterodimeric peptide (LFchimera) was constructed by linking the peptides LFcin17–30 and LFampin265–284 in such a way that it resembled the structural orientation in the native molecule (Bolscher et al. 2009). This construct displayed strikingly stronger antimicrobial activities against several microorganisms, including Gram-positive and Gram-negative bacteria, Candida albicans and parasites than its constituent peptides LFcin17–30 and LFampin265–284 (Bolscher et al. 2009, 2012; Leon-Sicairos et al. 2009; Flores-Villasenor et al. 2010; Silva et al. 2012a). We have shown previously that LFchimera possesses antibacterial and antibiofilm activities and can modulate Burkholderia pseudomallei colonization (Puknun et al. 2013, 2016; Kanthawong et al. 2014). However, there is no report on inhibitory effects of LFchimera against multispecies oral biofilm formation. Therefore, we aimed to investigate the potency of LFchimera towards multispecies oral biofilm obtained from clinical samples of periodontitis patients and to compare its potency with CHX and minocycline hydrochloride (MH). Their activities towards the periodontal pathogen A. actinomycetemcomitans grown planktonically or as monospecies biofilms were included for comparative purposes.
Materials and methods
Peptide synthesis and purification
The bovine lactoferrin peptides LFcin17–30, LFampin265–284 and LFchimera (Table 1) were synthesized using Fmoc-protected amino acids (Orpegen Pharma GmbH, Heidelberg, Germany) in a Syro II peptide synthesizer (Biotage, Uppsala, Sweden) and purified with an Ultimate 3000 RP-HPLC (Thermo Scientific, MA) to a purity of at least 95% as previously described (Bolscher et al. 2012; Puknun et al. 2013). The authenticity of the peptides was confirmed by Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry (MALDI-TOF MS) on a Microflex LRF mass spectrometer equipped with an additional gridless reflection (Bruker Daltonik, Bremen, Germany) as described previously (Bolscher et al. 2012).
Bacterial strain and growth condition
Aggregatibacter actinomycetemcomitans ATCC 43718 was maintained on tryptic soy serum bacitracin vancomycin (TSBV) agar. A single colony initially grown on TSBV agar was inoculated into brain–heart infusion (BHI) broth (Criterion, Hardy Diagnostics, CA), incubated overnight at 37 °C, 5% CO2, in an incubator and used as inoculum in the antimicrobial assay.
Antimicrobial assay
The killing activities of all peptides, CHX and MH against planktonic A. actinomycetemcomitans ATCC 43718 were determined by colony culturing assays as described previously (Puknun et al. 2013). Briefly, a bacterial suspension of approximately 105 CFU/ml was re-suspended in 1 mM potassium phosphate buffer (PPB), pH 7.0, supplemented with the tested agents to a final concentration of 5, 10, 20 and 50 µM. A bacterial suspension in PPB without peptide served as a control. Following incubation at 37 °C, 5% CO2, for 60 min, the incubated mixture was serially diluted in a physiological concentration of saline and plated on TSBV agar. Colonies were counted after 48 h of incubation at 37 °C, 5% CO2. The percentage killing or inhibiting effects of each agent were calculated using the formula [1 − (CFU sample/CFU control)] × 100%. Each assay was performed on three separate occasions, with triplicate determinations each time.
Effect of antimicrobial agents on viability and biofilm matrix of A. actinomycetemcomitans in a 1-day old biofilm
The most effective antimicrobial peptide and drug from the result of the antimicrobial assay was selected to determine the effect on viability and biofilm matrix of a 1-day old A.actinomycetemcomitans ATCC 43718 biofilm using a static Amsterdam Active Attachment (AAA) model (Exterkate et al. 2010; Silva et al. 2012b). A minimum contact time of 15 min was chosen to test bactericidal effect of LFchimera in comparison to CHX because the killing activity of LFchimera have been shown to reach its maximum effect already after 15 min for most bacteria (Bolscher et al. 2009). Moreover, bactericidal concentration of CHX on subgingival bacteria tested in serum at short contact times was determined at 10–60 min by another study (Oosterwaal et al. 1989).
