Abstract
Microalgae possess higher photosynthetic efficiency and accumulate more neutral lipids when supplied with high-dose CO2. However, the nature of lipid accumulation under conditions of elevated CO2 has not been fully elucidated so far. We now revealed that the enhanced lipid accumulation of Chlorella in high-dose CO2 was as efficient as under heterotrophic conditions and this may be attributed to the driving of enlarged carbon source. Both photoautotrophic and heterotrophic cultures were established by using Chlorella sorokiniana CS-1. A series of changes in the carbon fixation, lipid accumulation, energy conversion, and carbon-lipid conversion under high-dose CO2 (1–10 %) treatment were characterized subsequently. The daily carbon fixation rate of C. sorokiniana LS-2 in 10 % CO2 aeration was significantly increased compared with air CO2. Correspondingly, double oil content (28 %) was observed in 10 % CO2 aeration, close to 32.3 % produced under heterotrophic conditions. In addition, with 10 % CO2 aeration, the overall energy yield (Ψ) in Chlorella reached 12.4 from 7.3 % (with air aeration) because of the enhanced daily carbon fixation rates. This treatment also improved the energetic lipid yield (Ylipid/Es) with 4.7-fold, tending to the heterotrophic parameters. More significantly, 2.2 times of carbon-lipid conversion efficiency (ηClipid/Ctotal, 42.4 %) was observed in 10 % CO2 aeration, towards to 53.7 % in heterotrophic cultures, suggesting that more fixed carbon might flow into lipid synthesis under both 10 % CO2 aeration and heterotrophic conditions. Taken together, all our evidence showed that 10 % CO2 may push photoautotrophic Chlorella to display heterotrophic-like efficiency at least in lipid production. It might bring us an efficient model of lipid production based on microalgal cells with high-dose CO2, which is essential to sustain biodiesel production at large scales.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Background
Photoautotrophs collect solar energy and in turn convert to chemical energy which is stored in small organic molecules (e.g. glucose) for transient usage or in large-molecular-mass organic substances (e.g. lipids) for long-term usage (Ducharme et al. 2008). The average intensity of sunlight at the earth surface is 985.7 W/m2 (Nakkash et al. 2013). However, the overall photosynthetic efficiency of higher plants only represents 0.2–2 % of the total solar radiation, resulting in a huge waste of the solar energy. As the biofuel-producing organisms, microalgae have attracted a great deal of attentions for their higher growth rates, more biomass and lipid productions (Chen and Wu 2011; Chisti 2007; Huang et al. 2010). Of particular interest is the production of lipids accompanied with the production of short-chain alcohols and other by-products of the secondary metabolism (i.e., polyunsaturated fatty acids, β-carotenes or astaxanthin for human health) (Boelen et al. 2013; Guedes et al. 2011; Yuan et al. 2011). However, microalgae still carry out the relative inefficient photosynthesis compared with abundant solar radiation, although the overall photosynthetic efficiency of microalgae has reached 6 % of the total solar radiation, much higher than plants (Nakkash et al. 2013). Photosynthesis consists of the light and dark reactions, where the dark reaction probably is one of the rate-limiting steps due to low CO2 supply (Axelsson et al. 2001). Microalgal carbon fixation starts with Rubisco (ribulose-1,5-bisphosphate carboxylase/oxygenase), mitigating CO2 into Calvin cycle, where low CO2 concentration in air becomes a key limiting factor (Sforza et al. 2012). Hence, the supply of high-concentration CO2 may remove the substrate limitation and in turn improve photosynthetic efficiency, consequently with increases of biomass and products like neutral lipids.
Photoautotrophic microalgae can trap the light energy as the sole energy source and assimilate inorganic CO2 as the carbon source through photosynthesis. In addition, organic substrates like glucose can also be utilized as the carbon and energy sources through performing oxygenic phosphorylation by many microalgae (Yang et al. 2000). The typical characteristic of this heterotrophic mode is a significant increase in both biomass and lipid yields due to highly efficient chemical energy conversions (Xiong et al. 2008). As an example, both high biomass and cellular lipid contents were found in heterotrophic microalgal cultures (Li et al. 2007; Miao and Wu 2006; Xu et al. 2006). In the case of Chlorella protothecoides, heterotrophic growth on corn powder hydrolysates results in 3–4 times enhancement in biomass and lipid yields as compared with photoautotrophic growth (Liang et al. 2009). But the costs of organic carbon sources are higher when compared with all added nutrients in photoautotrophic cultures.
The previous investigations indicated that the supply of high-concentration CO2 promotes cell growth and lipid accumulation in a variety of microalgal species (Ho et al. 2010; Zeng et al. 2011). It thus brings us an important idea that elevated CO2 may improve solar energy conversion and storage into lipids in photoautotrophic microalgae. This process pushed by elevated CO2 may be as efficient as that in heterotrophic cultures. Our present comparative investigation is to reveal such high-efficiency in both increased lipid accumulation and cost saving evoked by high-dose CO2.
