Abstract
As one of the promising species of microalgae for biofuel production, Chlorella vulgaris CS-42 was cultivated phototrophically in two cylindrical photobioreactors with aeration of 5 % (v/v) CO2 or air for 13 days to evaluate the effects of CO2 supplementation on biomass, CO2 fixation performance, and biochemical content. Significant increases of specific growth rate and total carbon content in biomass resulting in a higher CO2 fixation rate were found with 5 % CO2. The maximum biomass concentration, carbohydrate and fatty acid contents with 5 % CO2 were significantly higher than those with air, while carbohydrate biosynthesis was most affected as compared to other biochemical components. Cytomic analysis revealed a rapid accumulation of neutral lipid in the late growth phase with more lipid bodies visualized by confocal laser scanning microscopy (CLSM), when nitrate consumption was accelerated with CO2 supplementation. Gas chromatography mass spectrometry (GC-MS) analysis indicated that 5 % CO2 favored the formation of C18:2, which led to a decrease in the degree of lipid unsaturation (DLU). These results proved that CO2 supplementation was one of the most efficient methods to significantly prompt the growth of microalgae and increase the C/N ratio in the medium, which in turn regulated the carbon metabolic flux to enhance neutral lipid and fatty acid production in C. vulgaris.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the rise of global energy crisis and concerns about sustainable and environment-friendly development, renewable energy including biofuels has received worldwide attentions (Huang et al. 2010; Tang et al. 2011b). Microalgae have emerged as an alternative feedstock of biodiesel with several advantages for crude oil production, such as high growth rate, high area yield of oil in biomass (Chisti 2007), and not competing with food crops and forestry for arable land and clean water (Chisti 2008; Huang et al. 2010). For low-cost production of oil-rich biomass of microalgae, the most attractive way is to produce desirable biofuels with value-added co-products simultaneously coupled with reducing CO2 emission from flue gas (Naik et al. 2010). Over 45 % of photosynthetic CO2 sequestration on earth may occur via algae (Matsuda et al. 2011). About 1.65 to 1.83 units (of mass) of carbon dioxide is captured by 1 unit of microalgal biomass (dry cell weight, DW) (Doucha et al. 2005). Therefore, high photosynthetic efficiency gives microalgae much potential to be a moderate, sustainable, and cost-effective energy source. However, carbon dioxide is a limiting factor for microalgal growth phototrophically due to its low concentration (0.04 %, v/v) in the air (Sforza et al. 2010). It can be reasoned that addition of carbon dioxide in the culture can accelerate microalgal biomass productivity and lipid content (Muradyan et al. 2004; Tang et al. 2011a).
Chlorella is a well-studied genus of green microalgae and has been cultivated in commercial raceway open ponds or closed photobioreactors for health food and animal feed production as well as wastewater treatment. Some species of Chlorella have been reported with high energy content (Malcata 2011). Lipid profile and productivity from Chlorella strains in response to CO2 levels have been studied. Compared to atmospheric CO2 concentration, aeration with 0.33 and 0.83 % (v/v) CO2 for Chlorella vulgaris cultivation enhanced the total intracellular lipid by 27 and 25 %, respectively, which was superior to N-starvation treatment, and that lipid productivity was proportionate to the increase of CO2 concentration (Widjaja et al. 2009). Moreover, high concentration of CO2 (30–50 %) facilitated the synthesis of unsaturated fatty acid in Chlorella pyrenoidosa (Tang et al. 2011a).
C. vulgaris has been suggested as a promising candidate for biodiesel production (Francisco et al. 2010). Although CO2 concentration is known to impact most microalgae during photoautotrophic growth, the intrinsic relationships between lipids and other biochemical components with regard to CO2 level still need to be well understood, which is crucial for biofuels and co-product production as well as CO2 mitigation via C. vulgaris. In the present work, C. vulgaris CS-42 was cultivated autotrophically in closed photobioreactors with aeration of air or 5 % (v/v) CO2 for 13 days. The aim of this study is to evaluate the effects of CO2 supplementation on microalgal biomass and biochemical compounds, especially carbohydrate and fatty acid production as well as CO2 fixation rate, and to obtain a informative understanding of regulation of carbon metabolic flux responding to CO2 supplementation within monitored culture systems.
