Abstract
Lactoferrin chimera (LFchimera), a hybrid peptide containing the two antimicrobial stretches of the innate immunity factor bovine lactoferrin, viz. LFampin265-284 and LFcin17-30, has strikingly high antimicrobial activity against the category B pathogen Burkholderia pseudomallei. The action mechanisms of LFchimera against B. pseudomallei is not fully understood. The aim of this study was to further investigate the effect of treated B. pseudomallei with LFchimera using (immune) electron microscopy. The effects of LFchimera on biofilm formation and against preformed biofilm of B. pseudomallei were also determined. After exposure to LFchimera, transmission electron microscopy revealed swelling of the periplasmic space of B. pseudomallei and a highly inhomogeneous electron density in the intracellular DNA region. Localization of LFchimera in B. pseudomallei using immunoelectron microscopy showed gold particles in intracellular structures without accumulation on the membranes. LFchimera also possessed stronger bactericidal activity than ceftazidime against B. pseudomallei grown in biofilm. Moreover, limited exposure of B. pseudomallei to LFchimera at subcidal concentration could reduce biofilm formation. Altogether, the results indicate that LFchimera possesses antibacterial and antibiofilm activities and can modulate B. pseudomallei colonization. Therefore, the efficacy of LFchimera merits further development of this agent for the therapy of melioidosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The environmental saprophytic bacterium Burkholderia pseudomallei is the causative agent of melioidosis, a fatal infectious disease of both humans and animals. It is a Gram negative, motile, non-spore-forming bacillus which can be isolated from soil and water in Southeast Asia and Northern Australia (Wiersinga et al. 2012). The clinical manifestations of melioidosis is variable and ranges from localized and chronic infection to acute fulminant sepsis (Limmathurotsakul and Peacock 2011). Burkholderia pseudomallei is classified by the Centers for Disease Control and Prevention as a category B warfare agent (Rotz et al. 2002). The treatment of melioidosis is quite difficult because B. pseudomallei is intrinsically resistant to a diverse group of antibiotics with those regularly used for the practical treatment of sepsis (Dance 2014). Currently, the most commonly used antimicrobials for treating melioidosis are ceftazidime (CAZ), imipenem, amoxicillin-clavulanate, doxycycline, and trimethoprimsulfamethoxazole, but there are several reports on in vitro and in vivo resistance to these drugs among B. pseudomallei isolates (White 2003; Cheng and Currie 2005; Limmathurotsakul and Peacock 2011). Moreover, B. pseudomallei are even more resistant to antibiotics when switched to the biofilm mode of growth (Sawasdidoln et al. 2010; Pibalpakdee et al. 2012; Bandeira Tde et al. 2013), which emphasizes the importance of treating melioidosis with antimicrobials that are effective against B. pseudomallei biofilms and less prone to develop resistance. Therefore, the development of novel anti-infective agents against B. pseudomallei both in planktonic and biofilm form is increasingly mandatory.
Lactoferricin (LFcin) and lactoferrampin (LFampin), two antimicrobial domains stretches of the innate immunity factor bLF, have been used for the development of series of antimicrobial peptides with broad spectrum activity against a range of Gram-positive and Gram-negative bacteria as well as yeast, like Candida albicans (Bellamy et al. 1992; Van Der Kraan et al. 2004; Valenti and Antonini 2005; van der Kraan et al. 2005; Van Der Kraan et al. 2006; Haney et al. 2007). A hybrid peptide (LFchimera) containing both LFampin265-284 and LFcin17-30 has strikingly higher antimicrobial activities than the individual peptides (Bolscher et al. 2009; Leon-Sicairos et al. 2009; Flores-Villasenor et al. 2010; Bolscher et al. 2012; Flores-Villasenor et al. 2012a, b; Puknun et al. 2013). By screening for the susceptibility of B. pseudomallei to LF-peptides and LFchimera, we have shown previously that this LFchimera possesses stronger killing activity against B. pseudomallei than its constituent peptides and the preferential antibiotic CAZ (Puknun et al. 2013). Moreover, we have found that the killing activity of LFchimera is similar for both B. pseudomallei and B. thailandensis and thought to be mediated by disruption of the plasma membrane and subsequently leakage of intracellular nucleotides leading to cell death (Kanthawong et al. 2014). However, the detailed mechanism by which the LFchimera kills B. pseudomallei is not clearly understood. Localization of LFchimera in B. pseudomallei as well as its effect on morphology analyzed by immunoelectron microscopy may help to clarify the mechanisms of cell death. In addition, the effect of LFchimera in inhibiting B. pseudomallei biofilm formation and against preformed biofilm has never been reported. Therefore, this study aims to further elucidate the mechanism of action of LFchimera by analyzing its effect on the above mentioned processes.