Doubled atmospheric CO2 concentration mimicking an increase in greenhouse gases in the open system has confirmed the stimulatory effect on growth of crop plants (O’Neill et al. 2010). Moreover, extremely high-doses of CO2 in flue gases (usually containing 10–20 %) emitted by intensified industry offer a unique source to further improve microalgal growth in the closed culture system (Demirbas 2011; Douskova et al. 2009). Microalgae are so far the sole species known to be able to utilize the high-dose CO2 up to 20 %. For example, a few microalgal species like Botryococcus braunii, Scenedesmus sp. and Chlorella sp. could grow well under 20 % CO2 even without any adjustments of culture pH (Westerhoff et al. 2010). These extensive studies, however, have focused on the effects of high-concentration CO2 on growth and photosynthetic rates of microalgae. The nature of carbon fixation and energy conversion in photoautotrophic microalgal cells under high-dose CO2 has not been fully elucidated (Chiu et al. 2008; Rosa et al. 2011; Sydney et al. 2010). We hypothesized that high-dose CO2 might drive more carbons into lipid synthesis, and therefore enhances the lipid production. This process pushed by elevated CO2 may be as efficient as that in heterotrophic cultures. To test the hypothesis, Chlorella sorokiniana CS-1 with both high-dose CO2 utilization and heterotrophic growth was used to establish the experimental systems. The growth kinetics, carbon fixation rates, lipid formation kinetics, energy conversion efficiencies and carbon-lipid conversion efficiencies were characterized and compared under both photoautotrophic and heterotrophic conditions. Through the investigation, the heterotrophy-like features of the lipid accumulation enhanced by elevated CO2 have been revealed in energy conversion efficiencies and carbon-lipid conversion efficiencies.
Methods
Identification of microalgae
The strain CS-1 isolated from fresh water with both high CO2 utilization and heterotrophic growth was identified by 18S rDNA. Oligonucleotides M18F and M18R were designed as described by Qiao and used to amplify the full sequence of 18S rDNA (Qiao et al. 2009). The genomic DNA was isolated using the plant genome extraction kit (Omega, USA). DNA was purified by the Gel Extraction Kit (Omega, USA). The PCR products were sent to the Beijing Genomics Institute (BGI, China), where sequencing was performed. Sequencing analysis was carried out using Blastn.
Optimization of microalgal culture conditions
The photoautotrophic and heterotrophic culture systems were established by using the identified microalga C. sorokiniana CS-1. For photoautotrophic system, C. sorokiniana CS-1 was inoculated at an initial density of 2.5 × 105 cells mL−1 in 500 mL air-lift photo-bioreactors and incubated under different illuminations with photoperiod of 12-h light/12-h dark. The autotrophic medium and temperature of the culture was BG-11 and 26 °C, respectively unless otherwise mentioned. After that, the effects of light intensities on biomass in phtoautotrophic cultures were investigated. The light intensities studied were 0, 40, 80, 120, 160, and 200 μE m−2 s−1. For heterotrophic system, C. sorokiniana CS-1 was also inoculated at an initial density of 2.5 × 105 cells mL−1 in 500 mL conical flasks and incubated on a rotary shaker at 200 r min−1 under the dark. The heterotrophic medium and temperature of the culture was BG-11 plus glucose and peptone and 26 °C, respectively. Optimization on glucose and peptone concentrations in heterotrophic cultures was conducted through the central composite design (CCD) (Khataee et al. 2010). The response function of interest was the biomass. The obtained data was analyzed using Design-Expert. In addition, the soluble sugar of peptone was determined as previously described (Yemm and Willis 1954).
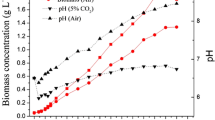
After establishment of the photoautotrophic and heterotrophic culture systems, C. sorokiniana CS-1 was inoculated at an initial density of 2.5 × 105 cells mL−1 in 500 mL cultures in both systems. The cultures were aerated with various concentrations of CO2 (air, 1, 2, 5, and 10 % CO2) under 120 μE m−2 s−1 illumination as photoautotrophic conditions (Fig. 1) or incubated on a rotary shaker at 200 r min−1 as heterotrophic conditions.
Analyses of biomass, total lipids and fatty acid profiles of microalgae
The biomass yield was determined by measuring dry cell weight. Cells were withdrawn daily and centrifuged at 4000g for 5 min. The precipitation were washed twice with deionized water and dried at 60 °C until constant weight. The concentrations of carbon, hydrogen, nitrogen and sulfur in biomass were determined in duplication for each experiment, using a vario macro cube (vario macro cube, Germany). The formula of C. sorokiniana CS-1 biomass was determined according to concentrations of C, H, N, S (Goudriaan et al. 1979).
To extract lipids, microalgal cells were gathered by centrifugation at 4000g for 5 min. Collected cells were washed twice with deionized water, lyophilized, and weighed. Dry microalgal cells (about 1 g) were resuspended in 5 mL concentrated HCl and heated at 70 °C for 20 min. After that 5 mL ethanol and 10 mL diethyl ether was added successively. The mixture was shaken for 1 min followed by centrifugation at 4000g for 2 min, and then the diethyl ether layer was taken into a round-bottom flask. This extraction process was repeated by adding another aliquot of 10 mL diethyl ether until no yellowish lipids left. Finally, the diethyl ether phase was combined and lipids were obtained by evaporating diethyl ether under vacuum in a rotary evaporator (Parsaeimehr et al. 2015).