Materials and methods
The unicellular green microalga C. vulgaris CS-42 was purchased from CSIRO Marine Laboratory, Australia. The lipophilic fluorescent dye BODIPY 505/515 (4,4-difluoro-1,3,5,7-tetramethyl-4-bora-3a,4a-diaza-sindacene) was from Invitrogen Molecular Probes (USA). HPLC-grade acetonitrile and methanol were from Merck (Germany). Nonadecanoic acid (C19:0) was from Sigma (USA). The other chemicals and solvents were of analytical grade. Distilled water was purified in-house using a Milli-Q system (Millipore, USA).
Seed culture in shaking flasks
The seed culture of C. vulgaris CS-42 with initial biomass concentration of 0.05 g L−1 started to grow in 100-mL basal medium (Shi et al. 1997) in 250mL Erlenmeyer flasks with continuous shaking at 130 rpm and illumination of ca. 70 μmol photons m−2 s−1 at 25 ± 0.5 °C for 6 days to reach the exponential phase (ca. 0.5 g L−1). The seed culture was transferred to 1 L of Erlenmeyer flasks containing 500- mL basal medium with 20 % (v/v) inoculum and cultured for 5 days under the same condition. The initial pH of the medium was adjusted to 6.5. Air was bubbled into the medium after filtration through a 0.22- μm membrane filter (Millipore).
Cultivation in photobioreactors and biomass harvest
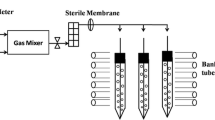
Two cylindrical glass photobioreactors (PBRs) were employed in parallel experiments for 13-day cultivation. The PBRs of 5 L (300 mm in height, 140 mm in diameter) were surrounded by cool white 8- W fluorescence lamps to provide continuous illumination with light intensity of about 100 μmol photons m−2 s−1 measured in the center of the PBR. The seed culture was simultaneously transferred into these PBRs individually containing 3.5 L of basal medium with initial biomass concentration of 0.05 g L−1. Air or 5 % (v/v) CO2 was bubbled into the medium after filtration through a 0.22- μm membrane filter at a flow rate of 0.5 vvm. Through a gas mixer, 5 % CO2 was generated by mixing CO2 from cylinder with ambient air. The medium was continuously blended by a stirrer in the center of the PBR at 100 rpm. pH was continuously monitored by pH electrodes (Hamilton, Switzerland). Three samples were separately taken as technical replicates every 24 h from each PBR for further analysis. PBRs, medium, and all items were autoclaved at 121 °C for 20 min before use.
Determination of cell growth and biochemical components
Cell growth was determined by measuring optical density (OD690) via microplate reader (Sunrise, Tecan, Sweden). Biomass in the sample was harvested by centrifugation and washed twice with distilled water prior to freeze drying. According to preliminary experiments, the relationship between OD690 and DW of C. vulgaris CS-42 was established as follows:
where y represents the dry cell weight (DWs) and x is the optical density (OD690). DWs were determined by weighing the lyophilized cells. Specific growth rate (μ, day−1) was calculated by the following formula:
where W t and W 0 are the final and initial biomass concentration (g L−1), respectively, and Δt is the cultivation time in days. The cell-free medium was used to determine nitrate concentration (Hecht and Mohr 1990) after centrifugation and filtration through a 0.22- μm membrane filter.
Total carbon content (C C, % w/w) in dry biomass was analyzed by an element analyzer (EuroEA3000, EuroVector S.p.A., Italy). As described by de Morais and Costa (2007a), CO2 fixation rate (R CO2, g L−1 day−1) was calculated using the following equation:
where P is the biomass productivity (g L−1 day−1), M C is the molecular weight of carbon, and M CO2 is the molecular weight of CO2.
Total chlorophyll content was measured by spectrophotometric method (Ritchie 2006). The soluble protein was extracted from biomass (Popovich et al. 2012) followed by spectrophotometric determination using the Coomassie Brilliant Blue dye (Bradford 1976). Measurement of total lipid content was carried out according to miniaturized Bligh-Dyer method (Burja et al. 2007). Carbohydrate content was determined by phenol-sulfuric method (Dubois et al. 1956). All assays above were operated in triplicates on lyophilized cells sampled at 312 h.