Materials and methods
Peptide synthesis, purification and labeling
LFchimera (Table 1) and fluorescent labeled variants were synthesized using Fmoc-protected amino acids (Orpegen Pharma GmbH, Heidelberg, Germany) using a Syro II peptide synthesizer (Biotage, Uppsala, Sweden) and purified with an Ultimate 3000 RP-HPLC (Thermo Scientific, MA) to a purity of at least 95 % as previously described (Bolscher et al. 2012; Puknun et al. 2013). The authenticity of the peptides was confirmed by Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry (MALDI-TOF MS) on a Microflex LRF mass spectrometer equipped with an additional gridless reflection (Bruker Daltonik, Bremen, Germany) as described previously (Bolscher et al. 2012).
Bacterial strains and growth conditions
Seven isolates of B. pseudomallei were used in this study (Table 2). Burkholderia pseudomallei were maintained on nutrient agar (NA) (Criterion, Hardy Diagnostics, CA) except for B. pseudomallei M10, SRM117 and PPKM which were grown on Luria–Bertani (LB) agar (Criterion, Hardy Diagnostics, CA) containing 15, 50 and 60 µg/ml tetracycline. A single colony of each bacteria initially grown on NA or LB was inoculated into brain–heart infusion (BHI) broth (Criterion, Hardy Diagnostics, CA) or Modified Vogel and Bonner’s medium (MVBM) (Lam et al. 1980), incubated overnight at 37 °C in a 200 rpm shaker-incubator and used as inoculum in all experiments.
Killing effects of LFchimera against planktonic bacteria
The killing activities of LFchimera against B. pseudomallei were determined by colony culturing assays as described previously (Puknun et al. 2013). Briefly, bacterial cells were washed three times and were re-suspended (approximately 105 CFU/ml) in 1 mM potassium phosphate buffer (PPB), pH 7.0. The bacterial suspension was then added to an equal volume of the test agents to reach a final concentration of 1, 5, 10 and 20 µM. A bacterial suspension in PPB without peptide served as a control. Following incubation at 37 °C for 60 min, the incubation mixture was serially diluted in physiological saline and plated in triplicate on NA. Colonies were counted after 24 h of incubation at 37 °C. The percentage killing effects were calculated using the formula [1 − (CFU sample/CFU control)] × 100 %. Each assay was performed on two separate occasions, with triplicate determinations each time.
Localization of LFchimera using fluorescence microscopy and immunoelectron microscopy
The localization of LFchimera in B. pseudomallei was studied by incubating B. pseudomallei (approximately 107 CFU/ml) in 1 mM PPB with 10 µM FITC-LFchimera for 15 min. Subsequently, bacteria were examined by a Leica fluorescence microscope (Wetzlar, Germany). Images were recorded using a cooled CCD camera (U4000, Apogee Instruments, CA). Bacterial suspension in PPB without peptide served as a control.
For transmission immunoelectron microscopy (TEM), B. pseudomallei cells were re-suspended in 1 mM PPB at 107 CFU/ml and incubated with 10 μM FITC-LFchimera for 30 min. Bacteria were harvested by centrifugation and fixed in 4 % paraformaldehyde solution in 0.1 M phosphate buffer pH 7.4 overnight in the refrigerator and then stored in 1 % paraformaldehyde fixative in the same buffer. Cryosections were prepared and incubated with ultra small gold-labelled mouse anti-FITC antibodies (Aurion, The Netherlands). The signal was enhanced with R-gent SE-EM (Aurion, The Netherlands) and sections were viewed in a FEI Technai-12 microscope (Technai Transmission Electron Microscopic, OR) equipped with a Lab6 filament, compu-stage, eagle 4 × 4 K bottom mount camera and a velata 2 × 2 K side-entry CCD camera for image acquisition.
Transmission electron microscopy
The effect of the LFchimera on the cell morphology of B. pseudomallei was investigated by TEM as previous described with some modifications (Shin et al. 1998). Burkholderia pseudomallei (approximately 107 CFU/ml) in 1 mM PPB was incubated with LFchimera at 5, 10, 20 µM at 37 °C for 1 h. A bacterial suspension in PPB without peptide served as a control. The bacteria were collected by centrifugation and washed with 0.1 M PPB, pH 7.0. The samples were fixed with 4 % paraformaldehyde and 1 % glutaraldehyde in 0.1 M sodium cacodylate buffer, pH 7.4 and then examined with a FEI Technai G2 Spirit BioTwin microscope operated at 100 kV and equipped with SIS MegaView II camera (Olympus Soft Imaging Solutions GmbH, Germany).