A one-step protocol was used for the fatty acid methylation (Halim et al. 2011). Dried lipids were re-dissolved in 500 μL chloroform followed by transferring it into a 1.5 mL glass vial. 1 mL of 1 M sulphuric acid–methanol was then added and incubated at 100 °C for 1 h under nitrogen protection. Subsequently, methyl ester was extracted with n-hexane, dried by nitrogen blowing and then weighed. The fatty acid composition of C. sorokiniana CS-1 was determined by a gas chromatography with FID detector (Agilent 7890) using methylundecanoate as internal standards.
Analysis of the energy and carbon-lipid conversion efficiencies
The obtained biomass was used to construct growth curves, from which we deduced the specific growth rates (μ). Similarly, the lipid formation curves and the specific formation rates (q) were also determined. Biomass productivity (P) was calculated as maximum productivity (Pmax, g/L/day), according to the Eq (1)
where X0 is the initial biomass at time t0 (d) and Xt is the biomass at any time t (d) subsequent to t0 (Ho et al. 1979).
The lipid yield on supplied energy to culture was designed as Ylipid/Es, where ES of photoautotrophic culture was estimated by Eq (2), (Yang et al. 2000):
where Is is the incident light intensity and A is the illuminated surface area. ES of heterotrophic culture was determined as the heat of combustion with consumed glucose. In order to determine the potential carbon source existed in peptone, the soluble sugar of peptone was tested. The result showed that only 0.071 g/L soluble sugar existed in heterotrophic medium when peptone was added at 5.32 g/L. It only occupied 0.4 % of glucose added in the medium. Thus, the carbon source in peptone can be ignored compared with the large amount of glucose (17.31 g/L). The energy yield in both system was simplified as Eq (3)
where Xm, S and tm represent the maximum biomass concentration, the corresponding residual substrate concentration and incubation time, X0, S0 and I0 represent the initial biomass concentration, the corresponding substrate concentration and light intensity, respectively, V and A represent the volume and area of culture, Qb and Qs represent the heat of combustion of Chlorella and glucose. Qb was determined as Eq (4)
where UO2 represents the g cell C burned/mol O2 consumed (DaHai et al. 2011). They were quantified by the molecular formula of C. sorokiniana CS-1 (Goudriaan et al. 1979). The contents of C, H, O, N, S in C. sorokiniana LS-2 was tested by elemental analyzer (vario mcro cube, Germany), and the molecular formula of C. sorokiniana LS-2 biomass was determined according to their contents (Goudriaan et al. 1979).
The carbon-lipid conversion efficiency (ηClipid/Ctotal) was determined as mol carbon in fatty acid (FA) per mol fixed carbon. The formula was Eq (5)
where F is the percent of each FA, M is the FA molecular mass, N is the carbon number in FA, X is the biomass, C is the carbon concentration in biomass and Mc is the molar mass of carbon. Cetane number (CN) representing biodiesel quality was calculated as Eq (6) (Parsaeimehr et al. 2015).
where SV and IV are Saponification and Iodine value. Here, SV = Σ(560 * N)/M and IV = Σ(254 * DN)/M, where D is number of double bonds, M is fatty acid (FA) molecular mass and N is the percent of each FA.
Carbon dioxide fixation
The molecular formula of C. sorokiniana CS-1 biomass was calculated using the CHNS data and the accumulation of fixed CO2 (FD, mM L−1 day−1) was determined as Eq (7)
(Yoo et al. 2010), where Xt is the biomass concentration at time t (d) after inoculation (t0), X0 represents the biomass concentration at initial time t0, mcbm represents the mass fraction of carbon in Chlorella biomass (g g−1), V represents the working volume of media, mCO2 and mC represent the molar mass of CO2 and carbon (g per mol). The maximum carbon fixation (FDmax, mM L−1 day−1) was also calculated.
Results
Establishment of photoautotrophic and heterotrophic culture systems
The strain CS-1 with abilities in high-dose CO2 utilization or heterotrophic growth was identified by using 18S rDNA. It showed 99 % identity with C. sorokiniana. So the strain was named C. sorokiniana CS-1 and the strain was sent to China General Microbiological Culture Collection Center (CGMCC) catalogued as No. CGMCC 9215.
To establish a photoautotrophic system, microalgal dry weight under light intensities of 0, 40, 80, 120, 160, and 200 μE m−2 s−1 was determined (Fig. 2). It was shown that biomass yield increased along with an increase of the light intensity from 0 to 120 μE m−2 s−1, while a decrease was observed when the light intensity went up to 200 μE m−2 s−1 intensity supply. Typical microalgal growth responses upon the light intensity could be divided into light limitation, light saturation and light inhibition phases. In the light limitation phase, growth rates was positively related with the light intensity, while in the light saturation phase growth rates were independent of the light intensity and the supply of high-dose CO2 became a limiting factor for microalgal cell growth. In the present investigation, biomass yields and growth rates of C. sorokiniana CS-1 reached maximum when the light intensity was monitored around 120 μE m−2 s−1 in the photoautotrophic system. Hence, all the data of the photoautotrophic system shown below were generated in the light intensity of 120 μE m−2 s−1.