Fatty acid (FA) composition in dry biomass was analyzed by gas chromatography mass spectrometry (GC-MS). Nonadecanoic acid (C19:0) was added as internal standard. Fatty acid methyl esters (FAMEs) were prepared and analyzed according to the protocol of Lu et al. (2012). The degree of lipid unsaturation (DLU) was calculated according to Kates and Baxter (1962):
Cytomic analysis based on fluorescent characteristics
The lipophilic fluorescent dye BODIPY 505/515 was used to stain the cells in order to assess the biosynthesis of intracellular neutral lipid according to the protocol of Govender et al. (2012). BODIPY 505/515 was dissolved in DMSO to prepare a stock solution of 0.1 g L−1 and stored in the dark. Aliquots of the stock solution were added to 1- mL cell suspensions (1 × 106 cells mL−1) in 2-mL Eppendorf tubes with a final dye concentration of 0.05 μg mL−1. The tubes were incubated for 3 min with constant shaking at room temperature in the dark. Fluorescence was measured on a fluorescence microplate reader (FMR, FLUOstar OPTIMA, BMG, Germany) with excitation wavelength at 485 ± 10 nm and emission wavelength at 530 ± 10 nm.
Flow cytometry (FC) was used to evaluate the relative quantity of intracellular neutral lipid in single cells. The harvested cells during cultivation were resuspended in distilled water at a density of ca. 5 × 106 cells mL−1. The staining with BODIPY 505/515 was the same as the method used above. After filtration through a 45- μm membrane filter, all samples were analyzed using BD Accuri C6 Flow Cytometer (BD Biosciences, USA) equipped with 488- nm solid-state blue laser. The acquisition settings were 104 events with a medium flow rate at 35 μL min−1 (16- μm core size). Nonalgal particles and dead cells were excluded from the analysis by gating on FL3 channel. All settings and compensations of all channels were preliminarily optimized and unchanged throughout the analysis.
To visualize the intracellular lipid bodies, fluorescence images of the stained cells were captured on a confocal laser scanning microscope (CLSM, TCS SP5; Leica Microsystems CMS, Germany) under HCX PL APO CS 100× NA 1.4 oil immersion with confocal pinhole set at Airy 1 and 3× zoom factor for improved resolution with eight bits. A blue excitation light was used through a band-pass filter (460–490 nm), and emission wavelengths were imaged through a long-pass filter (510–540 nm). Laser transmission and scan settings were constant in all scans.
Statistics
All values are expressed as mean ± standard deviation (SD). A Student’s t test was used to evaluate differentiation of biochemical contents between two conditions. A value of p < 0.05 was considered to be statistically significant.
Results and discussion
Cell growth and pH changes in the medium
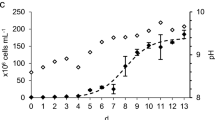
Growth curve of C. vulgaris with air or 5 % CO2 in two PBRs is shown in Fig. 1. Cells grew exponentially after 24 h in both conditions. The maximal biomass concentration reached 1.80 g L−1 at 264 h with 5 % CO2 supplementation, which was significantly higher than that (1.34 g L−1) at 312 h with air (Table 1). The maximal specific growth rate and biomass productivity reached 0.96 day−1 and 0.24 g L−1 day−1 in the exponential phase with 5 % CO2, respectively. The former is higher than that 0.31 day−1 from De Morais and Costa (2007b), who used vertical tubular reactors supplemented with 6 % CO2 to cultivate C. vulgaris LEB 12. The increase of the carbon dioxide concentration during cultivation is known to increase the specific growth rate and photosynthetic activity of microalgae. Used in laboratories, 1–5 % (v/v) CO2 has been shown to be optimal for culturing Chlorella and most of industrial microalgae (Hirata et al. 1996; Sydney et al. 2010). However, a few studies showed that Chlorella was sensitive to concentration of CO2 and exhibited inhibited growth when the CO2 level exceeded 5 % (Chiu et al. 2008). In the present study, the maximum specific growth rate, biomass concentration, and productivity with 5 % CO2 were 10, 34, and 46.9 % higher than those with air.
