Abstract
The insecticidal cry genes of Bacillus thuringiensis (Bt) have been successfully used for development of insect resistant transgenic rice plants. In this study, a novel cry2AX1 gene consisting a sequence of cry2Aa and cry2Ac gene driven by rice rbcS promoter was introduced into a rice cultivar, ASD16. Among 27 putative rice transformants, 20 plants were found to be positive for cry2AX1 gene. The expression of Cry2AX1 protein in transgenic rice plants ranged from 5.95 to 122.40 ng/g of fresh leaf tissue. Stable integration of the transgene was confirmed in putative transformants of rice by Southern blot hybridization analysis. Insect bioassay on T0 transgenic rice plants against rice leaffolder (Cnaphalocrosis medinalis) recorded larval mortality up to 83.33 %. Stable inheritance and expression of cry2AX1 gene in T1 progenies was demonstrated using Southern and ELISA. The detached leaf bit bioassay with selected T1 plants showed 83.33–90.00 % mortality against C. medinalis. The whole plant bioassay for T1 plants with rice leaffolder showed significant level of resistance even at a lower level of Cry2AX1 expression varying from 131 to 158 ng/g fresh leaf tissue during tillering stage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rice (Oryza sativa L.,) is one of the most important food crops in the world with more than half of the world population (nearly 3 billion people) depending on it as the primary staple food. Rice cultivation and consumption is mainly concentrated in the developing Asian countries, contributing more than 90 % of the world rice cultivation area. With ever increasing population and decreasing area of cultivable land the demand for rice continues to increase without any sustainable increment in its production. Rice yield has been immensely improved after the green revolution but the yield potential of improved varieties is not actually achieved in farmers’ field conditions. This is due to the fact that the productivity is adversely impacted by numerous biotic and abiotic factors. Approximately 52 % of the global production of rice is lost annually owing to the damage caused by biotic factors, of which almost 21 % is attributed to the attack of insect pests (Brookes and Barfoot 2003).
The major constraints in rice production, throughout the rice growing countries of the world, are the insect pests among which the most serious ones are lepidopterans viz. yellow stemborer (YSB) (Scirpophaga incertulas Walker), striped stemborer (SSB) (Chilo supressalis Walker) and rice leaffolder (RLF) (Cnaphalocrosis medinalis Guenee). Rice stem borers and leaffolder are potentially the most destructive insect pests, causing up to 30 and 10 % on an average respectively each year (Krishnaiah and Varma 2012). The use of chemical insecticides against these insects has not been found much effective mainly because of their nature of life cycle. Also chemical insecticide increases the cost of production, environmental pollution, and damage to the ecosystem. Moreover the harmful effects caused by the reckless application of the pesticides to control these pests pose threat to human health and biodiversity (Pingali and Roger 1995). Perhaps the most economic and efficient measure to protect rice from insect pests is the production of insect resistant rice varieties.
However the development of insect resistant lines in rice through conventional breeding has been a challenge as the existing rice varieties could not provide sufficient levels of insect resistance, which is mainly due to the scarcity in the diversity of germplasm. So, an alternative and the most attractive strategy is to employ the tools of genetic engineering to produce insecticidal proteins through the introduction of corresponding insecticidal genes into rice. Genetic engineering overcomes incompatibility barriers between different crop species (Muthukrishnan et al. 2001). Several crops such as tomato, cotton, maize and rice have been successfully transformed with different versions of crystal protein gene of B. thuringiensis (Christou et al. 2006).
Recent progress in rice transformation technologies has made it possible to produce genetically engineered (GE) rice cultivars with improved resistance to insect pests. The first transgenic rice plant with insect resistant Bt protein was reported by Fujimoto et al. (1993). Thereafter, many rice varieties have been transformed with genes encoding various Bt crystal proteins and have been shown to be resistant to one or more lepidopteran insect pests of rice, the most important of which are YSB, RLF and SSB (Nayak et al. 1997; Tu et al. 2000; Ye et al. 2003; Ramesh et al. 2004; Bashir et al. 2005; Kim et al. 2008; Xia et al. 2011; Yang et al. 2014; Wang et al. 2014). First field trial of Bt rice started in China in 1998 (Tu et al. 2000; Shu et al. 2000) and in 2009 China’s Ministry of Agriculture issued biosafety certificates for limited commercialization trial in Hubei Province in China for a 5-year period, 2009–2014 (Chen et al. 2011).
A major concern of Bt mediated insect resistant plants is the continuous use of similar Bt toxins against a target insect pest leading to breakdown of resistance. However, insects that develop resistance against one protein (Cry1A) are not cross-resistant to another (Cry2A) protein (Tabashnik et al. 2000). So, pyramiding of two or more Bt genes with different modes of action, is one of the strategies to delay the resistance development in insects. Commercial Bt crops expressing Cry1Ab, Cry1Ac, Cry1F, Cry2Ab and Cry3Bb proteins, either single or in combination, with different modes of action are now being grown worldwide for protection against a variety of insect pests. Similarly, hybrid Bt toxins produced through inclusion of a domain from another toxin result in increased potency of the fused protein (Bosch et al. 1994). A novel chimeric Bt gene cry2AX1 was constructed by using the sequences of cry2Aa and cry2Ac cloned from indigenous isolates of Bt (Udayasuriyan et al. 2010).