To establish a heterotrophic system, a central composite design (CCD) with two coded levels for glucose (A) and peptone (B) was used to optimize concentrations of carbon and nitrogen sources (Fig. 3). The levels of variables for the CCD were determined based on the initial results (Supplemental Table S1). The fitting of polynomial equations was described as Eq (8)
with the R2 = 0.9936. According to this model, the maximum biomass (6.14 g/L) was obtained at the glucose concentration of 17.31 g/L and peptone concentration of 5.32 g/L. Under this condition, the carbon source was excessive and would not become a limiting factor.
High-dose CO2 improves energy utilization and mimics a heterotrophic characteristic
The growth of C. sorokiniana CS-1 was improved by high-dose CO2
To investigate effects of high-dose CO2 on the growth of microalga, C. sorokiniana CS-1 was cultured under light and aerated with various concentrations of CO2 or cultured in heterotrophic medium under the dark. As microalgal cells grew up to the plateau stage, the maximum biomass (Xmax) and productivity (Pmax) in heterotrophic cultures reached to 6.12 g L−1 and 1.02 g L−1 day−1, respectively (Fig. 4a). Both parameters were much higher than those under air-aerated and light conditions, implying that C. sorokiniana CS-1 had higher energy conversion efficiency when utilizing glucose as a carbon source than that when air CO2 was used as a carbon source under light. The highest Xmax and Pmax (3.33 g L−1 and 0.56 g L−1 day−1) among a dose range of CO2 from air to 10 % were obtained when supplemented with 10 % CO2, an increase of 91.4 % in biomass, indicating that high-dose CO2 significantly promoted cell growth. The maximum specific growth rates (μmax) of C. sorokiniana CS-1 were 0.12, 0.13, 0.14 and 0.16 h−1 in the cultures aerated with 1, 2, 5 and 10 % CO2, respectively. An increase of 45.5 % in μmax was uncovered in the 10 % CO2 condition (Fig. 4b). Meanwhile, the maximum specific growth rate of the heterotrophic cultures was 0.19 ± 0.03 h−1, much higher than that under the air (0.11 h−1). Upon the treatment of high-dose CO2, the differences in the μmax between heterotrophy and photoautotrophy were reduced from 1.73 fold (air CO2) to 1.19 fold (10 % CO2), indicating the cell growth potential of photoautotrophic microalgae could be significantly improved by high-dose CO2 and somewhat comparable to that of heterotrophic growth. It also implied that such effectiveness of high-dose CO2 might reflect in the improvement of the carbon fixation and energy conversion. We therefore designed the following experiments to confirm the speculation.
The time courses of biomass production (a) and the specific growth rates (b) influenced by high-dose CO2 in Chlorella sorokiniana CS-1. The strain was cultured in modified BG-11 media aerated with 0.03 % (air), 1, 2, 5, or 10 % of CO2, or cultured in a heterotrophic medium. Data were presented as the mean ± standard error (n = 3) of three separated tests
Lipid production of C. sorokiniana CS-1 in high-dose CO2 condition mimics a heterotrophic characteristic
To investigate effects of high-dose CO2 on the lipid biosynthesis, parameters of the total lipid content, lipid accumulation curve and specific formation rate in C. sorokiniana CS-1 were also characterized. The total lipid content in the heterotrophic condition was 32 %, much higher than that under the air aeration and light (11 %) (Fig. 4a). Increased accumulation of total lipids could be attributed to sufficient supplies of carbon and energy. In photoautotrophic conditions, total lipid contents were positively correlated to CO2 concentrations and rose from 11 up to 28 % when aerated with 10 % CO2 (Fig. 5a). The significant increase in the total lipid content might be interpretated by an improvement in carbon supplies (e.g., 10 % CO2), whose situation is similar to heterotrophy where carbon supplies are usually excessive (e.g., 1.7 % glucose). The maximum specific formation rates (qmax) of lipids were 0.022, 0.049, 0.056, 0.061, and 0.066 g−1 h−1 in the cultures aerated with air CO2, 1, 2, 5 and 10 % CO2, respectively. It revealed a threefold increase in qmax at 10 % CO2 when compared with air CO2 (Fig. 5b, c). The qmax in heterotrophic conditions was 0.094 g−1 h−1. Obviously, the differences in the qmax between heterotrophy and photoautotrophy were significantly reduced from 4.27-fold of air CO2 to 1.42-fold of 10 % CO2. Taken together, sufficient carbon supplies in either high-dose CO2 or glucose generated similar influences on potential of the lipid formation, which in turn encourages us to compare the underlying energy conversions of the photoautotrophic and heterotrophic modes of microalgae.
