Abstract
Key message
Independent transgenic pigeonpea events were developed using two cry genes. Transgenic Cry2Aa-pigeonpea was established for the first time. Selected transgenic events demonstrated 100% mortality of Helicoverpa armigera in successive generations.
Abstract
Lepidopteran insect Helicoverpa armigera is the major yield constraint of food legume pigeonpea. The present study was aimed to develop H. armigera-resistant transgenic pigeonpea, selected on the basis of transgene expression and phenotyping. Agrobacterium tumefaciens-mediated transformation of embryonic axis explants of pigeonpea cv UPAS 120 was performed using two separate binary vectors carrying synthetic Bacillus thuringiensis insecticidal crystal protein genes, cry1Ac and cry2Aa. T0 transformants were selected on the basis of PCR and protein expression profile. T1 events were exclusively selected on the basis of expression and monogenic character for cry, validated through Western and Southern blot analyses, respectively. Independently transformed 12 Cry1Ac and 11 Cry2Aa single-copy events were developed. The level of Cry-protein expression in T1 transgenic events was 0.140–0.175% of total soluble protein. Expressed Cry1Ac and Cry2Aa proteins in transgenic pigeonpea exhibited significant weight loss of second–fourth instar larvae of H. armigera and ultimately 80–100% mortality in detached leaf bioassay. Selected Cry-transgenic pigeonpea events, established at T2 generation, inherited insect-resistant phenotype. Immunohistofluorescence localization in T3 plants demonstrated constitutive accumulation of Cry1Ac and Cry2Aa in leaf tissues of respective transgenic events. This study is the first report of transgenic pigeonpea development, where stable integration, effective expression and biological activity of two Cry proteins were demonstrated in subsequent three generations (T0, T1, and T2). These studies will contribute to biotechnological breeding programmes of pigeonpea for its genetic improvement.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pigeonpea (Cajanus cajan (L). Millsp.) is cultivated in semi-arid as well as rainfed regions, spanning mostly the developing nations. It is the sixth most important grain legume in the world and India contributes for 90% of the global production. This legume crop is an important source of protein for impoverished population of the world. Although production of pigeonpea has increased worldwide to 4.85 million tonnes, the yield per hectare has declined over the last decade (FAO 2014). India alone has to import 200,000 tonnes of grains annually to cope with the ever growing market demand of dry and split pigeonpea seeds (dahl) (Shiferaw et al. 2008). The major reason behind this slump in production is the susceptibility of this crop to the devastating Lepidopteran pest Helicoverpa armigera or the pod borer. This polyphagous pest is responsible for extensive economic loss to the tune of US$ 300 million, annually (Shanower et al. 1999).
Low level of genetic diversity in pigeonpea reflected by the absence of resistant germplasm to insect infestations was the main reason for failure in the conventional breeding strategies for its improvement. This narrow genetic base makes pigeonpea more prone to a variety of insect attacks, especially pod borer or H. armigera. Farmers resorted to excessive use of pesticides in the face of surging demands (Rondon et al. 2007). This practice has been a serious cause of public concern related to food safety and environmental pollution. Furthermore, Lepidopteran pests have a tendency to develop resistance against these compounds. In this context, genetic engineering was found to be an alternative strategy for crop improvement and new variety development.
Bacillus thuringiensis endotoxin (Bt) Cry1Ac has been preferred by researchers for developing resistance against Lepidopteran pests (Sanahuja et al. 2011). With the commercialization of various Bt transgenic crops, there were also reports of their decreasing efficacy towards pests due to field-evolved resistance (Tabashnik et al. 2013). Transgenic plant expressing more than one toxin, each with unique mode of action is of utmost importance to deal this obstacle. Cry2Aa toxin has been previously used in the development of genetically modified rice and chickpea (Bashir et al. 2004; Chen et al. 2005; Acharjee et al. 2010). Mode of action of Cry2Aa was found to be different to Cry1Ac owing to the limited sequence homology and different receptor binding epitopes (Morse et al. 2001). They interacted with different receptor binding sites in the insect gut epithelium, where there were no chance of cross-reactivity between these two proteins (English et al. 1994; Morse et al. 2001; Hernandez-Rodriguez et al. 2008). Moreover, Cry1Ac-resistant larvae did not survive on Bt-cotton plants producing Cry2Ab protein (Tabashnik et al. 2002). Thus, stacking of cry1Ac and cry2Aa in one crop is supposed to be effective for establishment of durable resistance management strategy against the devastating H. armigera.
Despite numerous efforts in last two decades, few reports of transgenic Bt-pigeonpea have been published (Krishna et al. 2010; Ghosh et al. 2014a). The cry genes that have been utilized until date are synthetic cry1E-C (replacement of 58 amino acids from Cry1Ea with 70 homologous amino acid of Cry1Ca), cry1Ab, cry1Ac, and chimeric cry1AcF (fusion of N-terminal and domain II from cry1Ac and the C-terminal domain from cry1F) (Surekha et al. 2005; Sharma et al. 2006; Krishna et al. 2011; Ramu et al. 2012). There has been inadequate evidences for validation of reported Cry1Ac transgenic pigeonpea events in terms of stability of protein expression and rate of insect mortality (Ghosh et al. 2014a). The present study delineates a reproducible strategy of Agrobacterium tumefaciens-mediated transformation of pigeonpea with two important cry genes, cry1Ac and cry2Aa. Established independent events of cry1Ac and cry2Aa transgenic pigeonpea expressed cry proteins at optimum level over three generations, corroborated by their remarkable effect on H. armigera larval mortality and weight loss.
Materials and methods
Seeds of the pigeonpea cultivar UPAS 120 obtained from the Indian Institute of Pulses Research (IIPR), Kanpur, India, were used for explant preparation. A. tumefaciens AGL-1 strain was used for plant transformation. Eggs of H. armigera were obtained from the National Bureau of Agricultural Insect Resources (NBAIR), Bangalore, India and used for larval mortality bioassay experiments.