Another concern is the expression of recombinant protein in unwanted tissue (e.g. grains). Transgene driven by tissue specific promoter will be expressed only in tissues where the transgene product is desired, leaving the rest of the tissues unmodified by transgene expression. Many experiments demonstrated that rbcS is a tissue-specific expression gene, especially present in green tissue and its expression pattern is regulated by light (Kyozuka et al. 1993; Huang et al. 1999; Nomura et al. 2000). Several earlier studies also showed that the Bt gene driven by green tissue specific rbcS promoter was successfully expressed in leaves and stems, but not in the seeds (Kim et al. 2009; Ye et al. 2009; Rawat et al. 2011).
In this background, attempts were made in the present study to generate transgenic indica rice cultivar, ASD16 with cry2AX1 gene driven by green tissue specific promoter with chloroplast transit peptide sequence for targeting Cry2AX1 protein in chloroplast and testing the efficacy against rice leaffolder.
Materials and methods
Construction of expression vectors and rice transformation
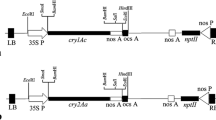
Codon optimized synthetic cry2AX1 gene (Accession No. GQ332539.1) under the control of green tissue specific rbcS-tp promoter (Jang et al. 1999) and nos terminator (3.46 kb), was cloned into the HindIII and SalI sites of the pCAMBIA1300 vector containing hpt (coding for hygromycin phosphotransferase) as a plant selectable marker gene, and the resultant plasmid was designated as pC1300-rbcS-tp-2AX1 (Fig. 1). This construct was mobilized, into the Agrobacterium strain LBA4404 for rice transformation.
T-DNA region of plant transformation construct pC1300-rbcS-tp-2AX1. The synthetic cry2AX1 gene is driven by a rice rbcS promoter and its transit peptide (tp) and terminated by the nopaline synthase (nos) terminator. The plant selectable marker gene, hptII is under the control of the CaMV35S promoter and tailed by the CaMV35S polyA. LB left border of T-DNA region, RB right border of T-DNA region
Agrobacterium-mediated rice transformation procedure suggested by Hiei and Komari (2008) was followed. The immature embryos of ASD16 were placed on cocultivation medium containing acetosyringone 100 mM and 5 µl of the Agrobacterium suspension was dropped on each embryo. The infected immature embryos were incubated at 25 °C in the dark for 7 days. Well developed embryogenic calli from co-cultivated immature embryos were sub-cultured twice on resting medium containing cefotaxime 250 mg l−1 for 15 days at 30 °C under continuous illumination. The proliferated embryogenic calli were sub-cultured twice on selection medium containing hygromycin 50 mg 1−1 and cefotaxime 250 mg l−1 for 17 days. The hygromycin resistant calli were transferred to pre-regeneration medium containing hygromycin 40 mg l−1 for 7 days. The proliferated calli with green spots were cultured on regeneration medium containing 30 mg l−1 hygromycin. The regenerated plantlets were transferred to half strength MS medium containing hygromycin 30 mg l−1 for rooting. Finally, the well developed rooted plants were transferred to soil in pots and grown to maturity in a transgenic greenhouse (Fig. 2).
Agrobacterium-mediated transformation of rice (Oriza sativa L. cv. ASD16). a Pre-treated immature embryos infected with Agrobacterium on cocultivation medium. b Immature embryos after cocultivation. c Subcultured calli on resting medium. d Callus proliferation on selection medium. e Embryogenic calli on pre-regeneration medium. f Regenerated transgenic rice plants. g Pre-hardening of transgenic rice plants in greenhouse. h Established transgenic rice plants in greenhouse
PCR and Southern blot analyses
PCR analysis were performed to demonstrate the presence of cry2AX1 and hptII genes in putative transgenic lines of ASD16 using gene specific primer (2AX1FP, 5′-CCTAACATTGGTGGACTTCCAG-3′ and 2AX1RP, 5′-GAGAAACGAGCTCCGTTATCGT-3′); (HPTFP, 5′-GCTGTTATGCGGCCATTGGTC-3′; HPTRP, 5′ GACGTCTGTCGAGAAGTTTG-3′) (Jayaprakash et al. 2014). These primers amplify 800 and 630 bp internal fragments from cry2AX1 and hptII genes, respectively. The plasmid DNA (pC1300-rbcS-tp-2AX1) was used as positive control and DNA isolated from non-transformed control plants was used as negative control. The amplified PCR products were resolved on 1.2 % agarose gel, visualized on UV transilluminator upon ethidium bromide staining.
Total genomic DNA was extracted from leaf tissue of transgenic and control plants using the method described by Dellaporta et al. (1983). For Southern blot analysis, 5 µg of genomic DNA was digested with KpnI, which has a recognition sequence at one end of the T-DNA. The digested products were separated on a 0.8 % agarose gel, and then transferred to a nylon membrane using 20X SSC following standard capillary transfer protocol. The transferred DNA was cross-linked by a UV crosslinker at 1200 μJ min−1 for 1 min. The prehybridisation was carried out for 1 h and hybridization for 18 h at 60 °C. For hybridization, PCR amplified 800 bp internal region of cry2AX1 gene was used as a probe. The probe DNA was labelled with α32P dCTP using Decalabel DNA labelling kit (Thermo Fischer Scientific Inc.) and added to hybridization solution. After hybridization, the blot was washed with 3X SSC + 0.1 % SDS and 2X SSC + 0.1 % SDS for 15 min each, followed by 10 min in 0.5X SSC + 0.1 % SDS. All washings were carried out at 60 °C and the blot was exposed to X-ray film.