The total lipid contents (a), time courses of total lipid contents (b) and the specific formation rates of lipid (c) affected by high-dose CO2 in microalgal Chlorella sorokiniana CS-1. The strain was cultured in modified BG-11 media aerated with 0.03 % (air), 1, 2, 5, or 10 % of CO2, or cultured in a heterotrophic medium. Data were presented as the mean ± standard error (n = 3) of three separated tests
Energy conversion efficiency was improved by high-dose CO2
To investigate effects of high-dose CO2 on the energy conversion efficiency, yields of total lipids were determined and compared under the photoautotrophic and heterotrophic conditions (Yang et al. 2000). Based on the heat of combustion with glucose, the Es (the total energy supplied to the reactor) of the heterotrophic culture was 271.8 kJ per liter at the consumption of 17.3 g glucose. The Es value of the photoautotrophic culture was 605.6 kJ per liter according to Eq (2). Not surprisingly, the energetic lipid yield (Ylipid/Es) (7.1 mg/kJ) in the heterotrophic culture was the highest due to the efficient conversion of chemical energy from glucose to lipid. In contrast, the lowest Ylipid/Es (0.32 mg/kJ) was formed in the photoautotrophic culture aerated with air CO2. Obviously, it’s the result of the inefficient conversion of light energy into lipids (Table 1). As expected, the Ylipid/Es value was significantly improved by high-dose CO2 from 0.32 in the air to 1.5 mg/kJ in 10 % CO2 because of the supplying of enough carbon sources. The differences in the Ylipid/Es between heterotrophy and photoautotrophy were significantly reduced from 22.19-fold of air CO2 to 4.73-fold of 10 % CO2. The data indicated that high-dose CO2 showed an obvious enhancement of conversion from light energy to lipids towards the conversion of chemical energy to lipids in heterotrophic conditions.
The carbon contents in biomasses were quantified and the molecular formulas of C. sorokiniana CS-1 was described as follows: CN0.13H1.83O0.64 (air), CN0.13H1.9O0.63 (1 % CO2), CN0.09H1.89O0.70 (2 % CO2), CN0.13H1.79O0.51 (5 % CO2), CN0.14H1.80O0.50 (10 % CO2), and CN0.15H1.88O0.65 (glucose). The energy yield (Ψ) was then calculated based on the molecular formulas of biomasses as well as Eq (3). The Ψ went up proportional to concentrations of aerated CO2 and the highest one was 12.4 % in 10 % CO2, an increase of 69.6 % compared with air aeration (7.3 %). High-dose CO2 improved the energy yield of photoautotrophy and also showed an enhancement towards the energy yield of heterotrophy, although it was not as obvious as Ylipid/Es.
Carbon-lipid conversion efficiency of C. sorokiniana CS-1 at high-dose CO2 mimics that of heterotrophy
The carbon-lipid conversion efficiency (ηClipid/Ctotal) of C. sorokiniana CS-1 was determined according to the Eq (5). The ηClipid/Ctotal values of cultures were 18.5, 26.8, 32.3, 40.8, 42.4 and 53.7 % under air, 1, 2, 5, 10 % CO2, or glucose, respectively. Again, the highest ηClipid/Ctotal value was observed in heterotrophic conditions, and the ηClipid/Ctotal value in photoautotrophic cultures also rose up greatly along with an increase of CO2 concentrations. The differences in the ηClipid/Ctotal between heterotrophy and photoautotrophy were also significantly decreased from 2.90-fold of air CO2 to 1.27-fold of 10 % CO2. The significant enhancement in the ηClipid/Ctotal under 10 % CO2 aeration hints that an efficient carbon flow from fixed carbon into lipid should occur compared with that of air aeration, just like the case of heterotrophy.
Fixed carbons as an energy carrier were improved by high-dose CO2
We then estimated the carbon fixation upon increased CO2 concentrations in photoautotrophic cultures by detecting the average carbon fixation (FDavg) and the maximum carbon fixation (FDmax) (Fig. 6). No doubt, the highest FDavg was 35.29 mM L−1 day−1 in the heterotrophic culture using glucose as the carbon source, while the lowest one belonged to the photoautotrophic culture aerated with air (11.76 mM L−1 day−1). The lowest value showed stable upon the fluctuation even the light intensity rose up to 160 μE m−2 s−1, suggesting that the CO2 supply would be a primary factor determining the carbon fixation efficiency. On the other hand, similar changes occurred in the FDmax values, that is, a nearly linear increase from 24.9 to 55.4 mM L−1 day−1 along with the increase of CO2 concentrations from air to 10 % CO2, suggesting a significant shift towards that of heterotrophy fed with glucose (83.0 mM L−1 day−1).
Changes of the average carbon fixation rate and the maximum carbon fixation rate affected by high-dose CO2 in microalgal Chlorella sorokiniana CS-1. The strain was cultured in modified BG-11 media aerated with 0.03 % (air), 1, 2, 5, or 10 % of CO2, or cultured in a heterotrophic medium. Data presented as the mean ± standard error (n = 3) of three separated tests
Influences of high-dose CO2 on fatty acid profiles
The fatty acid profiles in heterotrophic condition as well as in photoautotrophic conditions aerated with high-dose CO2 were monitored through the GC analysis (Table 2). It showed that C14, C16, and C18 fatty acids accounted for more than 99 % of extracted lipids. Along with the treatments of high-dose CO2, the obvious changes in the profiles occurred as decreased in the saturated C16:0 and increased in the unsaturated fatty acids (C16:2, C16:3, C18:1, C18:3), which resulted in the lower cetane number of derived biodiesel (e.g. 40.0 under 10 % CO2) when compared with biodiesels from cultures under the air CO2 (45.8) or heterotrophic conditions (48.2). The lower cetane number means lower quality of biodiesel (Amin 2009). These results implied that high-dose CO2 could improve yields of derived biodiesels at a cost of biodiesel quality. To solve this problem, alpha-linolenic acid (ALA, C18:3), the essential fatty acid for human health could be removed from the fatty acid profile. After that the rest fatty acid was used to produce biodiesel. If the ALA was removed from the fatty acid profile, the CN of the derived biodiesel from cultures under 10 % CO2 could be increased to 55.3, which is similar with that of heterotrophic conditions.