Effect of LFchimera on biofilm-forming capacity of B. pseudomallei
The bacterial suspensions adjusted to an optical density (OD) at 540 nm of 0.8–0.9 in PPB were added to an equal volume of LFchimera to reach final concentrations of 1 and 5 µM. A bacterial suspension in PPB without peptide served as a control. Following incubation at 37 °C for 1 h, LFchimera were removed by two cycles of dilution with PPB and centrifuged, washed and resuspended in PPB. The biofilm-forming capacity was determined according to the method as described previously (Taweechaisupapong et al. 2005). Briefly, 200 µl of each bacterial suspension were added into 96-well polystyrene flat-bottomed plate. Wells containing only PPB served as negative control. Bacteria were incubated aerobically at 37 °C for 3 h to allow adhesion. Next, the supernatant fluid of each well was aspirated gently to remove non-adherent bacteria, and replaced with 200 µl of PPB. After further incubation at 37 °C for an additional 21 h, non-adherent bacteria were removed again and the adherent bacteria were washed with 200 µl of sterilized deionized water and incubation in PPB for an additional 24 h. The supernatant was removed again and the wells were finally washed three times with 200 µl of sterilized deionized water. The attached bacteria, representing a 2-day biofilm culture, were fixed with 200 µl of 99 % methanol for 15 min and the plates were dried at room temperature. The biofilms were stained for 5 min with 200 µl of 2 % Hucker crystal violet. Excess stain was removed with running tap water. The plates were air dried and the dye bound to the adherent biofilm was solubilized with 200 µl of 33 % (v/v) glacial acetic acid per well. The OD in each well was measured at 620 nm using a microplate reader.
Antibacterial activities of LFchimera against B. pseudomallei grown in biofilm
The antibacterial activities of LFchimera against B. pseudomallei grown as a biofilm culture was investigated using a modification of the Calgary Biofilm Device (CBD) as previously described (Ceri et al. 1999; Kanthawong et al. 2012). In brief, the transferable solid-phase (TSP) pin lid (NUNC, Roskilde, Denmark) was placed into the 96-well microtiter plate containing the bacterial inoculum (approximately 107 CFU/ml) and incubated in an orbital incubator at 37 °C, 150 rpm, for 24 h. Following the period of incubation, the TSP pin lid with grown biofilms was rinsed with saline in another microtiter plate for approx. 1 min to remove loosely adherent bacteria and placed into a new 96-well microtiter plate (challenge plate) containing either 20 µM LFchimera, 20 CAZ or 1873 µM CAZ, then incubated at 37 °C for 24 h. The latter concentration of CAZ, 1873 µM which is about 1 mg/ml, was used as this order of magnitude is often used in antibiotic studies (Sawasdidoln et al. 2010; Kanthawong et al. 2012; Anutrakunchai et al. 2015). Antimicrobial agent-free incubations were included for growth control. After that, the TSP pin lid was removed and rinsed with saline in another microtiter plate. After rinsing, the TSP pin lid was placed into the new microtiter plate containing Muller–Hinton broth (recovery plate) and biofilms were disrupted from the TSP pin surface using an ultrasonic cleaner (SONOREX; Bandelin, Berlin, Germany) for 5 min. Viability of the biofilm bacteria was determined by plate counts. Colonies were counted after 24 h incubation at 37 °C. The effects of each concentration of LFchimera and CAZ was calculated as percentage killing using the formula [1 − (CFU sample/CFU control)] × 100 %. Each assay was performed on three separate occasions, with triplicate determinations each time.
Statistical analysis
The efficacy of the test agents against each isolate of B. pseudomallei grown in biofilm was analyzed using Kruskal–Wallis with Mann–Whitney U test to evaluate the differences in antibiofilm efficacy between the CAZ and LFchimera. P values <0.05 were considered as statistically significant.
Results
Antibacterial activities of LFchimera against planktonic B. pseudomallei
Killing activities of LFchimera against planktonic B. pseudomallei varied among the bacterial isolates (Table 2). At LFchimera concentration of 20 µM, 74–100 % killing was observed for all isolates tested. Among the wild-type B. pseudomallei (1026b, NF10/38 and H777), isolate 1026b was the most sensitive to LFchimera while isolate PPKM was the most sensitive among the mutants.