Detection of Cry2AX1 protein in transgenic rice plants
Fresh leaf tissue (30 mg) from transgenic and wild type ASD16 plants were collected separately, homogenized in 500 µl of extraction buffer, spun at 3000 g at 4 °C for 10 min and 100 µl of the supernatant was immediately used for assay. Each sample was replicated twice. Cry2AX1 protein expression in transgenic rice plants was determined by ELISA kit (Envirologix, USA) following standard procedures. The protein concentration was calculated on a linear standard curve, using the standards provided in the kit.
Detached leaf bit and whole plant bioassay
Adults of rice leaffolder were collected from the rice field at Paddy Breeding Station, TNAU, Coimbatore and they were released on TN1 rice plants maintained in insect cages (65 cm × 65 cm × 75 cm) for culturing. The adult moths were provided 10 % honey solution to enhance the egg laying capacity. After two generations, the neonates of C. medinalis larvae were used for the bioassay. Leaves of transgenic plants were cut into pieces (about 3 cm length) and three leaf bits were placed on a moist filter paper in a plastic petriplate. Ten neonate larvae of rice leaffolder were released in each petriplate. A control was maintained using leaf bits collected from non-transgenic rice ASD16. Three replications were maintained and the experiment was carried out at 25 °C ± 1, 60 % relative humidity. Larval growth and mortality was recorded every day up to 5 days. After 5 days, leaf area damage and surviving larval characteristics were evaluated in transgenic as well as control plants. Percentage of leaf area damage was calculated using the formula:
The whole plant bioassay was carried out with neonates and second instar larvae of C. medinalis. The 60 day old transgenic and control plants were kept in separate bioassay cages (75 cm × 75 cm × 100 cm) and 20 numbers of neonate or second instar larvae of C. medinalis were released on each treatment. The transgenic plants were evaluated 17 days after infestation. For transgenic and control plants, the level of insect mortality was recorded. The number of damaged leaves along with undamaged ones was recorded and % infestation was determined using the following formula.
Statistical analysis
The larval mortality and concentration of Cry2AX1 protein data were subjected to arc sine and square root transformations respectively, before analysis. Data analysis was done by analysis of variance (ANOVA) following the AGRES statistical package. Values for concentration of Cry2AX1 protein and mortality of rice leaffolder are reported as mean ± SD. The association between protein expression and insect mortality was studied by correlation analysis using the Statistical Package for Social Studies (SPSS) software version 16.
Results
Agrobacterium mediated transformation
The cry2AX1 gene was introduced into immature embryo of the ASD16 cultivar by Agrobacterium mediated method. A total 42 embryogenic calli were selected after two rounds of hygromycin selection, the selected embryogenic calli were transferred to pre-regeneration medium followed by regeneration medium for shoot induction. Finally, 27 putative transgenic rice lines were obtained and transferred to greenhouse for molecular analysis.
Molecular analysis
Total genomic DNA from putative rice transformants was subjected to PCR analysis with hptII and cry2AX1 gene specific primers. Out of 27 plants regenerated, 20 were found to be positive for the amplification of ~800 and ~630 bp internal sequences of cry2AX1 and hpt genes, respectively (Fig. 3). A similar band was also observed in positive control (pC1300-rbcS-tp-2AX1), whereas untransformed control plant did not show any amplification.
PCR analysis of cry2AX1 transgenic rice plants. a A 800 bp internal sequence of cry2AX1 gene was amplified by PCR from the DNA isolated from putative transgenic plants. Lanes 1 and 25 100 bp marker, Lanes 2–21 Putative transgenic plants of ASD16, Lane 22 Non transformed control plant, Lane 23 Negative control (water), Lane 24 pC1300-rbcS-tp-2AX1 plasmid as a positive control. b A 630 bp internal sequence of hptII gene was amplified by PCR from the DNA isolated from putative transgenic plants. Lane 1 and 25 100 bp marker, Lanes 2–21 Putative transgenic plants of ASD16, Lane 22 Non transformed control plant, Lane 23 Negative control (water), Lane 24, pC1300-rbcS-tp-2AX1 plasmid as a positive control
Southern hybridization analysis was performed to confirm T-DNA integration. Genomic DNA from ELISA positive T0 transgenic rice lines was digested by KpnI enzyme and subjected to Southern blot hybridization using [α-32P]- dCTP labelled 800 bp of cry2AX1 probe. The results showed a single hybridization signal in seven of the 19 plants analyzed (Rb 2, Rb 4, Rb 5, Rb 6, Rb 7, Rb 8 and Rb 20) whereas the remaining 12 plants had two to five signals. In addition to expected bands, a few unexpected hybridization signals, which were lesser than the expected size were observed (Fig. 4).