The AAA model consisted of stainless steel lid with clamps that contain 12 mm diameter round glass coverslips which were used as substratum to grow A. actinomycetemcomitans biofilm. The lid fits into standard polystyrene 24-well plates (Corning, USA). After assembling the lid and glass coverslips, the model was autoclaved. To allow adhesion on each glass coverslip, a final volume (1 ml) of bacterial suspension was added in each well of 24-well plate. The wells were then covered with the lid of the AAA model containing the glass coverslips and incubated at 37 °C, 5% CO2, for 3 h. After which, the lid was transferred to a new 24-well plate containing fresh media and incubated for another 21 h. The 1-day old biofilm were then placed on wells containing either 1.75 ml of 1 mM PPB (control), 5 µM LFchimera or 5 µM CHX for 15 min. The glass coverslips were then washed twice with sterile distilled water and subjected to staining.
To determine the viability of A. actinomycetemcomitans ATCC 43718, the 1-day old biofilm on the glass coverslips were submerged in 1.5 ml staining solution of LIVE/DEAD BacLight Bacterial Viability kit (Molecular Probes, Invitrogen, USA) and incubated at 25 °C in the dark for 15 min. After, it was fixed with 1% glutaraldehyde for 3 h at 4 °C. The 1-day old biofilm was examined using confocal laser scanning microscopy (Zeiss LSM 800, Zeiss, Germany) and quantification of bacterial viability was determined using the ZEN image software (Zeiss, Germany).
To determine the biofilm matrix of A. actinomycetemcomitans ATCC 43718, the 1-day old biofilm on the glass coverslips were fixed with 1% glutaraldehyde for 3 h at 4 °C and the extracellular polymeric substance of the biofilm was stained with fluorescein isothiocyanate-concanavalin A (FITC-ConA) for 15 min. The coverslips were examined using confocal laser scanning microscopy (Zeiss LSM 800, Zeiss, Germany) and the structure of the biofilm was analyzed using the ZEN image software.
Subjects and sampling of subgingival plaque
Subjects were healthy Thai adults who sought periodontal treatment at the Faculty of Dentistry, Khon Kaen University, Khon Kaen, Thailand. The criteria used for selection included: subjects between the ages of 35 and 45 years with no known systemic diseases, who had not received antibiotics within the previous 3 months, and who had not used medications that might influence the subgingival microbiota. The study protocol was approved by the Khon Kaen University ethical review committee (HE542176). Informed consent was obtained from all subjects. Subgingival plaque samples were collected from five individuals with periodontitis. The sampling sites had pocket probing depths ranging from 4 to 5 mm and clinical attachment loss ≥ 3 mm. The subgingival plaque was collected by method described previously (Taweechaisupapong et al. 2014). Briefly, a sterile absorbent paper point was inserted to the sulcus depth and moving it laterally along the tooth surface and the sulcular epithelial lining. The paper point sample was immediately placed into 1 ml thioglycollate medium (Criterion, Hardy Diagnostics, CA) and gently sonicated to disperse the bacterial cells.
Saliva collection and processing
Unstimulated saliva was obtained in 5-ml samples from the same subjects who had donated subgingival plaque and processed as previously described (Walker and Sedlacek 2007; Taweechaisupapong et al. 2014). In brief, each saliva sample was diluted (1:10) with pre-reduced, anaerobically-sterilized Ringer solution, containing 0.05% cysteine (Sigma Chemical Co, St Louis, MO) as a reducing agent, and centrifuged at 2000×g for 10 min to remove any particulate matter. The supernatant was passed through 0.2 µm sterile syringe filter.
PCR detection of A. actinomycetemcomitans in subgingival plaque
Subgingival plaque samples were suspended in 1 ml sterile double distilled water, pelleted, and resuspended in 200 µl of DNA isolation reagent (InstaGene Matrix, Bio-Rad Lab, Hercules, CA, USA). Total DNA was extracted according to the manufacturer’s instructions. The suspension was centrifuged and 5 µl of resulting supernatant was used for PCR. The PCR reactions (Taq PCR Core Kit, Qiagen, Valencia, CA, USA) were carried out as previously described using oligonucleotide primers specific for A. actinomycetemcomitans, sense 5′ AAA CCC ATC TCT GAG TTC TTC TTC 3′, antisense 5′ ATG CCA ACT TGA CGT TAA AT 3′ (Ashimoto et al. 1996). The PCR product was analyzed by 1% agarose gel electrophoresis. DNA of A. actinomycetemcomitans ATCC 43718 was used as positive control.