Localization of LFchimera in B. pseudomallei
Cellular localization of LFchimera in B. pseudomallei was achieved by fluorescence microscopy and immunoelectron microscopy. Both techniques used LFchimera in synthesis labeled with FITC, resulting in a labeling stoichiometry of 1:1, without any free FITC remaining. After incubation with 10 μM FITC-LFchimera for 15 min, all B. pseudomallei strains showed a more or less granular labeling intracellularly (data not shown). As shown previously for B. pseudomallei 1026b (Kanthawong et al. 2014) no accumulation of LFchimera in the membrane was observed in all strains tested.
To further specify the localization, bacteria treated with 10 μM FITC-LFchimera were incubated with gold-labeled anti-FITC antibodies and analyzed by TEM. The TEM-micrographs clearly show that all B. pseudomallei strains accumulated gold particles in intracellular structures (Fig. 1). Also here no accumulation in the bacterial membrane was found.
Ultrastuctural effects of LFchimera on B. pseudomallei
TEM micrographs of untreated B. pseudomallei in PPB showed a normal rod shape with an intact inner membrane and slightly waved outer membrane (Fig. 2). Burkholderia pseudomallei treated with 5–20 µM LFchimera showed membrane extrusion, membrane damage, swelling of periplasmic space and non-membrane-enclosed bodies. The bacteria seemed to lose their integrity and showed disordered structures. Cytoplasmic and DNA region appear to be inhomogeneous when compared to the control (Fig. 2). No major differences were found between the wild-type and mutant strains.
Transmission electron micrographs of B. pseudomallei treated with various concentration of LFchimera. B. pseudomallei incubated with PPB (control) showed a normal rod shaped morphology. B. pseudomallei treated with LFchimera showed swelling of periplasmic space of B. pseudomallei cells (arrows) and a highly inhomogeneous electron density in the intracellular DNA region, irregular membranes and a disturbance of the rod shape. Scale bar 500 nm
Effects of LFchimera on B. pseudomallei biofilm formation and established biofilm
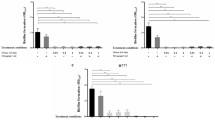
LFchimera at concentration of 1 µM exhibited 8 and 16 % killing activities against B. pseudomallei H777 and NF10/38, respectively, while 40 % killing was observed for isolate 1026b (Table 2). In addition, LFchimera at concentration of 5 µM exhibited <80 % killing activities against all isolates tested. Therefore, those subcidal concentrations of LFchimera (1 and 5 µM) were selected to determine their inhibitory effects on biofilm formation of B. pseudomallei H777, NF10/38 and 1026b after pretreatment of the bacteria with LFchimera for 1 h. The inhibitory effect of LFchimera on biofilm formation of B. pseudomallei appeared to be strain-dependent (Fig. 3). LFchimera at concentrations 1 and 5 µM exhibited 31 and 43 % inhibition on biofilm formation of B. pseudomallei 1026b, respectively, while the same concentrations of LFchimera showed less active against biofilm formation of B. pseudomallei H777 and NF10/38.
The effect of LFchimera against an established biofilm of B. pseudomallei was tested and compared with CAZ. The results showed that both agents affected B. pseudomallei in a strain-dependent killing activity (Fig. 4). LFchimera exhibited a higher killing effect in all isolates when compared to the CAZ-treated group. The average percentage killing activities of 20 µM LFchimera was about 80–90 % for all B. pseudomallei except the isolate 979b. In contrast, all bacterial isolates needed a high concentration of CAZ, 1873 µM, to reach 30–60 % killing activities. Interestingly, low concentration of CAZ (20 µM) increase CFU/ml of B. pseudomallei 1026b, SRM117 and M10 instead of inhibition when compared to untreated control. The difference of % killing between 20 µM LFchimera and 20 µM CAZ was statistically significant (P < 0.05) in all isolates. Even when compared to 1873 µM CAZ, significantly higher killing activities of 20 µM LFchimera were observed in 4 isolates (1026b, SRM117, NF10/38 and PPKM). These results indicated that LFchimera is an effective peptide against both planktonic and biofilm forms of B. pseudomallei and possessed stronger antibiofilm activity than CAZ.