Plant transformation
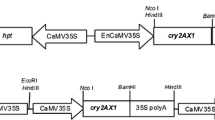
The plasmids pBINAR-cry1Ac and pBINAR-cry2Aa, obtained as generous gift from Dr. P. Ananda Kumar, NRCPB, New Delhi, were used for A. tumefaciens-mediated transformation. The plasmids contained the gene of interest, cry1Ac or cry2Aa, under the influence of CaMV35S promoter and nopaline synthase (nos) terminator. Neomycin phosphotransferase II (nptII) was used as selectable marker gene, flanked by nos promoter and terminator (Fig. 1). A. tumefaciens-mediated pigeonpea transformation was performed using embryonic axis explants as described by Ghosh et al. (2014b). Transformed explants were cultured on shoot regeneration medium [modified Murashige and Skoog (MS) medium (Murashige and Skoog 1962) supplemented with 1 mgl−1 6-benzylaminopurine (BAP) and 0.2 mgl−1 α-naphthaleneacetic acid] containing 100 mgl−1 kanamycin. After 6 weeks of incubation in regeneration medium, they were transferred to elongation medium [modified MS medium supplemented with 0.5 mgl−1 BAP and 0.5 mgl−1 gibberellic acid] containing 100 mgl−1 kanamycin and incubated for another 5 weeks. Properly elongated shoots were then grafted on non-transgenic rootstock and subsequently established in greenhouse.
Schematic representation of T-DNA region of binary vectors used for transformation. a pBINAR-cry1Ac and b pBINAR-cry2Aa. LB, left border of T-DNA; 35S P, cauliflower mosaic virus 35S promoter; nos A, nopaline synthase polyA terminator; ocs A, octopine synthase terminator; nptII, neomycin phosphotransferase II; nos P, nopaline synthase promoter; RB, right border of T-DNA
PCR analysis
Genomic DNA was extracted from leaves of transgenic pigeonpea plants of T0, T1, T2, and T3 generations following a modified CTAB protocol reported earlier by Chakraborti et al. (2006). Genomic DNA was also isolated from tissue culture regenerated, untransformed, control plants. The integration of cry1Ac or cry2Aa genes in pigeonpea genome, along with nptII in putative transformants, was examined by PCR using genomic DNA as template. Specific forward and reverse primers were designed to amplify the transgenes, cry1Ac/cry2Aa and selection marker, nptII (Table 1). The PCR conditions were, 95 °C for 5 min, followed by 35 cycles at 95 °C for 45 s, 57 °C for 45 s, and 72 °C for 1 min and a final extension of 72 °C for 10 min. PCR amplified products were analysed in 0.8% agarose gel, stained with ethidium bromide, and visualised on a UV transilluminator (UVP, LMS-20).
Qualitative detection of Cry protein using lateral flow strip
Expression of Cry proteins in T0 transformants was confirmed preliminarily using QuickStix™ strips (Envirologix, USA) meant for detecting Cry1Ac and Cry2A. Leaves of Bollgard II cotton plants were used as positive transgenic control, whereas untransformed pigeonpea plants were used as negative control. Control and transgenic leaf samples were crushed in buffer solution provided by manufacturer and lateral flow strips were dipped in slurry. It was kept for about 10 min, and observed for development of control and test bands as mentioned in manufacturer’s manual.
Western blot analysis
About 50 mg of fresh leaf tissue from plants of T0, T1, and T2 generations and untransformed control plants were ground in liquid nitrogen, to which 1 ml of extraction buffer (50 mM Tris Cl, 10 mM ethylenediaminetetraacetic acid, 0.05% Triton-X, 200 μM phenylmethylsulphonylfluoride, 10 μM Leupeptin, and 1 μM Pepstatin) was added. After centrifugation, supernatant containing total soluble protein (TSP) was collected and protein concentration was determined (Bradford 1976). Five hundred nanogram of Escherichia coli expressed and purified Cry protein (Cry1Ac/Cry2Aa) was used as positive control. Forty microgram of crude protein from each sample was separated by SDS-PAGE and blotted onto positively charged Hybond C Membrane (Amersham Sciences, UK). After blocking, the membrane was probed with anti-Cry1Ac/ anti-Cry2Aa polyclonal primary antibody (Amar Immunodiagnostics, India) at 1:10,000 dilution and anti-rabbit IgG-horse radish peroxidase (HRP) conjugate (Sigma-Aldrich, USA) as secondary antibody at 1:10,000 dilution. Bound secondary antibodies were detected by enhanced chemiluminescence (ECL kit, Amersham Biosciences, UK) reagents and developed on Kodak film.
Segregation analysis of T1 progenies
T1 seeds were collected from individual T0 plants. They were imbibed in water overnight, and seed coats were removed and submerged in 100 mgl−1 kanamycin for 5 h. Similarly, untransformed seeds were also treated under the same conditions. After 5 h of treatment, seeds were soaked in sterile tissue paper and sown on sterile soilrite. Three-week-old healthy seedlings were scored for kanamycin resistance (KanR), while the non-germinated or deformed seedlings were scored for kanamycin sensitivity (KanS). Resistant plants were allowed to grow and compared with untransformed plants treated with (negative control) and without (positive control) kanamycin. They were compared on the basis of their morphological differences, viz., plant height, number of leaves and leaflets, and formation of primary and secondary roots. Segregation patterns in the progeny plants were calculated and validated through χ 2 test using the Statistica Software v. 10.0 (StatSoft 2010).