Effects of antimicrobial agents on multispecies subgingival biofilm formation
The inhibitory effect of LFchimera, CHX and MH on biofilm formation in a simulated oral cavity condition was investigated. The experiment was designed to test the capacity of multispecies bacteria to re-establish a biofilm after 15 min pretreatment, subsequent dislodgment and reseating; which is similar to a possible scenario after SRP. In detail, subgingival plaque sample from each periodontitis patient was cultivated in patient matched saliva-coated 96-well microtiter plate under anaerobic atmosphere at 37 °C for 24 h to form a 1-day old biofilm. Thereafter, the supernatant in each well was removed and the wells were washed with 200 µl phosphate buffered saline (PBS). LFchimera, CHX or MH, was added at final concentrations as indicated to the adherent microorganisms for 15 min. The control wells were incubated with PBS without antimicrobial agents. Then the wells were washed with PBS and 200 µl of fresh tryptic soy broth was added in each well. Sonication was performed for 5 min to dislodge adherent microorganisms. The bacterial suspension was transferred to the new 96-well microtiter plates and cultivated under anaerobic atmosphere at 37 °C for 24 h to allow re-establishment of a new biofilm. Then supernatant in each well was removed and the wells were washed with PBS. The re-established biofilm was stained for 15 min with 150 µl of 1% crystal violet. Excess stain was removed with running tap water. The plates were air dried and the dye bound to the adherent cells was solubilized with 150 µl of 33% (v/v) glacial acetic acid per well. After transferring the dye solution to a new plate, the optical density (OD) of each well was measured at 595 nm using microplate reader (Varioskan Flash, Thermo Fisher Scientific Inc., USA). The experiments were repeated on three separate occasions, with triplicate determinations in each experiment.
Effects of LFchimera on survival of bacteria in multispecies subgingival biofilm
To determine the effects of the tested agents on survival of bacteria in an established multispecies biofilm, LIVE/DEAD BacLight Bacterial Viability kit was used. Subgingival plaque sample from each periodontitis patient was cultivated in saliva-coated 96-well microtiter plate under anaerobic atmosphere at 37 °C for 24 h and the tested agents were added as described above. The control wells were incubated with PBS without the tested agents. After contact with the 1-day old biofilm for 15 min, the tested agents in each well were removed. The wells were washed with PBS and aliquots of 100 μl of the staining solution were added to each well and mixed thoroughly. The plate was incubated at 25 °C in the dark for 15 min and the fluorescence was measured with a fluorescence microplate reader (Varioskan Flash, Thermo Fisher Scientific Inc., USA). The excitation/emission maxima for the dyes were 485/530 nm for SYTO 9 and 485/630 nm for propidium iodide. The experiments were repeated on three separate occasions, with triplicate determinations in each experiment.
Statistical analysis
Kruskal–Wallis analysis and Dunn’s multiple comparison tests were carried out for comparison of biofilm formation and bacterial survival among the test and control groups using GraphPad Prism 5 (GraphPad Software, Inc., San Diego, CA, USA). The level required for statistical significance was P < 0.05.
Results
The killing effect of different concentrations of LFchimera and its constituent peptides LFcin17–30 and LFampin265–284 in comparison to CHX and MH on A. actinomycetemcomitans ATCC 43718 is shown in Fig. 1. The antimicrobial activities of each agent or combination depended on the dose of the agents. At the highest concentration used (50 µM), all agents reached 100% killing. Differences in activity become visible at lower concentrations, suggesting that CHX has more killing potency than MH. The antimicrobial activity of LFchimera seems comparable to CHX but was strikingly stronger than its constituent peptides, or a combination of both. Of these two antimicrobial peptides LFcin17-30 was the more potent one suggesting that this peptide might contribute more than LFampin to the high activity of LFchimera.
Effects of different concentrations of LFchimera, LF-peptides, chlorhexidine digluconate and minocycline hydrochloride on A. actinomycetemcomitans ATCC 43718. Bacterial suspensions were incubated with 5–50 µM of each agent and processed as described in materials and methods. Percentage killing was calculated using the formula [1 − (CFU sample/CFU control)] × 100%. Data are the mean value of three independent experiments carried out in triplicate
The effects of LFchimera and CHX on viability and biofilm matrix of the 1-day old A. actinomycetemcomitans ATCC 43718 biofilm were shown in Figs. 2 and 3, respectively. Confocal laser micrographs of biofilm after treatment with both LFchimera and CHX showed significantly lower live/dead ratio compared to control (P < 0.05). However, no statistically significant difference on live/dead ratio was seen between LFchimera and CHX (Fig. 2). Visualization of the exopolysaccharide matrix using FITC-ConA showed that LFchimera is able to decrease biofilm thickness. Measurement of the biofilm thickness using the image software revealed that LFchimera and CHX treated biofilms have a mean thickness of 8.5 and 9.5 µm, respectively, while the control group has a mean thickness of 12.3 µm. Moreover, LFchimera treated biofilm showed less green fluorescence color than control and CHX treated biofilms (Fig. 3).