The killing activity of LFchimera against B. pseudomallei in biofilm compared with ceftazidime (CAZ). The biofilm was incubated with tested agents for 24 h and the viability of bacterial cells was determined by plate count technique. Data are presented as the mean and standard deviation of three independent experiments performed in triplicate. *P < 0.05 compared to 20 µM LFchimera
Discussion
From our previous study, we found that LFchimera, a chimerical structure containing LFcin17-30 and LFampin265-284, is very potent against B. pseudomallei (Puknun et al. 2013). In this study, immunoelectron and transmission electron microscopy were used as complementary techniques to gain insight into peptide action, revealing not only cell surface effects but also intracellular alterations. The cellular localization of LFchimera in all strains tested in this study using fluorescence microscopy and immunogold labeling confirmed our previous reports for B. pseudomallei 1026b that LFchimera was found mainly inside bacterial cells with no accumulation in the membranes (Kanthawong et al. 2014). Similar to our study, it has been reported that lactoferricin B (LFcin B), a 25 residue peptide derived from the N-terminal part of bovine lactoferrin, can be traced in the cytoplasm (Haukland et al. 2001) and is capable of inhibiting bacterial macromolecular biosynthesis in both Gram-positive and Gram-negative bacteria (Ulvatne et al. 2004). In this study, TEM images of the bacteria treated with LFchimera showed swelling of periplasmic space and the outer membrane extrusion (Fig. 2). The possible explanation may be that the peptide interacts with the inner membrane leading to a local disruption of the inner membrane and leakage of cytoplasm into the periplasmic space. Moreover, cytoplasmic and DNA region of bacterial cells treated with LFchimera appear to be inhomogeneous when compared to the control. In our previous study, a drastic reduction of intracellular level of nucleotides (AMP, ADP and ATP) was observed after treatment with 20 µM LFchimera for 1 h (Kanthawong et al. 2014). Direct leakage of intracellular nucleotides results in cell-death; whether binding to DNA or other intracellular materials is secondary or the cause of killing needs to be elucidated.
Biofilm formation is a survival strategy of bacteria. Within the biofilm, the bacterial cells are embedded in extracellular polymeric substances (EPS). EPS provides them with increased resistance to antimicrobial agents, and make them recalcitrant to host immune clearance mechanisms and highly difficult to eradicate with the currently available antimicrobial agents (Flemming and Wingender 2010; de la Fuente-Núñez et al. 2013; Kostakioti et al. 2013). Burkholderia pseudomallei is able to form biofilm both in vitro and in vivo (Vorachit et al. 1995). This has been proposed as a possible/main cause of relapse cases in melioidosis patients (Limmathurotsakul et al. 2014). We have demonstrated that B. pseudomallei growing in biofilm form was markedly resistant to several antimicrobial agents such as doxycycline, CAZ, imipenem and trimethoprim/sulfamethoxazole when compared to the corresponding planktonic cells of the same isolates (Sawasdidoln et al. 2010). One proposed mechanism of antibiotic resistance in bacterial biofilm is the slow growth rate and low metabolic activity of bacteria in biofilm because most antimicrobial agents, particularly the β-lactams, primarily target metabolically active cells (Lewis 2001; Walters et al. 2003). In the present study, LFchimera showed promising killing activities against biofilms of all B. pseudomallei isolates as compared to CAZ. A possible explanation may be that the effect of LFchimera is independent of growth, either slow- or even non-growing bacteria, due to its physico-chemical interaction with bio-membranes (Bolscher et al. 2009; Silva et al. 2013). Its ability to permeabilize and/or to form (transient) pores within the cytoplasmic membrane resulting in the fast penetration of the bacteria leading to cell death.
It has been shown that subinhibitory concentrations of antibiotics alter bacterial physiology, including the generation of genetic and phenotypic variability, synthesis and excretion of bacterial metabolites and their rate of growth (Lorian 1993; Andersson and Hughes 2014). From our previously published works, the minimum biofilm elimination concentration of CAZ for B. pseudomallei isolates range from 512 to >2048 µg/ml (Sawasdidoln et al. 2010; Anutrakunchai et al. 2015). In this study, we found that exposing bacteria to subinhibitory concentration of CAZ (20 µM or 10.93 µg/ml) enhance growth of B. pseudomallei 1026b, SRM117 and M10 instead of inhibition. Obviously low concentration of CAZ affect certain biological properties of B. pseudomallei which are able to interfere with some important bacterial cell functions. It is interesting to explore this further, especially because continuous growth in the presence of subinhibitory antibiotic levels is a crucial aspect of the current antibiotic resistance crisis.