Southern blot analysis
Southern blot analysis was performed according to Sambrook et al. (1989). Genomic DNA was isolated from young, green leaves of T1 and T2 plants. Tissue culture regenerated untransformed plant was used as control. Approximately 20 μg genomic DNA was digested with HindIII and separated on 0.8% (w/v) agarose gel and blotted onto positively charged nylon membrane (Hybond N+) (Amersham Biosciences, UK), following depurination, alkali denaturation, and neutralization. An amplicon of 662 bp obtained from pBINAR-cry1Ac vector was used as probe for hybridization with digested genomic DNA from Cry1Ac plants. Similarly, an amplicon of 600 bp obtained from pBINAR-cry2Aa vector was used as probe for hybridization with digested genomic DNA from Cry2Aa plants. Membrane was hybridized overnight with [α-32P]-dCTP-labelled cry1Ac/ cry2Aa specific probes at 68 °C. After hybridization, the membrane was washed with 2X SSC buffer (3 M sodium chloride and 0.3 M tri sodium citrate, dihydrate) and 0.1% sodium dodecyl sulphate (SDS) at room temperature for 1 h and at 68 °C for another hour using 0.1X SSC, 0.1% SDS, and then, the membrane was exposed to Kodak X-ray film, stored at −80 °C for 5 days, and developed subsequently.
In addition, membrane containing blotted DNA of T2 plants was probed with Digoxigenin (DIG) labelled above-mentioned DNA probes (Roche, DIG DNA Labelling and Detection Kit, version 19, 2004). Membrane was hybridized according to manufacturer’s instructions and incubated in dark, overnight. Reaction was stopped when desired band intensities were achieved on membrane.
Quantification of Cry proteins from total soluble protein extracts
Protein extracted from leaves of single-copy T1 and T2 lines was used for quantification analysis by indirect ELISA. Leaves from untransformed plants were used as control. Twenty microgram of crude protein extract of transgenic leaves and purified Cry proteins (serially diluted from 50 to 5 ng) were added to ELISA coating buffer (15 mM sodium carbonate, 35 mM sodium bicarbonate, 3 mM sodium azide; pH 9.5). They were coated onto 96-well microtiter plate and incubated overnight at 4 °C. After incubation, wells were washed thrice with 1X PBST (1X phosphate buffered saline and 0.1% Tween-20) and blocked with 5% (w/v) bovine serum albumin (BSA) in PBS for 2 h at 37 °C. The wells were again washed thrice with PBST, and incubated with anti-Cry1Ac/anti-Cry2Aa primary antibody in PBS for 1 h at 37 °C, followed by incubation in HRP-conjugated anti-rabbit antibody in PBS for 1 h at 37 °C after washing four times with PBST. Then, O-phenylenediamine hydrochloride tablets (OPD, Sigma-Aldrich, USA) dissolved in citrate buffer were added to each well. After the development of colour, the absorbance was read with a microplate reader (BioRad, CA, USA) at 415 nm.
Detached leaf bioassay of H. armigera
Eggs of H. armigera were hatched and reared on artificial diet (Armes et al. 1992) for 2–5 days. Second instar larvae were used for no-choice detached leaf feeding bioassay. Leaflets of 30-day-old individual T1 and T2 transgenic events and untransformed plants were placed in 3% water-agar in insect breeding boxes in five replicates. In each box, two healthy second instar larvae were placed. Each experiment was conducted for three times and percentage of larval mortality was recorded over a period of 7 days. Larvae fed on untransformed pigeonpea leaves served as control. The percentage of living insects was calculated for each bioassay box at 24 h intervals. Mean and standard error of survival percentage of larvae were calculated. Data were combined from the three independent repeat experiments and compared using analysis of variance (ANOVA, p = 0.05).
Furthermore, to determine the effect of Cry expression on the growth of H. armigera, detached leaf bioassay was conducted to observe the weight loss of third–fourth instar larvae over a period of 13 days. Each insect breeding box contained single larva, and five such boxes were used per experiment. Each experiment was conducted for three times. Weight of larvae was measured at every 24 h, and mortality was recorded. Larvae fed on untransformed leaves served as control.
Immunohistofluorescence analysis
Transverse leaflet sections of T3 transgenic events and untransformed plants were incubated in 10% (v/v) trichloroacetic acid at 4 °C for 2 h, followed by washing with 3:1 ethanol: acetic acid for three-to-four times or until complete removal of chlorophyll. The sections were then incubated successively through a series of graded ethanol to water 90, 70, 50, and 30% (v/v), respectively, each of 15 min duration and kept in blocking solution [3% (w/v) BSA in 1X PBS] at room temperature for 2 h. The tissue samples were then subjected to anti-Cry1Ac/anti-Cry2Aa antibody (1:10,000) dissolved in BSA for overnight at 4 °C. After washing with 1x PBS, the sections were treated with anti-rabbit IgG-FITC conjugated (1:20,000) (Sigma-Aldrich, USA) secondary antibody for 1 h at room temperature. Finally, leaf sections were examined under a fluorescence microscope (Axio Scope, Carl Zeiss, Germany) using excitation filter of 450–490 nm for FITC.
Results
Pigeonpea transformation and PCR analysis of T0 transgenic plants
Regenerated transformed shoots were grafted on non-transgenic rootstock after selection and established in greenhouse. Putative T0 transgenic plants were identified by PCR analysis. Fifty-two Cry1Ac and 55 Cry2Aa T0 plants successfully amplified 700 bp fragment of the nptII gene when specific primers were used to detect the selectable marker gene. All those T0 plants responded positively when cry1Ac or cry2Aa specific primers were used to amplify 662 bp or 600 bp internal fragment, respectively (Fig. 2).
Selection of primary transformants using Cry expression profile
T0 putative transformants positive for PCR analysis were further screened on the basis of their protein expressing capabilities. Lateral flow devices in the form of QuickStix strips were inducted to rapidly detect the expression of Cry proteins at preliminary stage of analysis. Twenty-one Cry1Ac and 32 Cry2Aa lines exhibited prominent control and test bands on the strips (Fig. 3 a, b). These batches of plants were primarily selected for the next round of screening by Western blot. Transformants that did not exhibit test bands were discontinued for further analyses. Western blot analysis of strip test-positive T0 transformants revealed 14 Cry1Ac and 18 Cry2Aa lines exhibiting the presence of ~66 and ~68 kDa bands, respectively (Fig. 3 c, d). No such corresponding bands were observed in case of the untransformed plant sample taken as negative control. T0 lines positive for PCR, strip assay, and Western blot were selected for further investigations. They were allowed to self-pollinate under restricted conditions in biocontainment facilities, and T1 seeds were harvested.