Confocal laser scanning micrographs of a representative section of a 1-day old A. actinomycetemcomitans ATCC 43718 biofilm after 15 min exposure to 1 mM PPB control (a), 5 µM LFchimera (b), and 5 µM chlorhexidine digluconate (c). The biofilms were stained with LIVE/DEAD BacLight Bacterial Viability kit. Green color indicates live bacteria and red color indicates dead bacteria. d Live/dead ratio calculation *P < 0.05 compared to control
Confocal laser scanning micrographs of a representative section of a 1-day old A. actinomycetemcomitans ATCC 43718 biofilm after 15 min exposure to 1 mM PPB control (a), 5 µM LFchimera (b), and 5 µM chlorhexidine digluconate (c). The biofilms were stained with FITC-ConA. Green color indicates exopolysaccharide of biofilm matrix
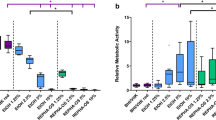
Next we tested the potency of LFchimera on multispecies biofilm of clinical samples from periodontitis patients, all harboring A. actinomycetemcomitans in the subgingival plaque samples as verified by PCR (Fig. 4). After pretreatment of the 1-day old biofilm derived from the subgingival plaque of periodontitis patients with 5–100 µM LFchimera for 15 min, biofilm was dislodged by sonication and bacteria were tested for the capacity to form a biofilm again in fresh culture medium under anaerobic conditions during the next 24 h period (Fig. 5). Whereas 15 min treatment with 5 µM LFchimera showed a similar biofilm forming capacity as the control, significant less biofilm was formed after a pretreatment with higher concentrations of LFchimera (20–100 µM). Similar inhibition of biofilm formation was found with 20–100 µM CHX; using the common clinical concentration (1337 µM, corresponding to 0.12% solution) an even higher inhibitory effect was found. In contrast, biofilm formation after pretreatment with MH was not significantly different compared with untreated control at all tested concentrations (5–100 µM).
Detection of A. actinomycetemcomitans DNA using agarose gel electrophoresis. The gel shows staining for A. actinomycetemcomitans DNA amplified by conventional PCR (Ashimoto et al. 1996) from five subjects. Lane 1 a 100 bp DNA ladder as a molecular weight marker; Lane 2 A. actinomycetemcomitans ATCC 43718; Lane 3–7 subgingival plaque samples from subjects No. 1–5, respectively; Lane 8 negative control
Effects of different concentrations of LFchimera, chlorhexidine digluconate and minocycline hydrochloride on multispecies oral biofilm formation. The bacteria in a 1-day old biofilm was pretreatment with the tested agents for 15 min, then adherent microorganisms were dislodged and transferred to the new microtiter plates and cultivated under anaerobic atmosphere at 37 °C for 24 h. The biofilm formation were measured at 24 h. Results are from 5 periodontitis patients (N = 5). The upper and lower box margins represent 75th percentile and 25th percentile, respectively. The horizontal line inside each box indicates the median (50th percentile). * P < 0.05 compared to control
Next we tested the capacity of 5–50 µM LFchimera to kill the bacteria in the 1-day old multispecies oral biofilm, using a LIVE/DEAD staining. Treatment for only 15 min with LFchimera at concentrations as low as 5 µM already showed a significant lower live/dead ratio of multispecies bacteria in biofilm compared to untreated control. Similar treatment with CHX at concentrations up to 50 µM had no killing effect; only the high concentration of 1337 µM showed a significant increase in killing. MH did not show any killing effect of the bacteria in biofilm (Fig. 6).