Southern blot analysis of T0 transgenic rice plants expressing cry2AX1 gene. DNA sample isolated from transgenic and non-transgenic plants digested with KpnI restriction enzyme, fractionized by electrophoresis, transferred to nylon membrane and allowed to hybridize with a radioactively labelled 800 bp internal sequence of cry2AX1 gene. a M λ/HindIII marker, Lanes 1–10 Genomic DNA from transgenic rice plants Rb1, Rb2, Rb3, Rb4, Rb5, Rb6, Rb7, Rb8 and Rb9, respectively, Lane 10 Non-transformed control plant, Lane 11 Positive control pC1300-rbcS-tp-2AX1. b M λ/HindIII marker, Lanes 1–10 Genomic DNA from transgenic rice plants Rb10, Rb11, Rb12, Rb13, Rb14, Rb15, Rb16, Rb17, Rb18 and Rb20, respectively, Lane 11 Non-transformed control plant, Lane 12 Positive control pC1300-rbcS-tp-2AX1
Expression analysis
Among the 20 PCR positive rice lines screened by Cry2A quantitative ELISA kit, 19 were found positive for the expression of Cry2AX1 protein in young rice leaf tissue. The expression of Cry2AX1 protein in these transgenic rice lines ranged from 5.95 ± 0.15 to 122.40 ± 2.40 ng/g fresh leaf tissue indicating expression of cry2AX1 gene in transgenic rice lines (Table 1).
Insect bioassay
In order to determine the insecticidal activity of Cry2AX1 protein in transgenic rice plants, detached leaf bit bioassay carried out using neonate larvae of rice leaffolder on the ELISA positive plants showed larval mortality ranging from 20.00 ± 0.00 to 83.33 ± 5.77 % (Table 1). A significant, positive correlation was found between the concentration of Cry2AX1 protein and insect mortality in T0 transgenic plants (r = 0.942, p = 0.01). There was no larval mortality on control plants and the major portion of the leaf tissue was consumed by the surviving larvae over a period of 5 days (Fig. 5).
Analysis of the T1 generation plants
Thirty plants of T1 progenies derived from Rb16 (selected based on relatively higher level expression of Cry2AX1 protein in T0 generation) were established in the greenhouse for further analyses of gene inheritance, expression and efficacy. An amplicon of ~800 bp was found in all the T1 progenies for the presence of cry2AX1 gene, whereas no amplification was found in non-transgenic control plant. Among the 30 PCR positive T1 plants of Rb16, five were subjected to analysis of Cry2AX1 expression at three phenological stages viz, vegetative, tillering and reproductive and the expression varied from 131.00 ± 5.00 to 236.00 ± 0.00 ng/g fresh leaf tissue, 47.20 ± 0.80 to 158.40 ± 0.00 and 36.08 ± 0.00 to 124.00 ± 0.80 ng/g, respectively (Table 2). The Cry2AX1 protein was not detected in seeds of transgenic plants. Southern blot hybridization analysis of selected T1 progenies (Rb 16-8 and Rb 16-9) showed four and five hybridization signals of ~5.6, ~6.0, ~7.5, ~10.0 and ~14.0 kbp size (Fig. 6).
Southern blot analysis of T1 transgenic rice plants expressing cry2AX1 gene. DNA digested with KpnI and probed with a radioactively labelled 800 bp internal sequence of cry2AX1 gene. M λ/HindIII marker, Lane 1 Rb 16-8, Lane 2 Rb 16-9, Lane 3 Control plant, Lane 4, Positive control pC1300-rbcS-tp-2AX1
Detached leaf bit bioassay for two T1 plants (which expressed relatively higher levels of Cry2AX1 protein) were tested for efficacy of Cry2AX1 protein against the neonates of rice leaffolder. Mortality in rice leaffolder varied from 83.33 ± 2.35 to 90.00 ± 0.00 % in the transgenic rice plants (Table 3). The percentage of leaf area damaged by rice leaffolder larvae varied from 10.68 ± 0.43 to 17.51 ± 0.85 in the transgenic lines compared to 92.73 ± 0.43 % in control plants (Supplementary Figure 1).
Whole plant bioassay was performed on two T1 plants Rb 16-9 and Rb 16-8 against the neonates and second instar larvae of rice leaffolder, respectively (Tables 4, 5). Mortality was recorded after 17 days. The transgenic rice lines showed 60 and 40 % mortality against neonate and second instar larvae of rice leaffolder whereas no mortality was recorded in the non-transgenic control. In case of neonates, the leaf infestation was 22.22 and 76.47 % in transgenic line and non-transgenic control, respectively. In second instar larvae, leaf infestation were 29.82 and 80.76 % in transgenic and control plants, respectively. Inhibition of growth was observed in larval survivors which fed on transgenic lines compared to normal growth in non-transgenic control (Fig. 7).