Discussion
Quite large amounts of the solar energy are wasted and this case leads to an extremely low photosynthetic efficiency of higher plants (Long et al. 2006). As revealed in the previous investigation, the shortage of the carbon supply limits light energy conversions and storage in lipids (Goudriaan and Ajtay 1979). We have confirmed here high-dose CO2 (e.g., 10 %) could improve the energy yield from 7.3 % of the air control to 12.4 %. The enlarged carbon supply also facilitates the energy flow from light energy to lipid (Ylipid/Es increased from 0.32 to 1.5 mg/kJ), and consequently the lipid content is at least doubled. This investigation has shown several significances. Firstly, high-dose CO2 might offer a practical approach leading to higher lipid yields, which is the foundation for an engineering photosynthetic system with the industrial efficiency (Wang et al. 2008). As stated above, photosynthesis is usually low in light energy utilization but in nature is a sustainable biosystem because of endless light energy inputs (Kumar et al. 2010). In contrast, heterotrophic fermentation is with high energy conversion efficiency from glucose to lipid (Xiong et al. 2010), however in nature is unsustainable since the organic carbons (e.g., glucose) should be sourced from photoautotrophic organisms. Secondly, microalgae with stimulated neutral lipid accumulation by high-dose CO2 might become an effective lipid production system, in which the carbon limitation was greatly alleviated (Dragone et al. 2010). It thus appears superior to the nitrogen defective lipid production system, a popular system where lipid accumulation is at a cost of growth and biomass (Rodolfi et al. 2009). Thirdly, the effective biofixation of high-dose CO2 by microalgae ensures a promising strategy for the carbon sequestration since high-dose CO2 could be derived from a variety of flues gases (Doucha et al. 2005) and the massive transfer of CO2 into photobioreactors has also been designed (Chen et al. 2011).
There should be multiple steps from the inorganic carbon fixation to neutral lipid (e.g., triacylglycerol) formation to complete light energy conservation and storage. It also remains unknown which components are critical to the enhanced lipid accumulation by high-dose CO2. As revealed in the current work, the carbon flow is significantly enhanced by 10 % CO2. In the photosynthetic routes, fatty acids are formed from the Calvin cycle product glyceraldehyde-3-phosphate (GAP) by the following reaction sequence: 18 CO2 + 52 NADPH (NADH) + 71ATP C18:0 (Xiong et al. 2008). The net carbon stoichiometry shows that generation of one molecule of stearic acid (C18:0) requires 71 molecules of ATP and 52 molecules of reductant (Xiong et al. 2008). This result suggested CO2 initiated lipid synthesis consumed great amounts of cofactors, which limited the carbon conversion ratio of CO2 to lipid. In the heterotrophic mode, the net stoichiometry is: 9 Glucose 18 CO2 + C18:0 (Xiong et al. 2008). In this case, ATP and reductant are almost balanced between lipid biosynthesis and glucose oxidation. Therefore, the maximum carbon conversion ratio of glucose to lipid (Clipid/Cglucose) is estimated up to 66.67 %, much higher than that of photoautotrophic cultures. In the photoautotrophic mode, since much reducing power and ATP molecules are obtained through photosynthesis, the respiratory metabolism might not be an essential source of reducing power and ATP. The photophosphorylation is a less efficient energy-producing pathway than the mitochondrial oxidative phosphorylation (Yang et al. 2000). The increased carbon fixation by high-dose CO2 might accelerate the whole cell metabolism processes including photosynthesis, glucose metabolism and oxidative phosphorylation. Therefore, more reducing power and ATP molecules for lipid synthesis might be generated from efficient oxidative phosphorylation, which improves the carbon-lipid conversion efficiency. This case might be analogous to heterotrophy where reducing power and ATP are generated from the oxidative phosphorylation and consequently high-dose CO2 makes C. sorokiniana CS-1 mimic a heterotrophy-like characteristic in the carbon-lipid conversion. A global comparative analysis of microalgal genomics will be initiated to clarify these interesting questions.
The development of the high-dose CO2 effectiveness into an engineering technology is another important concern in the microalgal biosystem. A series of scaleup experiments are required to test and strengthen the principle uncovered in this investigation. For example, the facilities for the CO2 microbubble generation and massive transfer will be optimized and coupled to photobioreactors (Chen et al. 2011; Sun et al. 2014).
Conclusions
High-dose CO2 significantly enhanced the light energy conversion and storage into lipid, which probably in a manner mimicked the characteristic of heterotrophy in C. sorokiniana CS-1. The underlying mechanisms have been uncovered by analyzing a series of parameters indicative of improved efficiencies in the carbon fixation, energy yield, and carbon-lipid conversion. Its significances are also addressed to build up an efficient photosynthetic system for biofuel production at large scales and a bio-fixation system for carbon sequestration of flue gases.