Effects of different concentrations of LFchimera, chlorhexidine digluconate and minocycline hydrochloride on viability of multispecies bacteria in a 1-day old biofilm after treatment with the tested agents for 15 min. Results are from five periodontitis patients (N = 5). The upper and lower box margins represent 75th percentile and 25th percentile, respectively. The horizontal line inside each box indicates the median (50th percentile). * P < 0.05 compared to control
Discussion
In this paper we have investigated LFchimera as an antimicrobial agent to combat periodontal pathogens in biofilms. The potency of this chimerical combination of two antimicrobial domains of the mammalian defense protein lactoferrin emerged from reports describing a strikingly higher antimicrobial effect in comparison to both constituent peptides against a series of pathogens including parasites, yeast, as well as Gram positive and Gram negative bacteria including A. actinomycetemcomitans (Bolscher et al. 2009, 2012; Leon-Sicairos et al. 2009; Flores-Villasenor et al. 2010; Silva et al. 2012a). Here we confirmed the enhanced killing activity of LFchimera towards A. actinomycetemcomitans and showed that its activity was comparable to that of CHX and significant higher than MH, two conventional agents used in oral hygiene to combat oral pathogens (Da Rocha et al. 2015; Lecic et al. 2016).
CHX is the most commonly used antibacterial agent and the most effective antiplaque agent until date. However, its disadvantages on long-term usage are staining of teeth and altered taste sensation. In rare cases, serious adverse effects like oral mucosal erosion, parotid swelling and enhanced supragingival calculus has been reported (Chauhan et al. 2013). For MH, several studies have yielded promising results with the local application of MH in the treatment of periodontitis (Pandit et al. 2013; Abbas et al. 2016). However, the long term use of antimicrobial oral care products contributes to the development of multidrug resistance (Webber et al. 2015; Saleem et al. 2016). In contrast, bacteria seems to be largely unable to develop resistance to AMPs (Peschel and Sahl 2006), encouraging the exploration of therapeutics based on AMPs.
In the present study, it was clearly evident that LFchimera affects viability and the structure of monospecies A. actinomycetemcomitans ATCC 43718 biofilm. Although no statistically significant difference on live/dead ratio was seen between LFchimera and CHX, LFchimera treated biofilm showed less exopolysaccharide matrix production than CHX treated biofilms (Fig. 3). From our previous reports in Gram negative bacteria, the bacteria treated with LFchimera showed accumulation of the peptide in intracellular structures (Puknun et al. 2016; Reyes-Cortes et al. 2017). These results suggested that less exopolysaccharide matrix production found in LFchimera treated biofilm in this study may not only depend on the viability of bacteria but also could depend on intracellular effects of LFchimera.
To further explore LFchimera as an agent to combat periodontal pathogens in multispecies biofilms, an in vitro multispecies biofilm model described by Walker and Sedlacek was used because this model closely mimics the composition of the in vivo state (Walker and Sedlacek 2007). Our previous studies demonstrated that LFchimera is an effective peptide against monospecies biofilm form of B. pseudomallei, possessing a stronger antibiofilm activity than the conventional antibiotic ceftazidime (Puknun et al. 2013, 2016). The inhibitory effects of LFchimera on in vitro subgingival biofilm formation and against an established multispecies biofilm found in this study are in accordance with its effects on monospecies A. actinomycetemcomitans ATCC 43718 biofilm and our previous report (Puknun et al. 2016).
In this study, multispecies subgingival biofilm formation was quantified in 96-well microtiter plates using crystal violet staining. However, the 1-day old monospecies A. actinomycetemcomitans ATCC 43718 biofilm could not be quantified by crystal violet staining in 96-well microtiter plates (data not shown). This may be because of the thinner biofilm formation of monospecies A. actinomycetemcomitans than multispecies and the inherent limitation of the detection method. The very thin biofilm formation of monospecies A. actinomycetemcomitans was confirmed using AAA model and examined by confocal laser scanning microscope (Fig. 3). Several studies have demonstrated higher extracellular polymeric substances (EPS) production in multispecies biofilms compared to monospecies (Bridier et al. 2012; Alavi and Hansen 2013; Wang et al. 2013; Jahid et al. 2014) Additional research has found that multispecies biofilms can induce the formation of compact 3-dimensional structures (Schwering et al. 2013; Ibusquiza et al. 2012) as well as increased biomass compared to monospecies biofilms (Burmølle et al. 2006; Kuznetsova et al. 2013) Moreover, multispecies biofilms are more resistant to antimicrobial agents than monospecies, possibly due to higher EPS production, denser and thicker biofilms maturation (van der Veen and Abee 2011; Schwering et al. 2013; Wang et al. 2013). Our findings are consistent with those studies because in the monospecies A. actinomycetemcomitans biofilm after treatment with 5 µM CHX, significantly lower live/dead ratio compared to control was found (Fig. 2) while in multispecies biofilms, only the highest concentration of CHX exhibited a significant higher killing activity in comparison to the controls (Fig. 6).