In the presents study, B. pseudomallei H777, NF10/38 and 1026b wild type were selected to test the effects of LFchimera on biofilm formation because B. pseudomallei M10 and PPKM mutant were defect in biofilm production (Taweechaisupapong et al. 2005; Tunpiboonsak et al. 2010). It was found that exposure of bacterial cells to subcidal concentration of LFchimera (1 and 5 μM) reduced biofilm forming capacity of the cells. Therefore, LFchimera might be used to prevent B. pseudomallei biofilm associated infection.
Another promising feature of LFchimera is that concentrations up to 20 µM did not show any significant lysis or permeabilization of human red blood cells (Kanthawong et al. 2014) or cytotoxicity to rat hepatocytes (Leon-Sicairos et al. 2009). Recently, LFchimera was proposed to inhibit damage caused by enteropathogenic E. coli (EPEC) in HEp-2 cells by suppressing EPEC attachment to HEp-2 cells and blocking EPEC-mediated actin polymerization. Moreover, LFchimera blocked the hemolysis of human red blood cells produced by EPEC (Flores-Villasenor et al. 2012a).
In conclusion, the present work indicates that LFchimera possessed antibacterial and antibiofilm activities and could modulate B. pseudomallei colonization. Therefore, it is a promising peptide for the prevention and treatment of melioidosis.
References
Andersson DI, Hughes D (2014) Microbiological effects of sublethal levels of antibiotics. Nat Rev Microbiol 12:465–478
Anutrakunchai C, Sermswan RW, Wongratanacheewin S, Puknun A, Taweechaisupapong S (2015) Drug susceptibility and biofilm formation of Burkholderia pseudomallei in nutrient-limited condition. Trop Biomed 32:300–309
Bandeira Tde J, Moreira CA, Brilhante RS, Castelo-Branco Dde S, Neto MP, Cordeiro Rde A, Rodrigues Tde J, Rocha MF, Sidrim JJ (2013) In vitro activities of amoxicillin-clavulanate, doxycycline, ceftazidime, imipenem, and trimethoprim-sulfamethoxazole against biofilm of Brazilian strains of Burkholderia pseudomallei. Antimicrob Agents Chemother 57:5771–5773
Bellamy W, Takase M, Wakabayashi H, Kawase K, Tomita M (1992) Antibacterial spectrum of lactoferricin B, a potent bactericidal peptide derived from the N-terminal region of bovine lactoferrin. J Appl Bacteriol 73:472–479
Bolscher JG, Adao R, Nazmi K, van den Keybus PA, van ‘t Hof W, Nieuw Amerongen AV, Bastos M, Veerman EC (2009) Bactericidal activity of LFchimera is stronger and less sensitive to ionic strength than its constituent lactoferricin and lactoferrampin peptides. Biochimie 91:123–132
Bolscher J, Nazmi K, van Marle J, van ‘t Hof W, Veerman E (2012) Chimerization of lactoferricin and lactoferrampin peptides strongly potentiates the killing activity against Candida albicans. Biochem Cell Biol 90:378–388
Ceri H, Olson ME, Stremick C, Read RR, Morck D, Buret A (1999) The Calgary Biofilm Device: new technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. J Clin Microbiol 37:1771–1776
Cheng AC, Currie BJ (2005) Melioidosis: epidemiology, pathophysiology, and management. Clin Microbiol Rev 18:383–416
Dance D (2014) Treatment and prophylaxis of melioidosis. Int J Antimicrob Agents 43:310–318
de la Fuente-Núñez C, Reffuveille F, Fernández L, Hancock REW (2013) Bacterial biofilm development as a multicellular adaptation: antibiotic resistance and new therapeutic strategies. Curr Opin Microbiol 16:580–589
DeShazer D, Brett PJ, Carlyon R, Woods DE (1997) Mutagenesis of Burkholderia pseudomallei with Tn5-OT182: isolation of motility mutants and molecular characterization of the flagellin structural gene. J Bacteriol 179:2116–2125
DeShazer D, Brett PJ, Woods DE (1998) The type II O-antigenic polysaccharide moiety of Burkholderia pseudomallei lipopolysaccharide is required for serum resistance and virulence. Mol Microbiol 30:1081–1100