Lateral flow strip test (a, b) and Western blot analysis (c, d) performed on total soluble protein of T0 putative transformants. a Lanes 3–13, Cry1Ac transformants. b Lanes 3–12, Cry2Aa transformants. Lanes 1 and 2 (a, b), untransformed plant as negative control and Bollgard II cotton as positive control, respectively. c Lane 1, purified Cry1Ac (~66 kDa) as positive control; lanes 3–17, Cry1Ac transformants. d Lane 1, purified Cry2Aa (~68 kDa) as positive control; lanes 3–17, Cry2Aa transformants. Lane 2 (c, d), untransformed plant as negative control
Segregation analysis of T1 progenies and inheritance of transgene
Segregation pattern of T1 seeds derived from self-fertilization of the T0 plants (14 Cry1Ac and 18 Cry2Aa lines) was studied by germinating them in the presence of 100 mgl−1 kanamycin. Later, those were sown on soilrite. Positive control seeds (not treated with kanamycin) germinated normally and attained optimum height (~14 cm) within 3 weeks. Transgenic seedlings were comparable with positive control plants with distinct cotyledonary leaves. These seedlings later matured into green plants with expected height, leaf shape, and number without deformities. Long primary tap roots with numerous secondary roots were observed in these plants (Supplementary Fig. 1). Seeds of null segregant lines failed to set appropriate cotyledonary leaves and were distinctly dwarfed and stunted in appearance with deformed rudimentary roots. They were unable to attain the desired height within 3 weeks. Leaflets were shrivelled up and the apical buds started to disintegrate, ultimately leading to death. Negative control seeds (treated with kanamycin) responded similarly to the null segregants. They failed to germinate showing deformed cotyledonary leaves and absence of primary roots.
After 3 weeks of germination, 12 Cry1Ac and 15 Cry2Aa T1 events showed segregation in 3:1 ratio (KanR:KanS) according to Chi-square test (p ≥ 0.05) (Table 2). These T1 transformants were chosen for PCR analysis with nptII specific primers, which yielded 700 bp amplicon. Similarly, PCR with cry1Ac or cry2Aa specific primers resulted in the amplification of 662 or 600 bp fragment, respectively. The individual progenies of these T1 events were identified for their molecular attributes and grown to maturity in greenhouse to obtain T2 and T3 seeds.
Molecular analyses of cry-pigeonpea in T1 generation
Western blot analysis of leaf extracts of matured T1 progeny lines demonstrated that Cry1Ac/Cry2Aa protein was expressed efficiently in the transgenic pigeonpea lines. TSP obtained from T1 progenies indicated the presence of ~66 and ~68 kDa bands of expressed Cry1Ac and Cry2Aa proteins, respectively, in transgenic events (Fig. 4). No such band was observed in case of untransformed plant samples when probed with anti-cry1Ac/cry2Aa antibody. T1 progenies obtained from 12 Cry1Ac and 11 Cry2Aa T0 parental events were able to express Cry1Ac and Cry2Aa proteins, respectively.
Western blot analysis of T1 transgenic pigeonpea events. a Total soluble protein (TSP) of 12 Cry1Ac events. Lane ‘+ve’, purified Cry1Ac (~66 kDa) as positive control; lane –ve, untransformed plant as negative control. b TSP of 11 Cry2Aa events. Lane ‘+ve’, purified Cry2Aa (~68 kDa) as positive control; lane −ve, TSP from untransformed plant as negative control
Genomic DNA of 12 Cry1Ac and 11 Cry2Aa Western-positive T1 events was digested with HindIII, due to the presence of unique HindIII site located at the 3′ end of the ocs terminator. After hybridization with radiolabelled gene probes, all Cry1Ac and Cry2Aa events showed single-copy transgene integration (Fig. 5). The hybridization signal of T1 transgenic plants unveiled single-copy insertion of cry1Ac ranging between 4 and 9 kb. Integration of single-copy cry2Aa ranged between 3 and 9 kb. Untransformed plant did not show any hybridization signal in both occasions. Purified PCR product of cry genes (662 bp and 600 bp for cry1Ac and cry2Aa, respectively) as well as HindIII digested binary vectors (~14 kb) used as positive controls in this experiment showed positive signals.
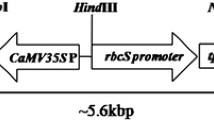
Southern blot analysis of T1 transgenic pigeonpea events. a Schematic representation of HindIII recognition site and 662 bp probe sequence (marked in grey) obtained from binary vector pBINAR-cry1Ac using probe specific forward (F1) and reverse (R1) primers. b, c HindIII digested genomic DNA of T1 progenies of 12 Cry1Ac transgenic events. Lanes +ve(1) and +ve(2), PCR purified 662 bp cry1Ac specific amplicon, and HindIII digested 14.3 bp pBINAR-cry1Ac plasmid, respectively, as positive controls. d Schematic representation of HindIII recognition site and 600 bp probe sequence (marked in grey) obtained from binary vector pBINAR-cry2Aa using probe specific forward (F2) and reverse (R2) primers. e, f HindIII digested genomic DNA of T1 progenies of 11 Cry2Aa transgenic events. Lanes +ve(3) and +ve(4), PCR purified 600 bp cry2Aa specific amplicon and HindIII digested 14.4 bp pBINAR-cry2Aa plasmid as positive controls, respectively. Lane −ve, HindIII digested genomic DNA of untransformed plant as negative control
The kanamycin-resistant and Western blot positive single-copy transgenic events were analysed by indirect ELISA. Twelve events expressed Cry1Ac in the range of 0.150–0.172% of TSP and another 11 events exhibited Cry2Aa in the range of 0.138–0.170% of TSP (Fig. 6).
Transgene integration and expression analyses in T2 generation
HindIII digested genomic DNA of T2 progenies was probed with non-radioactive DIG DNA probes. All T2 progenies exhibited single-copy integration of transgene. Three progenies each from T1 Cry1Ac [143-2S/1 and 143-43M(1)/2] and Cry2Aa events [137-5/4 and 70-2/5] exhibited single-copy band identical to the parental line (Fig. 7a–d). Untransformed plant did not show any hybridization signal.