Discussion
One of the major concerns in using constitutive promoter is that the expression is throughout the plant which includes tissues which are not fed by insects. Compared with the temporal or spatial specific expression, the constitutive expression of foreign proteins in transgenic plants may cause adverse effects, such as the metabolic burden imposed on plants for constant synthesis of foreign gene products and may increase the potential risk of resistance of the target insects to Bt. There is also undue concern about the food safety of genetically modified plants. Therefore, in certain circumstances, it is desirable to use tissue-specific promoters which drive the expression of foreign gene in specific plant tissues or organs. The rbcS gene, which encodes the small subunit of ribulose-bisphosphate carboxylase (Rubisco), is expressed only in leaf mesophyll cells. Indeed, the expression of the Bt gene by tissue-specific promoters enhanced the rice resistance to insects. Kim et al. (2009) reported that use of rbcS promoter-transit peptide sequence in transgenic rice increased the cry1Ac transcript and protein level by 25- and 100-fold, respectively. Similarly, Ye et al. (2009) reported that the insect resistant gene, Cry1C under rice rbcS promoter was transformed into Zhonghua 11 (Oryza sativa L. ssp. japonica) and transgenic plants were resistant to yellow stem borer, striped stem borer and leaffolder. But the levels of Cry1C were undetectable in endosperm. In this direction an attempt was made to express the cry2AX1 gene by green tissue specific rice rbcS promoter and target the expressed Cry2AX1 protein to chloroplast using their own transit peptide and to study its efficacy in transgenic rice plants.
Agrobacterium mediated transformation was used for introducing the synthetic cry2AX1 gene in rice. Out of the twenty-seven putative transformants of rice generated under hygromycin selection, 20 plants were found to be positive for the cry2AX1 gene in PCR analysis and nineteen of them were found positive in ELISA for expression of Cry2AX1 protein in leaf tissue. The concentration of Cry2AX1 protein varied among the transformants from 5.95 to 122.40 ng/g fresh leaf tissue. Bioassay of selected T0 transgenic plants showed significant level of mortality in neonates of rice leaffolder ranging from 20.00 to 83.33 %. The bioassay results indicated considerable variation in the level of insect resistance among the independent T0 transgenic rice lines. The highest larval mortality of 83.33 % was observed on transgenic plant Rb16. The differences in the level of toxicity (morality) observed among the different transgenic lines could be attributed to differences in the level of Bt gene expression whereas non transformed control plants did not show any mortality and major portion of leaf tissue was consumed by surviving larvae. The surviving larvae fed on transgenic leaf bits were severely stunted in growth. Correlation between the Cry2AX1 protein expression and larval mortality showed a highly significant positive relationship (r = 0.942 at 1 percent level) indicating that higher the concentration of Cry2AX1 protein has resulted in higher mortality of the larvae. Transformants expressing higher levels of Cry2AX1 protein invariably induced higher larval mortality. The result of bioassay suggests that the Cry2AX1 protein produced in T0 transgenic rice plants is functionally active.
The ELISA positive T0 plants were further analyzed by Southern using the coding sequences of cry2AX1 gene as a probe for T-DNA integration analysis. Southern results indicated that the transgene was inserted in three to five locations of the rice genome in most of the analyzed plants, although in a few cases only one insertion was found. However, smaller in size of hybridizing signal (~3.5 kb) was also observed in few cases of transgenic lines. The smaller fragment in Rb12 and Rb18 might be a result of the transfer of a truncated T-DNA. The difference in size and banding pattern of T-DNA integration into the rice genome indicated that they were derived from different independent transformants and not from entophytic Agrobacterium contamination. It was also observed that the expression of Cry2AX1 transgenic plants appeared to be dependent on the number of cry2AX1 gene integration into the plant genome. The transgenic plant Rb16 with five insertion of cry2AX1 gene, expressed relatively higher amounts of Cry2AX1 protein (122.40 ng/g) compared to the other plants with one to four insertion of the transgene. Further studies on the regions flanking the transgene insertion sites in the plant genome might be useful in assessing any relationship between the integration number and the transgene expression levels. Zaidi et al. (2009) also reported that the level of Cry1C expression was higher in transgenic rice plant with three copies of cry1C gene when compared to single copy plants. In general, multiple integration of gene might cause unstable inheritance or transgene silence. Therefore, transgenic plants with the single insertion are more important for stable resistance to pest (Ye et al. 2009).
The stable inheritance of cry2AX1 gene was demonstrated by progenies analysis through PCR. The Mendelian segregation ratio could not be determined due to smaller size (30) of the T1 progeny of the plants and also due to multiple integration of cry2AX1 gene. Southern hybridization analysis revealed four and five integrations of cry2AX1 gene in two of the T1 progenies tested (event Rb 16).
The expression of Cry2AX1 protein in transgenic rice plants was analyzed at different phenological stages to measure the temporal variation of Cry protein. The expression of Cry2AX1 protein was relatively higher in vegetative stage followed by tillering and reproductive stage. The expression was drastically decreased in tillering stage when compared to vegetative stage. One of the progenies Rb16-8 recorded maximum level of expression of Cry2AX1 protein i.e., 236.00, 158.40 and 124.00 ng/g fresh leaf tissue during vegetative, tillering and reproductive stage, respectively. The ELISA results indicated that the expression of Cry2AX1 protein in leaves decreased towards the end of growing season. Similar results were also reported in previous researches on Bt rice (Wu et al. 2001; Zhao et al. 2004; Han et al. 2009). Wan et al. (2005) reported that expression pattern of Bt toxin in progenies of cotton varies within the season with higher concentration at the beginning and lower at the latter stages. The mechanisms of variation in Cry protein concentration in plants are rather complicated. The possible reason for reduction of Bt protein expression is that the levels of cry gene transcripts decreased in plant tissues as they mature and is likely because of decreased activity of the promoters, which is probably hindered by adverse physiological conditions in the rice plants (Christensen et al. 1992; Han et al. 2009). Bakhsh et al. (2012) reported that the reduction of Bt protein content in later season of cotton tissue could be attributed to the over expression of the Bt gene at earlier stage, which leads to gene regulation at post-transcriptional level and consequently results in gene silencing at a later stage.