Flemming H-C, Wingender J (2010) The biofilm matrix. Nat Rev Microbiol 8:623–633
Flores-Villasenor H, Canizalez-Roman A, Reyes-Lopez M, Nazmi K, de la Garza M, Zazueta-Beltran J, Leon-Sicairos N, Bolscher JG (2010) Bactericidal effect of bovine lactoferrin, LFcin, LFampin and LFchimera on antibiotic-resistant Staphylococcus aureus and Escherichia coli. Biometals 23:569–578
Flores-Villasenor H, Canizalez-Roman A, de la Garza M, Nazmi K, Bolscher JG, Leon-Sicairos N (2012a) Lactoferrin and lactoferrin chimera inhibit damage caused by enteropathogenic Escherichia coli in HEp-2 cells. Biochimie 94:1935–1942
Flores-Villasenor H, Canizalez-Roman A, Velazquez-Roman J, Nazmi K, Bolscher JG, Leon-Sicairos N (2012b) Protective effects of lactoferrin chimera and bovine lactoferrin in a mouse model of enterohaemorrhagic Escherichia coli O157:H7 infection. Biochem Cell Biol 90:405–411
Haney EF, Lau F, Vogel HJ (2007) Solution structures and model membrane interactions of lactoferrampin, an antimicrobial peptide derived from bovine lactoferrin. Biochim Biophys Acta (BBA)-Biomembr 1768:2355–2364
Haukland HH, Ulvatne H, Sandvik K, Vorland LH (2001) The antimicrobial peptides lactoferricin B and magainin 2 cross over the bacterial cytoplasmic membrane and reside in the cytoplasm. FEBS Lett 508:389–393
Kanthawong S, Nazmi K, Wongratanacheewin S, Bolscher JG, Wuthiekanun V, Taweechaisupapong S (2009) In vitro susceptibility of Burkholderia pseudomallei to antimicrobial peptides. Int J Antimicrob Agents 34:309–314
Kanthawong S, Bolscher JG, Veerman EC, van Marle J, de Soet HJ, Nazmi K, Wongratanacheewin S, Taweechaisupapong S (2012) Antimicrobial and antibiofilm activity of LL-37 and its truncated variants against Burkholderia pseudomallei. Int J Antimicrob Agents 39:39–44
Kanthawong S, Puknun A, Bolscher JG, Nazmi K, van Marle J, de Soet JJ, Veerman EC, Wongratanacheewin S, Taweechaisupapong S (2014) Membrane-active mechanism of LFchimera against Burkholderia pseudomallei and Burkholderia thailandensis. Biometals 27:949–956
Kostakioti M, Hadjifrangiskou M, Hultgren SJ (2013) Bacterial biofilms: development, dispersal, and therapeutic strategies in the dawn of the postantibiotic era. Cold Spring Harb Perspect Med 3:a010306
Lam J, Chan R, Lam K, Costerton JW (1980) Production of mucoid microcolonies by Pseudomonas aeruginosa within infected lungs in cystic fibrosis. Infect Immun 28:546–556
Leon-Sicairos N, Canizalez-Roman A, de la Garza M, Reyes-Lopez M, Zazueta-Beltran J, Nazmi K, Gomez-Gil B, Bolscher JG (2009) Bactericidal effect of lactoferrin and lactoferrin chimera against halophilic Vibrio parahaemolyticus. Biochimie 91:133–140
Lewis K (2001) Riddle of biofilm resistance. Antimicrob Agents Chemother 45:999–1007
Limmathurotsakul D, Peacock SJ (2011) Melioidosis: a clinical overview. Br Med Bull 99:125–139
Limmathurotsakul D, Paeyao A, Wongratanacheewin S, Saiprom N, Takpho N, Thaipadungpanit J, Chantratita N, Wuthiekanun V, Day NP, Peacock SJ (2014) Role of Burkholderia pseudomallei biofilm formation and lipopolysaccharide in relapse of melioidosis. Clin Microbiol Infect 20:O854–O856
Lorian V (1993) Medical relevance of low concentrations of antibiotics. J Antimicrob Chemother 31(Suppl D):137–148
Pibalpakdee P, Wongratanacheewin S, Taweechaisupapong S, Niumsup PR (2012) Diffusion and activity of antibiotics against Burkholderia pseudomallei biofilms. Int J Antimicrob Agents 39:356–359
Puknun A, Bolscher JG, Nazmi K, Veerman EC, Tungpradabkul S, Wongratanacheewin S, Kanthawong S, Taweechaisupapong S (2013) A heterodimer comprised of two bovine lactoferrin antimicrobial peptides exhibits powerful bactericidal activity against Burkholderia pseudomallei. World J Microbiol Biotechnol 29:1217–1224