Southern (a–d) and Western blot (e, f) analyses of T2 transgenic pigeonpea events. a Schematic representation of HindIII recognition site and 662 bp probe sequence (marked in grey) obtained from binary vector pBINAR-cry1Ac using probe specific forward (F1) and reverse (R1) primers. b HindIII digested genomic DNA of T2 progenies of two Cry1Ac transgenic events. Lanes +ve(1) and +ve(2) PCR purified 662 bp cry1Ac specific amplicon and HindIII digested 14.3 bp pBINAR-cry1Ac plasmid, respectively, as positive controls. c Schematic representation of HindIII recognition site and 600 bp probe sequence (marked in grey) obtained from binary vector pBINAR-cry2Aa using probe specific forward (F2) and reverse (R2) primers. d HindIII digested genomic DNA of T2 progenies of two Cry2Aa transgenic events. Lanes +ve(3) PCR purified 600 bp cry2Aa specific amplicon. Lane –ve (a, b), HindIII digested genomic DNA of untransformed plant as negative control. e Total soluble protein (TSP) of T2 progenies of two Cry1Ac events. Lane +ve, purified Cry1Ac (~66 kDa) as positive control. f TSP of T2 progenies of two Cry2Aa events. Lane ‘+ve’, purified Cry2Aa (~68 kDa) as positive control. Lane –ve (c, d), TSP from untransformed plant as negative control
Western blot analysis of leaf extracts of mentioned T2 progenies demonstrated that Cry1Ac/Cry2Aa protein was expressed efficiently in these pigeonpea lines similar to their T1 parental events (Fig. 7e, f). ELISA revealed T2 progenies of mentioned events contained Cry1Ac in the range of 0.160–0.171% of TSP, and Cry2Aa in the range of 0.156–0.172% of TSP.
Efficacy of the transformed plants against Helicoverpa armigera
Biological efficacy of 12 Cry1Ac and 11 Cry2Aa T1 events carrying single-copy transgene was analysed. The survivability of second instar larvae inside the bioassay setup was monitored at an interval of 24 h (Fig. 8). Untransformed plants were used as negative control. The difference in survival percentage of the insects incubated on T1 leaves was statistically significant after 72 h in comparison with control leaves according to ANOVA analysis (p ≥ 0.05). Insect survival on untransformed pigeonpea was between 98–100%. Three independent events of Cry1Ac and two events of Cry2Aa were found to have 100% mortality on the second instar larvae at the end of the bioassay period (Table 3). Five Cry1Ac and four Cry2Aa events exhibited mortality between 80–90% (Table 3). In rest of the events, i.e., four Cry1Ac and five Cry2Aa, mortality was below 80%. T1 events exhibiting more than 80% mortality were selected for further analysis and their T2 progenies were taken for bioassay studies.
Representative images illustrating detached leaf bioassay performed on T1 transgenic pigeonpea events to determine rate of mortality of Helicoverpa armigera second instar larvae. a Insects feeding on untransformed plant as control. b–d Events 143-2S/1 (Cry1Ac), 143-43M(1)/2 (Cry1Ac), and 70-2/5 (Cry2Aa), respectively, exhibiting mortality of larvae. Arrowheads indicate dead insects
Three Cry1Ac T2 progenies each from two T1 events [143-2S/1 and 143-43M(1)/2] were observed to have 90–100% mortality. Similarly, three Cry2Aa T2 progenies each from two T1 events [137-5/4 and 70-2/5] exhibited 100% mortality (Fig. 9a, c). The difference in survival percentage of the insects incubated on T2 leaves was found to be statistically significant after 72 h in comparison with control leaves according to ANOVA analysis (p ≥ 0.05). Development of surviving larvae that fed on transgenic plants was critically impaired when correlated with the control larvae. Drastic weight loss followed by untimely death of third–fourth instar larvae was observed within 13 days. (Fig. 9b, d; Fig. 10). Loss of weight was recorded to be statistically significant after 9th day of incubation in comparison with control plant, according to ANOVA analysis (p ≥ 0.05). Weight of larvae fed on control plants increased gradually. They matured normally on their host, and were able to pupate.
Bioassay of T2 events against H. armigera. a, b T2 progenies of two Cry1Ac transgenic events manifesting 90–100% larval mortality and weight loss, respectively, in comparison with untransformed control. c, d T2 progenies of two Cry2Aa transgenic events manifesting 99–100% larval mortality and weight loss, respectively, in comparison with untransformed control
Immunohistofluorescence study of T3 plants
The spatial expression patterns of cry1Ac and cry2Aa driven by CaMV35S promoter in PCR positive (data not shown), T3 transgenic events [143-43M(1)/2/2/1 and 70-2/5/2/1] were determined by in situ immunohistofluorescence localization of Cry proteins in TCA-fixed leaf tissue sections. Immunohistofluorescence analysis of the leaf tissue sections showed the expected constitutive expression pattern with green fluorescence spreading in all tissue types (Fig. 11). Leaf sections revealed the presence of Cry1Ac in trichomes, epidermis, cortex, and vascular bundles. Similar expression pattern was found in case of Cry2Aa leaf sections. No Cry accumulation was detected in the tissues of untransformed control plant.