In this study the expression of Cry2AX1 protein was detected only in the leaves of the transgenic plants and not in the seeds. Therefore the expression cry2AX1 gene driven by the rbcS promoter and transit peptide could help to reduce the release of Bt toxin into the environment through the seeds and pollen of transgenic plants. Similar results were also reported by earlier workers, when the cry1C and cry1Ac genes driven by the rbcS promoter were transformed into rice (Ye et al. 2009; Kim et al. 2009; Qi et al. 2012; Yang et al. 2014).
The detached leaf bioassay against neonate larvae of rice leaffolder on selected T1 transgenic rice plants expressing the Cry2AX1 protein indicated that Rb 16-8 recorded the highest mortality of 90 % followed by Rb 16-9 (83.33 %). The mortality data correlated well with data recorded for leaf area damage. The transgenic plants showed low level of leaf damage which ranged from 10.68 to 17.51 % whereas in non-transformed control plant the level of leaf damage was significantly higher (92.73 %). The present study indicated that neonate larvae were highly susceptible to Cry2AX1 protein even at low concentration. Earlier workers reported that Bt crops (Davidson et al. 2002; Kranthi et al. 2005, Meiyalaghan et al. 2006; Katageri et al. 2007; Zaidi et al. 2009; Jafari et al. 2009) offered significant level of protection against neonate larvae. Tang et al. (2006) also reported that transgenic indica rice plants expressing cry1C gene were highly resistant to C. medinalis and S. incertulas under laboratory conditions.
In whole plant bioassay, the larval mortality recorded on T1 plants showed 60 and 40 % against neonate and second instar larvae of rice leaffolder, respectively. Significant difference in damage symptoms like scrapping and folding was noticed in the transgenic lines and non-transgenic control. Transgenic lines showed less damage symptoms compared to non-transgenic control. The surviving larval growth and development were severely affected when fed on transgenic leaf tissues whereas survivors from non transformed control plants grew normally and consumed major portion of leaf tissue. The results suggested that the level of cry2AX1 gene expression in the transformants were not high enough to confer complete protection against rice leaffolder. In leaf bit bioassay, the larvae are forced to feed on selected leaf bits (leaves selected from top canopy of the plant). The whole plant bioassay may provide a clear picture as the larvae are not restricted to feed particular leaves and also it imposes a situation like in a field. The difference in mortality of leaffolder between leaf bit bioassay and whole plant bioassay could be due to the settlement of larvae in middle or still lower canopy of the plant where the expression of protein may be comparably low.
In earlier report, use of the rbcS-tp sequence for expressing cry1Ac accumulated Cry1Ac protein in chloroplasts up to 2 % of the total soluble proteins. In the present study the expression level of Cry2AX1 protein in transgenic rice was very low (varying from 36 to 236 ng/g of fresh leaf tissue in different phonological stages among the T1 plants) when compared to earlier studies. Therefore it is suggested that further modification of cry2AX1 gene construct with suitable 5′ UTR (untranslated region) sequence and matrix attachment region to overcome position effect of gene integration (Streatfield 2007), coupled with high frequency generation of large number of cry2AX1 transformants of rice is necessary to identify an elite event with desirable level of Cry2AX1 expression.