Abbreviations
- Es:
-
The total energy supplied to the reactor
- ATP:
-
Adenosine triphosphate
- CCD:
-
Central composite design
- Xmax:
-
The maximum biomass
- Pmax:
-
The maximum productivity
- μ:
-
Specific growth rate
- qmax :
-
Specific formation rate of lipid
- Es:
-
The total energy supplied to the reactor
- Ylipid/Es :
-
Energetic lipid yield
- Qb and Qs:
-
The heat of combustion of C. sorokiniana and glucose
- Ψ:
-
Energy yield
- ηClipid/Ctotal :
-
Carbon-lipid conversion efficiency
- FDavg and FDmax:
-
The average and maximum carbon fixation
- CCMs:
-
CO2 concentrating mechanisms
- DIC:
-
Dissolved inorganic carbon
- CBB:
-
The Calvin–Benson–Bassham
References
Amin S (2009) Review on biofuel oil and gas production processes from microalgae. Energy Convers Manag 50(7):1834–1840
Axelsson L, Beer S (2001) Carbon Limitations. In: Rai LC, Gaur JP (eds) Algal adaptation to environmental stresses. Springer, Berlin, pp 21–43
Boelen P, van Dijk R, Damsté JSS, Rijpstra WIC, Buma AG (2013) On the potential application of polar and temperate marine microalgae for EPA and DHA production. AMB Express 3:1–9
Chen Y-F, Wu Q (2011) Production of biodiesel from algal biomass: current perspectives and future. In: Pandey A, Larroche C, Ricke SC, Dussap CG, Gnansounou E (eds) Biofuels: alternative feedstocks and conversion processes. Elsevier, pp 399–413
Chen CY, Yeh KL, Aisyah R, Lee DJ, Chang JS (2011) Cultivation, photobioreactor design and harvesting of microalgae for biodiesel production: a critical review. Bioresour Technol 102(1):71–81
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25:294–306
Chiu S, Kao C, Chen C, Kuan T, Ong S, Lin C (2008) Reduction of CO2 by a high-density culture of Chlorella sp. in a semicontinuous photobioreactor. Bioresour Technol 99:3389–3396
DaHai T, Wei H, PengLin L, XiaoLing M, JianJiang Z (2011) CO2 biofixation and fatty acid composition of Scenedesmus obliquus and Chlorella pyrenoidosa in response to different CO2 levels. Bioresour Technol 102:3071–3076
Demirbas A (2011) Biodiesel from oilgae, biofixation of carbon dioxide by microalgae: a solution to pollution problems. Appl Energy 88(10):3541–3547
Doucha J, Straka F, Lívanský K (2005) Utilization of flue gas for cultivation of microalgae Chlorella sp.) in an outdoor open thin-layer photobioreactor. J Appl Phycol 17(5):403–412
Douskova I, Doucha J, Livansky K, Machat J, Novak P, Umysova D, Zachleder V, Vitova M (2009) Simultaneous flue gas bioremediation and reduction of microalgal biomass production costs. Appl Microbiol Biotechnol 82:179–185
Dragone G, Fernandes B, Vicente A, Teixeira JA (2010) Third generation biofuels from microalgae. In: Vilas AM (ed) Current research, technology and education topics in applied microbiology and microbial biotechnology. Formatex Research Center, Badajoz, pp 1355–1366
Ducharme NA, Bickel PE (2008) Minireview: lipid droplets in lipogenesis and lipolysis. Endocrinology 149:942–949
Goudriaan J, Ajtay G (1979) The possible effect of increased CO2 on photosynthesis. In: Bolin B, Degens ET, Kempe S, Ketner P (eds) The global carbon cycle. Wiley, Chichester, pp 237–249
Guedes AC, Amaro HM, Malcata FX (2011) Microalgae as sources of carotenoids. Mar Drugs 9:625–644
Halim R, Gladman B, Danquah MK, Webley PA (2011) Oil extraction from microalgae for biodiesel production. Bioresour Technol 102:178–185
Ho K, Payne W (1979) Assimilation efficiency and energy contents of prototrophic bacteria. Biotechnol Bioeng 21:787–802
Ho SH, Chen WM, Chang JS (2010) Scenedesmus obliquus CNW-N as a potential candidate for CO2 mitigation and biodiesel production. Bioresour Technol 101:8725–8730
Huang G, Chen F, Wei D, Zhang X, Chen G (2010) Biodiesel production by microalgal biotechnology. Appl Energy 87:38–46
Khataee AR, Dehghan G, Ebadi E, Pourhassan M (2010) Central composite design optimization of biological dye removal in the presence of macroalgae Chara sp. Clean Soil Air Water 38:750–757
Kumar A, Ergas S, Yuan X, Sahu A, Zhang Q, Dewulf J, Van Langenhove H (2010) Enhanced CO2 fixation and biofuel production via microalgae: recent developments and future directions. Trends Biotechnol 28(7):371–380
Li X, Xu H, Wu Q (2007) Large-scale biodiesel production from microalga Chlorella protothecoides through heterotrophic cultivation in bioreactors. Biotechnol Bioeng 98:764–771
Liang Y, Sarkany N, Cui Y (2009) Biomass and lipid productivities of Chlorella vulgaris under autotrophic, heterotrophic and mixotrophic growth conditions. Biotechnol Lett 31:1043–1049