Immunohistofluorescence localization of Cry proteins in transverse section of leaf of T3 events, 143-43M(1)/2/2/1 (Cry1Ac) and 70-2/5/2/1 (Cry2Aa). The presence of Cry protein is indicated by green fluorescence. Lamina of untransformed control plant (a, c), Cry1Ac (b) and Cry2Aa (d) events. Vascular bundle of leaf of untransformed control plant (e, g), Cry1Ac (f), and Cry2Aa (h) events
Discussion
Non-availability of insect-resistant traits in the germplasm of pigeonpea had restricted its genetic improvement. The described study delineates a reproducible strategy to develop transgenic pigeonpea with two important cry genes, cry1Ac and cry2Aa. The transgenic events were screened on the basis of the following criteria: (1) ability of transgenic plants to express Cry protein; (2) Mendelian segregation pattern in T1 progenies; (3) single-copy insertion status; and (4) high rate of insect mortality. The putative transformants of pigeonpea were selected on the basis of Cry expression in T0 generation. It involved the usage of QuickStix™ strips for rapid detection of Cry1Ac and Cry2Aa proteins in primary transformants those amplified the transgenes through PCR. These lateral flow devices were designed to give yes/no result, whether the sample contained the desired protein at or above a standard threshold level (Lipton et al. 2000). Usage of strips was found to be reliable during quick detection of Bt proteins in transgenic corn, Bollgard® cotton and Roundup-Ready® soybean (Bernardi et al. 2015; Stave 2002; Lipp and Anklam 2000). This helped to narrow down the search for the lines having detectable transgene expression, instead of carrying many primary transformants to the next generation.
It is an essential requirement of any transgenic research to prove faithful transmission of integrated transgene in the successive generations of the transgenic plants. The study was undertaken to determine the inheritance and segregation of the cry insecticidal gene in transgenic pigeonpea events. The kanamycin-resistant phenotype in Cry1Ac—and Cry2Aa—pigeonpea segregated as simple dominant Mendelian trait. Twelve Cry1Ac and 15 Cry2Aa T1 lines exhibited 3:1 segregation pattern. Stable expression of cry gene in putative T1 single-copy lines was confirmed by Western blot analysis before proceeding to Southern blot. Twelve Cry1Ac and 11 Cry2Aa lines that showed 3:1 segregation pattern in kanamycin selection demonstrated Cry1Ac and Cry2Aa expression, respectively. Transformation is considered to be a random event and each transgenic plant is the result from a separate insertion event (Kohel et al. 2000). The number and sites of gene insertions into the plant genome were found to influence gene expression in transgenic plants (Benedict et al. 1996). In the present study, Southern blot hybridization with HindIII digested genomic DNA in selected T1 progenies indicated copy number of T-DNA integration events. Twelve Cry1Ac [142-6/4, 142-7/3, 142-7(1)/4, 142-10/2, 142-10(1)/5, 142-11/1, 143-1S/1, 143-2S/1, 143-8S/3, 143-11S/1, 143-43M/1 and 143-43M(1)/2] and 11 Cry2Aa [129-1/1, 137-2/2, 137-4/1, 137-5/4, 137-7/1, 137-10/1, 137-11/1, 137-20/1, 140-3/2, 140-14/1, and 70-2/5] events carried single-copy T-DNA integrations, concurring the findings in segregation analysis. Furthermore, Southern blot hybridization in selected T2 events indicated stable foreign gene integration. The size of each insert was unique in individual event confirming independent origin of the transgenic lines. Earlier, in the field of transgenic pigeonpea development, Sharma et al. (2006) performed Southern blot of four single-copy T1 Cry1Ab events; Surekha et al. (2005) reportedly identified 18 Southern positive, single-copy T1 Cry1E-C plants and Krishna et al. (2011) demonstrated four T0 Cry1Ac events with single-copy integration. Previously, Ramu et al. (2012) performed extended analysis during the production of transgenic pigeonpea containing the fused cry1Ac-F gene. In T3 generation, they designated four transgenic lines to be promising on the basis of larval mortality bioassay, but Southern analysis was reportedly done on three selected plants at T3 stage, while data revealed two events, with different integration patterns.
Western blot analysis, followed by ELISA, of T2 events confirmed the stable expression of cry genes. Expression study of transgenic events revealed that Cry expression remained constitutive, stable, and consistent through three generations. Among all T2 progenies, the highest level of Cry1Ac expression was detected in, 143-43M(1)/2/2 (0.171% of TSP), whereas maximum Cry2Aa expression was recorded in 70-2/5/2 (0.172% of TSP). Deployment of expression based selection ensured the identification of high expressing events in the present study. Furthermore, immunohistofluorescence study confirmed the constitutive localization of Cry1Ac and Cry2Aa proteins in the leaf tissues of transgenic pigeonpea. Previously, researchers demonstrated the use of CaMV35S promoter to drive the constitutive expression of Cry1Ab (Sharma et al. 2006) and haemagglutinin (Satyavathi et al. 2003) in transgenic events of pigeonpea at considerably higher level (0.1–0.49% of TSP). Amidst very few reports of recovery of transgenic pigeonpea with trait genes, Surekha et al. (2005), Sharma et al. (2006), and Ramu et al. (2012) reported the expression and inheritance of Cry1 E-C, Cry1Ab, and Cry1AcF, respectively, beyond the T0 generation. Although, expression of Cry1Ac in pigeonpea had been reported by Krishna et al. (2011), the study was limited to T0 generation only. Among other legumes, Stewart et al. (1996) reported the expression of Cry1Ac at 0.0046% in transgenic soybean and Kar et al. (1997) reported the constitutive expression of Cry1Ac up to 0.0045% in chickpea. Chen et al. (2005) reported the presence of Cry2Aa at 12.11 µg g−1 of leaf fresh weight in transgenic rice. In the present study, we report for the first time the stable expression of Cry1Ac in transgenic pigeonpea beyond the T0 generation. This is also the first ever report of T1 transgenic pigeonpea events expressing Cry2Aa.
The biology and ecology of H. armigera had been extensively reviewed, and general features did not differ when pigeonpea was used as a host (Fitt 1989). Females were found to oviposit on leaves at night and fecundity was high, with up to 3000 eggs reported from a single female (Sison and Shanower 1994). This made leaves of pigeonpea the primary target of growing pod borer larvae. Larvae of first–third instar generally feed on leaves and gradually advance to other parts of the plant including flowers and pods. In this context, the use of constitutive CaMV35S promoter to express insecticidal proteins throughout the plant body to confer maximum resistance against pod borer was found to be essential.