Rotz LD, Khan AS, Lillibridge SR, Ostroff SM, Hughes JM (2002) Public health assessment of potential biological terrorism agents. Emerg Infect Dis 8:225–230
Sawasdidoln C, Taweechaisupapong S, Sermswan RW, Tattawasart U, Tungpradabkul S, Wongratanacheewin S (2010) Growing Burkholderia pseudomallei in biofilm stimulating conditions significantly induces antimicrobial resistance. PLoS One 5:e9196
Shin K, Yamauchi K, Teraguchi S, Hayasawa H, Tomita M, Otsuka Y, Yamazaki S (1998) Antibacterial activity of bovine lactoferrin and its peptides against enterohaemorrhagic Escherichia coli O157:H7. Lett Appl Microbiol 26:407–411
Silva T, Adao R, Nazmi K, Bolscher JG, Funari SS, Uhrikova D, Bastos M (2013) Structural diversity and mode of action on lipid membranes of three lactoferrin candidacidal peptides. Biochim Biophys Acta 1828:1329–1339
Taweechaisupapong S, Kaewpa C, Arunyanart C, Kanla P, Homchampa P, Sirisinha S, Proungvitaya T, Wongratanacheewin S (2005) Virulence of Burkholderia pseudomallei does not correlate with biofilm formation. Microb Pathog 39:77–85
Tunpiboonsak S, Mongkolrob R, Kitudomsub K, Thanwatanaying P, Kiettipirodom W, Tungboontina Y, Tungpradabkul S (2010) Role of a Burkholderia pseudomallei polyphosphate kinase in an oxidative stress response, motilities, and biofilm formation. J Microbiol 48:63–70
Ulvatne H, Samuelsen O, Haukland HH, Kramer M, Vorland LH (2004) Lactoferricin B inhibits bacterial macromolecular synthesis in Escherichia coli and Bacillus subtilis. FEMS Microbiol Lett 237:377–384
Valenti P, Antonini G (2005) Lactoferrin: an important host defence against microbial and viral attack. Cell Mol Life Sci 62:2576–2587
Van Der Kraan MIA, Groenink J, Nazmi K, Veerman ECI, Bolscher JGM, Nieuw Amerongen AV (2004) Lactoferrampin: a novel antimicrobial peptide in the N1-domain of bovine lactoferrin. Peptides 25:177–183
Van der Kraan MI, Nazmi K, Teeken A, Groenink J, van ‘t Hof W, Veerman EC, Bolscher JG, Nieuw Amerongen AV (2005) Lactoferrampin, an antimicrobial peptide of bovine lactoferrin, exerts its candidacidal activity by a cluster of positively charged residues at the C-terminus in combination with a helix-facilitating N-terminal part. Biol Chem 386:137–142
Van Der Kraan MIA, Nazmi K, Van’t Hof W, Nieuw Amerongen AV, Veerman ECI, Bolscher JGM (2006) Distinct bactericidal activities of bovine lactoferrin peptides LFampin 268-284 and LFampin 265-284: Asp-Leu-Ile makes a difference. Biochem Cell Biol 84:358–362
Vorachit M, Lam K, Jayanetra P, Costerton JW (1995) Electron microscopy study of the mode of growth of Pseudomonas pseudomallei in vitro and in vivo. J Trop Med Hyg 98:379–391
Walters MC 3rd, Roe F, Bugnicourt A, Franklin MJ, Stewart PS (2003) Contributions of antibiotic penetration, oxygen limitation, and low metabolic activity to tolerance of Pseudomonas aeruginosa biofilms to ciprofloxacin and tobramycin. Antimicrob Agents Chemother 47:317–323
White NJ (2003) Melioidosis. Lancet 361:1715–1722
Wiersinga WJ, Currie BJ, Peacock SJ (2012) Melioidosis. N Engl J Med 367:1035–1044
Acknowledgments
This work was supported by the Commission on Higher Education granting under the CHE-Ph.D.-SW and the Higher Education Research Promotion and National Research University Project of Thailand, Office of the Higher Education Commission, through the Health Cluster (SHeP-GMS), Khon Kaen University. JGMB, KN and ECIV are supported by a grant from the University of Amsterdam for research into the focal point Oral Infections and Inflammation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Puknun, A., Kanthawong, S., Anutrakunchai, C. et al. Ultrastructural effects and antibiofilm activity of LFchimera against Burkholderia pseudomallei . World J Microbiol Biotechnol 32, 33 (2016). https://doi.org/10.1007/s11274-015-1988-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-015-1988-x