Our present data demonstrate that LFchimera is able to inhibit in vitro subgingival multispecies biofilm formation and reduce viability of multispecies bacteria in biofilm better than CHX and MH. Moreover, concentrations up to 20 µM LFchimera does not induce significant lysis or permeabilization of human red blood cells (Kanthawong et al. 2014), nor cytotoxicity on rat hepatocytes (Leon-Sicairos et al. 2009). Therefore, it could be considered for development as a new potential locally delivered therapeutic agent that may be used as an adjunctive treatment for periodontitis.
References
Abbas S, MahenDra J, Ari G (2016) Minocycline ointment as a local drug delivery in the treatment of generalized chronic periodontitis—A clinical study. J Clin Diagn Res 10:ZC15-19
Aimetti M (2014) Nonsurgical periodontal treatment. Int J Esthet Dent 9:251–267
Alavi HED, Hansen LT (2013) Kinetics of biofilm formation and desiccation survival of Listeria monocytogenes in single and dual species biofilms with Pseudomonas fluorescens. Serratia proteamaculans or Shewanella baltica on food-grade stainless steel surfaces. Biofouling 29:1253–1268
Ashimoto A, Chen C, Bakker I, Slots J (1996) Polymerase chain reaction detection of 8 putative periodontal pathogens in subgingival plaque of gingivitis and advanced periodontitis lesions. Oral Microbiol Immunol 11:266–273
Bellamy W, Takase M, Wakabayashi H, Kawase K, Tomita M (1992) Antibacterial spectrum of lactoferricin B, a potent bactericidal peptide derived from the N-terminal region of bovine lactoferrin. J Appl Bacteriol 73:472–479
Bolscher JG, Adao R, Nazmi K, van den Keybus PA, van ‘t Hof W, Amerongen AVN, Bastos M, Veerman EC (2009) Bactericidal activity of LFchimera is stronger and less sensitive to ionic strength than its constituent lactoferricin and lactoferrampin peptides. Biochimie 91:123–132
Bolscher J, Nazmi K, van Marle J, van ‘t Hof W, Veerman E (2012) Chimerization of lactoferricin and lactoferrampin peptides strongly potentiates the killing activity against Candida albicans. Biochem Cell Biol 90:378–388
Bridier A, del Pilar Sanchez-Vizuete M, Le Coq D, Aymerich S, Meylheuc T, Maillard JY, Thomas V, Dubois-Brissonnet F, Briandet R (2012) Biofilms of a Bacillus subtilis hospital isolate protect Staphylococcus aureus from biocide action. PLoS ONE 7:e44506
Brogden KA (2005) Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol 3:238–250
Bruni N, Capucchio MT, Biasibetti E, Pessione E, Cirrincione S, Giraudo L, Corona A, Dosio F (2016) Antimicrobial activity of lactoferrin-related peptides and applications in human and veterinary medicine. Molecules 21:E752
Burmølle M, Webb JS, Rao D, Hansen LH, Sørensen SJ, Kjelleberg S (2006) Enhanced biofilm formation and increased resistance to antimicrobial agents and bacterial invasion are caused by synergistic interactions in multispecies biofilms. Appl Environ Microbiol 72:3916–3923
Chauhan AS, Bains VK, Gupta V, Singh GP, Patil SS (2013) Comparative analysis of hyaluronan gel and xanthan based chlorhexidine gel, as adjunct to scaling and root planing with scaling and root planing alone in the treatment of chronic periodontitis: a preliminary study. Contemp Clin Dent 4:54–61
Da Rocha HA, Silva CF, Santiago FL, Martins LG, Dias PC, De Magalhaes D (2015) Local drug delivery systems in the treatment of periodontitis: a literature review. J Int Acad Periodontol 17:82–90
Exterkate RA, Crielaard W, Ten Cate JM (2010) Different response to amine fluoride by Streptococcus mutans and polymicrobial biofilms in a novel high-throughput active attachment model. Caries Res 44:372–379
Farnaud S, Evans RW (2003) Lactoferrin—a multifunctional protein with antimicrobial properties. Mol Immunol 40:395–405