In the described study, eight Cry1Ac events [142-10/2, 143-2S/1,143-43M(1)/2, 142-7/3, 142-7(1)/4, 143-8S/3, 142-11/1, and 143-1S/1] showed 80–100% mortality against the second instar H. armigera larvae. In addition, six Cry2Aa events [70-2/5, 137-5/4, 137-10/1, 137-11/1, 137-7/1, and 137-20/1] demonstrated 80–100% mortality of the second instar larvae. Some of the mentioned events exhibited drastic weight loss of the fourth instar larvae leading to death. T1 events selected based on 100% larval mortality were transferred to T2 generation to check the inheritance of phenotype. The evidences of bioassay experiment on transgenic pigeonpea by previous workers exhibited mortality of Spodoptera litura to be 80% on Cry1E-C events (Surekha et al. 2005) while Cry1Ac T0 plants demonstrated 55% mortality of H. armigera (Krishna et al. 2011). Ramu et al. (2012) reported bioassay of T2 and T3 pigeonpea expressing Cry1AcF, where mortality of H. armigera ranged between 80–100%. Recently, Chakraborty et al. (2016) reported about 90% mortality of H. armigera larvae fed on chickpea events expressing Cry1Ac under the control of RuBisCo small subunit promoter. Earlier, 100% larval mortality of H. armigera in chickpea (Acharjee et al. 2010) and yellow stem borer in rice (Chen et al. 2005) were demonstrated using constitutive expression of Cry2Aa.
This is a novel report of transgenic pigeonpea development, successfully expressing Cry2Aa protein at levels lethal to H. armigera. The approach of screening T1 transgenic pigeonpea events on the basis of antibiotic selection and protein expression phenotypes helped to narrow down the search for promising events. The events were later on confirmed by Southern blot to ensure their monogenic character, followed by extensive bioassay experiments confirming efficiency of the expressed proteins. The expression of Cry proteins in three successive generations of transgenic pigeonpea conferred substantial resistance against H. armigera, not only in terms of increased mortality, but also weight loss of larvae. Furthermore, the cry1Ac and cry2Aa genes were successfully inherited to T3 generations. These Cry-transgenic events can serve as resources for the development of pyramided pigeonpea in the near future. A multi-toxin deployment system is supposed to enhance the efficacy of transgenic pigeonpea and eventually slow down the rate of resistance evolution in pod borers.
References
Acharjee S, Sarmah BK, Ananda Kumar P, Olsen K, Mahon R, Moar WJ, Moore A, Higgins TVJ (2010) Transgenic chickpeas (Cicer arietinum L.) expressing a sequence-modified cry2Aa gene. Plant Sci 178:333–339
Armes NJ, Bond GS, Cooters RJ (1992) The laboratory culture and development of Helicoverpa armigera. National Resources Institute Bulletin no. 57. National Resources Institute, Chatham
Bashir K, Husnain T, Fatira T, Latif Z, Mehdi SA, Riazuddin S (2004) Field evaluation and risk assessment of transgenic indica basmati rice. Mol Breed 13:301–312
Benedict JH, Sachs ES, Altman DW, Deaton WR, Kohel RJ, Ring DR, Berberich SA (1996) Field performance of cottons expressing transgenic Cry IA insecticidal proteins for resistance to Heliothis virescens and Helicoverpa zea (Lepidoptera: Noctuidae) on transgenic Bt cottons. J Econ Entomol 89:230–238
Bernardi D, Salmeron E, Horikoshi RJ, Bernardi O, Dourado PM, Carvalho RA et al (2015) Cross-Resistance between Cry1 Proteins in Fall Armyworm (Spodoptera frugiperda) may affect the durability of current pyramided Bt maize hybrids in Brazil. PLoS ONE 10(10):e0140130. doi:10.1371/journal.pone.0140130
Bradford MM (1976) A rapid and sensitive method for the quantitation of proteins using the principle of protein–dye binding. Anal Biochem 72:248–254
Chakraborti D, Sarkar A, Gupta S, Das S (2006) Small and large scale genomic DNA isolation protocol for chickpea (Cicer arietinum L.), suitable for molecular marker and transgenic analyses. Afr J Biotechnol 5:585–589
Chakraborty J, Sen S, Ghosh P, Sengupta A, Basu D, Das S (2016) Homologous promoter derived constitutive and chloroplast targeted expression of synthetic cry1Ac in transgenic chickpea confers resistance against Helicoverpa armigera. Plant Cell Tiss Organ Cult 125(3):521–535
Chen H, Tang W, Xu CG, Li XH, Lin YJ, Zhang Q (2005) Transgenic indica rice plants harboring a synthetic cry2A gene of Bacillus thuringiensis exhibit enhanced resistance against lepidopteran rice pests. Theor Appl Genet 111:1330–1337
FAO (2014) FAOSTAT data in 2014. Available at: http://faostat3.fao.org. Accessed November 2016
Fitt GP (1989) The ecology of Heliothis in relation to agroecosystems. Annu Rev Entomol 34:17–52
Ghosh G, Purohit A, Chaudhuri RK, Chakraborti D (2014a) Advances in genetic transformation of important pulse crop pigeonpea. OA Biotechnol 12:5
Ghosh G, Purohit A, Ganguly S, Chaudhuri RK, Chakraborti D (2014b) In vitro shoot grafting on rootstock: An effective tool for Agrobacterium-mediated transformation of pigeonpea (Cajanus cajan (L.) Millsp.). Plant Biotechnology 31:301–308
Hernandez-Rodriguez CS, Vliet AV, Bautsoens N, Rie JV, Ferre J (2008) Specific Binding of Bacillus thuringiensis Cry2A Insecticidal proteins to a common site in the midgut of Helicoverpa species. Appl Env Microbiol 74(24):7654–7659
Kar S, Basu D, Das S, Ramakrishnan NA, Mukherjee P, Nayak P, Sen SK (1997) Expression of cryIA(c) gene of Bacillus thuringiensis in transgenic chickpea plants inhibit development of pod-borer (Heliothis armigera) larvae. Transgenic Res 6:177–185