Long SP, Zhu XG, Naidu SL, Ort DR (2006) Can improvement in photosynthesis increase crop yields? Plant Cell Environ 29(3):315–330
Miao X, Wu Q (2006) Biodiesel production from heterotrophic microalgal oil. Bioresour Technol 97(6):841–846
Nakkash N, Wang Z, Naterer G (2013) Solar thermal energy-based high purity CO2 release from carbonate sorbents. http://www3.aiche.org/Proceedings/content/Annual-2013/extended-abstracts/P319102.pdf
O’Neill BF, Zangerl AR, Dermody O, Bilgin DD, Casteel CL, Zavala JA, DeLucia EH, Berenbaum MR (2010) Impact of elevated levels of atmospheric CO2 and herbivory on flavonoids of soybean (Glycine max Linnaeus). J Chem Ecol 36:35–45
Parsaeimehr A, Sun Z, Dou X, Chen Y-F (2015) Simultaneous improvement in production of microalgal biodiesel and high-value alpha-linolenic acid by a single regulator acetylcholine. Biotechnol Biofuels 8:11. doi:10.1186/s13068-015-0196-0
Qiao H, Wang G, Zhang X (2009) Isolation and characterization of Chlorella sorokiniana Gxnn01 (Chlorophyta) with the properties of heterotrophic and microaerobic growth. J Phycol 45:1153–1162
Rodolfi L, Chini Zittelli G, Bassi N, Padovani G, Biondi N, Bonini G, Tredici MR (2009) Microalgae for oil: strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnol Bioeng 102(1):100–112
Rosa APCD, Carvalho LF, Goldbeck L, Costa JAV (2011) Carbon dioxide fixation by microalgae cultivated in open bioreactors. Energy Convers Manag 52:3071–3073
Sforza E, Bertucco A, Morosinotto T, Giacometti GM (2012) Photobioreactors for microalgal growth and oil production with Nannochloropsis salina: from lab-scale experiments to large-scale design. Chem Eng Res Des 90:1151–1158
Sun X, Cao Y, Xu H, Liu Y, Sun J, Qiao D, Cao Y (2014) Effect of nitrogen-starvation, light intensity and iron on triacylglyceride/carbohydrate production and fatty acid profile of Neochloris oleoabundans HK-129 by a two-stage process. Bioresour Technol 155:204–212
Sydney EB, Sturm W, de Carvalho JC, Thomaz-Soccol V, Larroche C, Pandey A, Soccol CR (2010) Potential carbon dioxide fixation by industrially important microalgae. Bioresour Technol 101:5892–5896
Wang B, Li Y, Wu N, Lan CQ (2008) CO2 bio-mitigation using microalgae. Appl Microbiol Biotechnol 79(5):707–718
Westerhoff P, Hu Q, Esparza-Soto M, Vermaas W (2010) Growth parameters of microalgae tolerant to high levels of carbon dioxide in batch and continuous-flow photobioreactors. Environ Technol 31:523–532
Xiong W, Li X, Xiang J, Wu Q (2008) High-density fermentation of microalga Chlorella protothecoides in bioreactor for microbio-diesel production. Appl Microbiol Biotechnol 78:29–36
Xiong W, Gao C, Yan D, Wu C, Wu Q (2010) Double CO2 fixation in photosynthesis–fermentation model enhances algal lipid synthesis for biodiesel production. Bioresour Technol 101(7):2287–2293
Xu H, Miao X, Wu Q (2006) High quality biodiesel production from a microalga Chlorella protothecoides by heterotrophic growth in fermenters. J Biotechnol 126(4):499–507
Yang C, Hua Q, Shimizu K (2000) Energetics and carbon metabolism during growth of microalgal cells under photoautotrophic, mixotrophic and cyclic light-autotrophic/dark-heterotrophic conditions. Biochem Eng J 6:87–102
Yemm EW, Willis AJ (1954) The estimation of carbohydrates in plant extracts by anthrone. Biochem J 57(3):508–514
Yoo C, Jun SY, Lee JY, Ahn CY, Oh HM (2010) Selection of microalgae for lipid production under high levels carbon dioxide. Bioresour Technol 101:S71–S74
Yuan JP, Peng J, Yin K, Wang JH (2011) Potential health-promoting effects of astaxanthin: a high-value carotenoid mostly from microalgae. Mol Nutr Food Res 55:150–165
Zeng X, Danquah MK, Chen XD, Lu Y (2011) Microalgae bioengineering: from CO2 fixation to biofuel production. Renew Sust Energy Rev 15:3252–3260
Acknowledgments
This work was supported by the Jiangsu Agricultural Science and Technology Innovation Funds (CX (12) 3041), the Natural Science Foundation of Jiangsu Province (BK20130712), and the Open Foundation of Jiangsu Key Laboratory for Microbes and Functional Genomics Key Laboratory (164070303402). Dr. Ali Parsaeimehr and Dr. Sitwat Aman critically read the manuscript. Undergraduate student Dongling Liang assisted in the experiments.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sun, Z., Dou, X., Wu, J. et al. Enhanced lipid accumulation of photoautotrophic microalgae by high-dose CO2 mimics a heterotrophic characterization. World J Microbiol Biotechnol 32, 9 (2016). https://doi.org/10.1007/s11274-015-1963-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-015-1963-6