Flores-Villasenor H, Canizalez-Roman A, Reyes-Lopez M, Nazmi K, de la Garza M, Zazueta-Beltran J, Leon-Sicairos N, Bolscher JG (2010) Bactericidal effect of bovine lactoferrin, LFcin, LFampin and LFchimera on antibiotic-resistant Staphylococcus aureus and Escherichia coli. Biometals 23:569–578
Goodson JM, Gunsolley JC, Grossi SG, Bland PS, Otomo-Corgel J, Doherty F, Comiskey J (2007) Minocycline HCl microspheres reduce red-complex bacteria in periodontal disease therapy. J Periodontol 78:1568–1579
Greenstein G (2000) Nonsurgical periodontal therapy in 2000: a literature review. J Am Dent Assoc 131:1580–1592
Hoiby N, Bjarnsholt T, Givskov M, Molin S, Ciofu O (2010) Antibiotic resistance of bacterial biofilms. Int J Antimicrob Agents 35:322–332
Hyvarinen K, Laitinen S, Paju S, Hakala A, Suominen-Taipale L, Skurnik M, Kononen E, Pussinen PJ (2009) Detection and quantification of five major periodontal pathogens by single copy gene-based real-time PCR. Innate Immun 15:195–204
Ibusquiza PS, Herrera JJ, Vazquez-Sanchez D, Cabo ML ((2012) Adherence kinetics, resistance to benzalkonium chloride and microscopic analysis of mixed-species biofilms formed by Listeria monocytogenes and Pseudomonas putida. Food Control 25:202–210
Jahid IK, Han N, Srey S, Ha SD (2014) Competitive interactions inside mixed-species biofilms of Salmonella typhimurium and cultivable indigenous microorganisms on lettuce enhance microbial resistance of their sessile cells to ultraviolet C (UV-C) irradiation. Food Res Int 55:445–454
Kanthawong S, Puknun A, Bolscher JG, Nazmi K, van Marle J, de Soet JJ, Veerman EC, Wongratanacheewin S, Taweechaisupapong S (2014) Membrane-active mechanism of LFchimera against Burkholderia pseudomallei and Burkholderia thailandensis. Biometals 27:949–956
Kuznetsova MV, Maslennikova IL, Karpunina TI, Nesterova LY, Demakov VA (2013) Interactions of Pseudomonas aeruginosa in predominant biofilm or planktonic forms of existence in mixed-species with Escherichia coli in vitro. Can J Microbiol 59:604–610
Lecic J, Cakic S, Janjic Pavlovic O, Cicmil A, Vukotic O, Petrovic V, Cicmil S (2016) Different methods for subgingival application of chlorhexidine in the treatment of patients with chronic periodontitis. Acta Odontol Scand 74:502–507
Leon-Sicairos N, Canizalez-Roman A, de la Garza M, Reyes-Lopez M, Zazueta-Beltran J, Nazmi K, Gomez-Gil B, Bolscher JG (2009) Bactericidal effect of lactoferrin and lactoferrin chimera against halophilic Vibrio parahaemolyticus. Biochimie 91:133–140
Oosterwaal PJ, Mikx FH, van den Brink ME, Renggli HH (1989) Bactericidal concentrations of chlorhexidine-digluconate, amine fluoride gel and stannous fluoride gel for subgingival bacteria tested in serum at short contact times. J Periodontal Res 24:155–160
Paju S, Pussinen PJ, Suominen-Taipale L, Hyvonen M, Knuuttila M, Kononen E (2009) Detection of multiple pathogenic species in saliva is associated with periodontal infection in adults. J Clin Microbiol 47:235–238
Pandit N, Dahiya R, Gupta R, Bali D, Kathuria A (2013) Comparative evaluation of locally delivered minocycline and metronidazole in the treatment of periodontitis. Contemp Clin Dent 4:48–53
Peschel A, Sahl HG (2006) The co-evolution of host cationic antimicrobial peptides and microbial resistance. Nat Rev Microbiol 4:529–536
Petersen PE, Bourgeois D, Ogawa H, Estupinan-Day S, Ndiaye C (2005) The global burden of oral diseases and risks to oral health. Bull World Health Organ 83:661–669
Pradhan-Palikhe P, Mantyla P, Paju S, Buhlin K, Persson GR, Nieminen MS, Sinisalo J, Pussinen PJ (2013) Subgingival bacterial burden in relation to clinical and radiographic periodontal parameters. J Periodontol 84:1809–1817
Puknun A, Bolscher JG, Nazmi K, Veerman EC, Tungpradabkul S, Wongratanacheewin S, Kanthawong S, Taweechaisupapong S (2013) A heterodimer comprised of two bovine lactoferrin antimicrobial peptides exhibits powerful bactericidal activity against Burkholderia pseudomallei. World J Microbiol Biotechnol 29:1217–1224