Kohel RJ, Quisenberry JE, Cartwright G, Yu J (2000) Linkage analysis of transgenes inserted into cotton via Agrobacterium tumefaciens transformation. J Cotton Sci 4:65–69
Krishna G, Reddy PS, Ramteke PW, Bhattacharya PS (2010) Progress of tissue culture and genetic transformation research in pigeon pea [Cajanus cajan, L. Millsp.]. Plant Cell Rep 29:1079–1095
Krishna G, Reddy SP, Ramteke WP, Rambabu P, Tawar KB, Bhattacharya P (2011) Agrobacterium-mediated genetic transformation of pigeonpea [Cajanus cajan (L.) Millsp.] for resistance to legume pod borer Helicoverpa armigera. J Crop Sci. Biotech 14:197–204
Lipp M, Anklam E (2000) Validation of an Immunoassay for detection and quantitation of a genetically modified soybean in food and food fractions using reference materials: interlaboratory study. J AOAC Int 83:4
Lipton CR, Dautlick JX, Grothaus GD, Hunst PL, Magin KM, Mihaliak CA, Rubio FM, Stave JW (2000) Guidelines for the validation and use of immunoassays for determination of introduced proteins in biotechnology enhanced crops and derived food ingredients. Food Agric Immunol 12:153–164
Morse RJ, Yamamoto T, Stroud RM (2001) Structure of Cry2Aa suggests an unexpected receptor binding epitope. Structure 9:409–417
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiol Plant 15:473–497
Ramu SV, Rohini S, Keshavareddy G, Neelima MG, Shanmugam NB, Kumar ARV, Sarangi SK, Ananda Kumar P, Udayakumar M (2012) Expression of a synthetic cry1AcF gene in transgenic pigeonpea confers resistance to Helicoverpa armigera. J Appl Entomol 136:675–687
English L, Robbins HL, Vontersch MA, Kuleseza CA, Avi D, Coyle D, Jany CS, Slatin SL (1994) Mode of action of cry11A: a Bacillus thuringiensis endotoxin. Insect Biochem Mol Biol 24:1025–1035
Rondon SI, DeBano SJ, Clough GH, Hamm PB, Jensen A, Schreiber A, Alvarez JM, Thornton M, Barbour J, Dogramaci M (2007) Biology and management of the potato tuberworm in the Pacific Northwest. PNW.594
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
Sanahuja G, Banakar R, Twyman RM, Capell T, Christou P (2011) Bacillus thuringiensis: a century of research, development and commercial applications. Plant Biotechnol J 9:283–300
Satyavathi VV, Prasad V, Khandelwal A, Shaila MS, Lakshmi Sita G (2003) Expression of hemagglutinin protein of Rinder pest virus in transgenic pigeonpea pea (Cajanus cajan (L.) Millsp.) plants. Plant Cell Rep 21:651–658
Shanower TG, Romeis J, Minja EM (1999) Insect pests of pigeon pea and their management. Ann Rev Entomol 44:77–96
Sharma KK, Lavanya M, Anjaiah V (2006) Agrobacterium mediated production of transgenic pigeonpea (Cajanus cajan L. Millsp.) expressing the synthetic Bt Cry1AB gene. In Vitro Cell Dev Biol Plant 42:165–173
Shiferaw B, Okello J, Muricho G, Omiti J, Silim SN, Jones R (2008) Unlocking the potential of high value legumes in the semi-arid regions: analyses of the pigeon pea value chains in Kenya. International Crops Research Institute for the Semi Arid Tropics
Sison MLJ, Shanower TG (1994) Development and survival of Helicoverpa armigera (Lepidoptera: Noctuidae) on short-duration pigeonpea. J Econ Entomol 87:1749–1753
StatSoft (2010) Statistica for Windows (Computer program manual). Statsoft, Inc, Tulsa
Stave JW (2002) Protein immunoassay methods for detection of biotech crops: applications, limitations, and practical considerations. J AOAC Int 85:780–786
Stewart CN Jr, Adang MJ, All JN, Boerma HR Cardineau G, Tucher D, Parrott WA (1996) Genetic transformation, recovery, and characterization of fertile soybean transgenic for a synthetic Bacillus thuringiensis cryIAc gene. Plant Physiol 112:121–129
Surekha C, Beena MR, Arundhati A, Singh PK, Tuli R, Dutta-Gupta A, Kirti PB (2005) Agrobacterium-mediated genetic transformation of pigeon pea (Cajanus cajan (L.) Millsp.) using embryonal segments and development of transgenic plants for resistance against Spodoptera. Plant Sci 169:1074–1080
Tabashnik BE, Dennehy TJ, Sims MA, Larkin K, Head GP, Moar WJ, Carrière Y (2002) Control of resistant pink bollworm (Pectinophora gossypiella) by transgenic cotton that produces Bacillus thuringiensis toxin Cry2Ab. Appl Environ Microb 68:3790–3794
Tabashnik BE, Brévault T, Carriere Y (2013) Insect resistance to Bt crops: lessons from the first billion acres. Nat Biotechnol 31:510–521
Acknowledgements
The authors thank the Indian Council of Agricultural Research (No. NFBSFARA/PB2010/2010-11) for financial assistance and St. Xavier’s College (Autonomous) for providing infrastructure. Mr. Joydeep Chakraborty, Division of Plant Biology, Bose Institute, Kolkata is duly acknowledged for his help during various experiments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Author contribution statement
GG, SG, AP, and DC conceived and designed the experiments. GG and SG conducted all the experiments. GG and DC drafted the manuscript. SD, RKC, and DC were responsible for data analysis, manuscript editing, and supervision of the work. All authors read and approved the final manuscript.
Additional information
Communicated by Leandro Peña.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Ghosh, G., Ganguly, S., Purohit, A. et al. Transgenic pigeonpea events expressing Cry1Ac and Cry2Aa exhibit resistance to Helicoverpa armigera . Plant Cell Rep 36, 1037–1051 (2017). https://doi.org/10.1007/s00299-017-2133-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-017-2133-0