Abbreviations
- rbcS :
-
Ribulose biphosphate carboxylase
- SSC:
-
Saline-sodium citrate
- SDS:
-
Sodium dodecyl sulphate
- dCTP:
-
Deoxycytidine triphosphate
References
Bakhsh A, Rao AQ, Shahid AA (2012) Spatio temporal expression pattern of an insecticidal gene (cry2A) in transgenic cotton lines. Not Sci Biol 4:115–119
Bashir K, Husnain T, Fatima TN, Riaz N, Makhdoom R, Riazuddin S (2005) Novel indica basmati line (B-370) expressing two unrelated genes of B. thuringiensis is highly resistant to two lepidopteran insects in the field. Crop Prot 24:870–879
Bosch D, Schipper B, van der Kleij H, de Maagd R, Stiekema WJ (1994) Recombinant Bacillus thuringiensis crystal proteins with new properties: possibilities for resistance management. Biotechnol 12:915–918
Brookes P, Barfoot GB (2003) GM rice, will this be the way for global acceptance of GM crop technology. ISAAA Briefs no. 28:ISAAA, Ithaca
Chen M, Shelton A, Ye GY (2011) Insect-resistant genetically modified rice in China: from research to commercialization. Ann Rev Entomol 56:81–101
Christensen AH, Sharrock RA, Quail PH (1992) Maize polyubiquitin genes: structure, thermal perturbation of expression and transcript splicing, and promoter activity following transfer to protoplasts by electroporation. Plant Mol Biol 18:675–689
Christou P, Capell T, Kohli A, Gatehouse J, Gatehouse A (2006) Recent developments and future prospects in insect pest control in transgenic crops. Trends Plant Sci 11:302–308
Davidson M, Jacobs J, Reader J, Butler R, Frater CM, Markwick NP, Wratten SD, Conner AJ (2002) Development and evaluation of potatoes transgenic for a cry1Ac9 gene conferring resistance to potato tuber moth. J Am Soc Hort Sci 127:590–596
Dellaporta SL, Wood J, Hicks JB (1983) A plant DNA minipreparation: version II. Plant Mol Biol Rep 1:873–876
Fujimoto H, Itoh K, Yamamoto M, Kyozuka J, Shimamoto K (1993) Insect resistant rice generated by introduction of a modified gene of B. thuringiensis. Biotechnol 11:1151–1155
Han LZ, Liu PL, Wu KM, Peng YF, Wang F (2009) Population dynamics of Sesamia inferens on transgenic rice expressing Cry1Ac and CpTI in southern China. Environ Entomol 37:1361–1370
Hiei Y, Komari T (2008) Agrobacterium-mediated transformation of rice using immature embryos or calli induced from mature seed. Nat Protoc 3:824–834
Huang F, Buschman LL, Higgins RA, McGaughey WH (1999) Inheritance to Bacillus thuringiensis toxin (Dispel ES) in European corn borer. Science 284:965–967
Jafari M, Norouzi P, Malboobi MA (2009) Enhanced resistance to a Lepidopteran pest in transgenic sugar beet plants expressing synthetic cry1Ab gene. Euphytica 165:333–344
Jang IC, Nahm BH, Kim JK (1999) Sub-cellular targeting of green fluorescent protein to plastids in transgenic rice plants provides a high-level expression system. Mol Breed 5:453–461
Jayaprakash SP, Nandeesha P, Sozhavendan AE, Naveenkumar A, Illakiya Priya A, Balakrishnan N, Balasubramani V, Singh PK, Sudhakar D, Balasubramanian P, Udayasuriyan V (2014) Expression of a novel synthetic cry gene of Bacillus thuringiensis in transgenic tobacco confers resistance to Helicoverpa armigera and Spodoptera litura. J Pure Appl Microbiol 8:4687–4692
Katageri IS, Vamadevaiah HM, Udikeri SS, Khadi BM, Kumar PA (2007) Genetic transformation of an elite Indian genotype of cotton (Gossypium hirsutum L.) for insect resistance. Curr Sci 93:25
Kim S, Kim C, Li W, Kim T, Li Y, Zaidi MA, Altosaar I (2008) Inheritance and field performance of transgenic Korean Bt rice lines resistant to rice yellow stemborer. Euphytica 164:829–839
Kim EH, Suh SC, Park BS, Shin KS, Kweon SJ, Park SH, Kim YS, Kim JK (2009) Chloroplast-targeted expression of synthetic cry1Ac in transgenic rice as an alternative strategy for increased pest protection. Planta 230:397–405
Kranthi KR, Naidu S, Dhawad CS, Butler R, Frater CM, Markwick NP, Wratten SD, Conner AJ (2005) Temporal and intra-plant variability of Cry1Ac expression in Bt-cotton and its influence on the survival of the cotton bollworm, Helicoverpa armigera (Noctuidae: Lepidoptera). Curr Sci 89:291–298
Krishnaiah K, Varma NRG (2012) Changing insect pest scenario in the rice ecosystem—a national perspective. Directorate of Rice Research Rajendranagar, Hyderabad, pp 2–8
Kyozuka J, McElroy D, Hayakawa T, Xie Y, Wu R, Shimamoto K (1993) Light regulated and cell-specific expression of tomato rbcS-gusA and rice rbcS-gusA fusion gene in transgenic rice. Plant Physiol 102:991–1000
Meiyalaghan S, Jacobs JME, Butler RC, Wratten SD, Conner AJ (2006) Transgenic potato lines expressing cry1Ba1 or cry1Ca5 genes are resistant to potato tuber moth. Potato Res 49:203–216
Muthukrishnan S, Liang GH, Trick HN, Gill BS (2001) Pathogenesis related proteins and their genes in cereals. Plant Cell Tissue Org 64:93–114
Nayak KP, Basu D, Das S, Basu A, Ghosh D, Ramakrishna NA, Ghosh M, Sen SK (1997) Transgenic elite indica rice plants expressing CryIAc δ-endotoxin of B. thuringiensis are resistant against yellow stemborer (Scirpophaga incertulas). Proc Natl Acad Sci USA 94:2111–2116