Puknun A, Kanthawong S, Anutrakunchai C, Nazmi K, Tigchelaar W, Hoeben KA, Veerman EC, Bolscher JG, Taweechaisupapong S (2016) Ultrastructural effects and antibiofilm activity of LFchimera against Burkholderia pseudomallei. World J Microbiol Biotechnol 32:33
Reyes-Cortes R, Acosta-Smith E, Mondragon-Flores R, Nazmi K, Bolscher JG, Canizalez-Roman A, Leon-Sicairos N (2017) Antibacterial and cell penetrating effects of LFcin17–30, LFampin265–284, and LF chimera on enteroaggregative Escherichia coli. Biochem Cell Biol 95:76–81
Saleem HG, Seers CA, Sabri AN, Reynolds EC (2016) Dental plaque bacteria with reduced susceptibility to chlorhexidine are multidrug resistant. BMC Microbiol 16:214
Schwering M, Song J, Louie M, Turner RJ, Ceri H (2013) Multi-species biofilms defined from drinking water microorganisms provide increased protection against chlorine disinfection. Biofouling 29:917–928
Silva T, Abengozar MA, Fernandez-Reyes M, Andreu D, Nazmi K, Bolscher JG, Bastos M, Rivas L (2012a) Enhanced leishmanicidal activity of cryptopeptide chimeras from the active N1 domain of bovine lactoferrin. Amino Acids 43:2265–2277
Silva TC, Pereira AF, Exterkate RA, Bagnato VS, Buzalaf MA, Machado MA, Ten Cate JM, Crielaard W, Deng DM (2012b) Application of an active attachment model as a high-throughput demineralization biofilm model. J Dent 40:41–47
Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL Jr. (1998) Microbial complexes in subgingival plaque. J Clin Periodontol 25: 134–144
Taweechaisupapong S, Pinsuwan W, Suwannarong W, Kukhetpitakwong R, Luengpailin S (2014) Effects of Streblus asper leaf extract on the biofilm formation of subgingival pathogens. S Afr J Bot 94:1–5
Torrungruang K, Jitpakdeebordin S, Charatkulangkun O, Gleebbua Y (2015) Porphyromonas gingivalis, Aggregatibacter actinomycetemcomitans, and Treponema denticola/Prevotella intermedia co-infection are associated with severe periodontitis in a Thai population. PLoS ONE 10:e0136646
van der Veen S, Abee T (2011) Mixed-species biofilms of Listeria monocytogenes and Lactobacillus plantarum show enhanced resistance to benzalkonium chloride and peracetic acid. Intl J Food Microbiol 144:421–431
van der Kraan MI, Groenink J, Nazmi K, Veerman EC, Bolscher JG, Amerongen AVN (2004) Lactoferrampin: a novel antimicrobial peptide in the N1-domain of bovine lactoferrin. Peptides 25:177–183
Walker C, Sedlacek MJ (2007) An in vitro biofilm model of subgingival plaque. Oral Microbiol Immunol 22:152–161
Wang R, Kalchayanand N, Schmidt JW, Harhay DM (2013) Mixed biofilm formation by Shiga toxin-producing Escherichia coli and Salmonella enterica serovar Typhimurium enhanced bacterial resistance to sanitization due to extracellular polymeric substances. J Food Prot 76:1513–1522
Wara-aswapati N, Pitiphat W, Chanchaimongkon L, Taweechaisupapong S, Boch JA, Ishikawa I (2009) Red bacterial complex is associated with the severity of chronic periodontitis in a Thai population. Oral Dis 15:354–359
Webber MA, Whitehead RN, Mount M, Loman NJ, Pallen MJ, Piddock LJ (2015) Parallel evolutionary pathways to antibiotic resistance selected by biocide exposure. J Antimicrob Chemother 70:2241–2248
Acknowledgements
This work was supported by Khon Kaen University. JGMB and KN are supported by a grant from the University of Amsterdam for research into the focal point Oral Infections and Inflammation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ruangcharoen, S., Suwannarong, W., Lachica, M.R.C.T. et al. Killing activity of LFchimera on periodontopathic bacteria and multispecies oral biofilm formation in vitro. World J Microbiol Biotechnol 33, 167 (2017). https://doi.org/10.1007/s11274-017-2334-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-017-2334-2