Nomura M, Katayama K, Nishimura A (2000) The promoter of rbcS in a C3 plant (rice) directs organ-specific, light-dependent expression in a C4 plant (maize), but does not confer bundle sheath cell-specific expression. Plant Mol Biol 44:99–106
Pingali PL, Roger PA (1995) Impact of pesticides on farmer health and the rice environment. IRRI, Manila Philippines
Qi YB, Chen L, He XL, Jin QS, Zhang XM, He ZH (2012) Marker-free, tissue-specific expression of Cry1Ab as a safe transgenic strategy for insect resistance in rice plants. Pest Manag Sci 69:135–141
Ramesh S, Nagadhara D, Reddy V, Ra K (2004) Production of transgenic indica rice resistant to yellow stem borer and sap sucking insects, using super binary vectors of A. tumfaciens. Plant Sci 166:1077–1085
Rawat P, Singh AK, Ray K, Chaudhary B, Kumar SV, Gautam T, Kanoria S, Kaur G, Kumar P, Pental D, Burma PK (2011) Detrimental effects of expression of Bt endotoxin Cry1Ac on in vitro regeneration, in vivo growth and development of tobacco and cotton transgenics. J Biosci 36:363–376
Shu Q, Ye G, Cui H, Cheng X, Xiang Y, Wu D, Goa M, Xia Y, Hu C, Sardana R, Altosaar I (2000) Transgenic rice plants with a synthetic cry1Ab gene from B. thuringiensis were highly resistant to eight Lepidopteran pest species. Transgenic Res 9:433–439
Streatfield SJ (2007) Approaches to achieve high-level heterologous protein production in plants. Plant Biotechnol J 5:2–15
Tabashnik BE, Liu YB, de Maagd RA, Dennehy TJ (2000) Cross-resistance of pink bollworm (P. gossypiella) to B. thuringiensis toxins. Appl Environ Microbiol 66:4582–4584
Tang W, Chen H, Xu CG, Lin J, Zhang QF (2006) Development of insect-resistant transgenic indica rice with a synthetic cry1C gene. Mol Breed 18:1–10
Tu J, Zhang G, Data K, Xu C, He Y, Zhang Q, Khush GS, Datta SK (2000) Field performance of transgenic elite commercial hybrid rice expressing B. thuringiensis δ-endotoxin. Nat Biotechnol 18:1101–1104
Udayasuriyan V, Indra Arulselvi P, Balasubramani V, Sudha DR, Balasubramanian P, Sangeetha P (2010) Construction of new chimeric cry2AX1 gene of B. thuringiensis encoding protein with enhanced insecticidal activity. Indian Patent number 244427
Wan P, Zhang Y, Wu K, Huang M (2005) Seasonal expression profiles of insecticidal protein and control efficacy against Helicoverpa armigera for Bt cotton in China. J Econ Entomol 98:195–201
Wang Y, Zhang L, Li Y, Liu Y, Han L, Zhu Z, Wang F, Peng Y (2014) Expression of Cry1Ab protein in a marker-free transgenic Bt rice line and its efficacy in controlling a target pest, Chilo suppressalis (Lepidoptera: Crambidae). Environ Entomol 43:528–536
Wu G, Cui HR, Shu QY, Ye GY, Xie XB, Xia YW, Gao MW, Altosaar I (2001) Expression patterns of cry1Ab gene in progenies Kemingdao and the resistance to striped stem borer. Sci Agric Sin 34:465–468
Xia H, Rong L, Kai X, Wang W, Yang X, Yang C, Luo J, Lai F, Ye W, Fu Q (2011) Enhanced yield performance of Bt rice under target-insect attacks: implications for field insect management. Transgenic Res 20:655–664
Yang YY, Mei F, Zhang W, Shen Z, Fang J (2014) Creation of Bt rice expressing a fusion protein of Cry1Ac and Cry1I-Like using a green tissue-specific promoter. J Econ Entomol 107:1674–1679
Ye GY, Yao HW, Shu QY (2003) High levels of stable resistance in transgenic rice with a cry1Ab gene from B. thuringiensis Berliner to rice leaffolder, Cnaphalocrocis medinalis (Guenee) under field conditions. Crop Prot 22:171–178
Ye R, Huang H, Yang Z, Chen T, Liu L, Li X, Chen H, Lin Y (2009) Development of insect-resistant transgenic rice with Cry1C-free endosperm. Pest Manag Sci 65:1015–1020
Zaidi MA, Ye GY, Yao HW (2009) Transgenic rice plants expressing a modifed cry1Ca1 gene are resistant to Spodoptera litura and Chilo suppressalis. Mol Biotechnol 43:232–242
Zhao HY, Zhang YJ, Wu KM, Zhao KJ, Peng YF, Guo YY (2004) Expression of CrylAc protein in Cry1Ac/CpTI transgenic rice and its resistance in different stages to Chilo suppressalis. J Agric Biotechnol 12:76–79
Acknowledgments
We thank Prof. Dr. S. Suresh and Dr. R. P. Soundararajan, Assistant Professor (Department of Entomology, TNAU, Coimbatore) for providing facilities for rearing of rice leaffolder. We thank Dr. K. K. Kumar, Assistant Professor (Department of Plant Biotechnology, TNAU) for his help in Southern analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors certify that there is no conflict of interest in our manuscript.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Manikandan, R., Balakrishnan, N., Sudhakar, D. et al. Development of leaffolder resistant transgenic rice expressing cry2AX1 gene driven by green tissue-specific rbcS promoter. World J Microbiol Biotechnol 32, 37 (2016). https://doi.org/10.1007/s11274-015-2006-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-015-2006-z