The pH changes during the cultivation in two PBRs are depicted in Fig. 1. The pH dramatically decreased from 6.5 to 5.9 in the first 12 h with 5 % CO2 but then slowly increased and remained at an optimal pH range from 6.1 to 6.8, while a steady increase of pH from 6.5 to 8.5 was observed with air. There is a complex relationship between CO2 concentration and pH in the aqueous culture environment, due to the chemical equilibria among the inorganic carbon species CO2, HCO3 −, and CO3 2−, which play an important role in microalgal growth. According to the thermodynamic model, HCO3 − is the dominant fraction when pH is between 6.1 and 6.8, where the ratio of [HCO3 −]/[CO2] in the medium ranges from approximately 1 to 6.3 (pK*1 = 6.1, T = 25 °C). As most microalgae seem to grow well under low-CO2 and HCO3 −-enriched environment (Ota et al. 2009), such near neutral pH range is beneficial to microalgal growth. In the present study, although no attempt was made to maintain the microalga at an appropriate pH, the supplementation of 5 % CO2 did not appear to have an adverse effect on cell growth, in contrast, contributed to a stable and optimal range of pH for cell growth.
Carbon content in biomass and CO2 fixation rate
The mean total carbon content (C C) in biomass harvested at 312 h in two conditions is shown in Table 1. The results show that C C with 5 % CO2 presented a significant increase (p < 0.05) as compared to that with air. As shown in Fig. 2a, a higher CO2 fixation rate was observed with the mean and maximum value at 0.28 and 0.51 g L−1 day−1 with 5 % CO2, which was 34.8 and 57.1 % higher than that with air (0.21 and 0.33 g L−1 day−1), respectively. The maximum CO2 fixation rate occurred during the exponential phase, indicating that Chlorella exhibited high-level photosynthetic activity at that time. The ratio of CO2 fixation rate over biomass productivity throughout the exponential phase was calculated and is shown in Fig. 2b, indicating the conversion efficiencies (CEs) from carbon dioxide to biomass. As shown in this figure, CO2 supplementation resulted in higher CEs than that with air, ranging from 1.80 to 2.05. That is, 1 g (DW) of biomass captured approximately 1.80 to 2.05 g of CO2 as a result of CO2 supplementation, suggesting that 5 % CO2 supplementation to the medium elevated the utilization of CO2 by the microalga.
During the exponential phase of microalgal growth when photosynthetic rate is high, the accumulation of photosynthesis-generated oxygen occurs in the culture (Kumar et al. 2010). Ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCO) (EC 4.1.1.39) is the key enzyme of CO2 fixation, catalyzing the reaction of CO2 assimilation to produce two molecules of 3-phosphoglycerate, which are subsequently catalyzed as substrates for carbohydrate and FA biosynthesis. Oxygen in the culture can compete with CO2 for RuBisCO in Calvin cycle, which is also known as photorespiration, eventually reducing CO2 fixation and compromising the photosynthetic efficiency (Zeng et al. 2011). Therefore, higher CO2 concentration in the medium is beneficial and diminishes photorespiration. In the present study, when compared with air, 5 % CO2 resulted in higher CO2 concentration in the medium which elevated CO2 fixation rate and consequently accelerated cell growth and biochemical biosynthesis.
Cytomic study on neutral lipid accumulation
Cytomic analysis based on FMR and FC was performed to track the accumulation of neutral lipid in C. vulgaris. BODIPY 505/515 is a highly lipophilic fluorophore and spectrally distinguishable from algal chlorophyll auto-fluorescence (Brennan et al. 2012). In this study, BODIPY 505/515 was chosen instead of Nile Red based on our preliminary studies that Nile Red was not suitable for staining C. vulgaris CS-42. With the help of the fluorescent probe, the total fluorescence (TF) measured by FMR reflects the accumulative signals from algal population, while the mean fluorescence (MF) measured by FC provides a reference for relative amount of neutral lipid from individual cells. As depicted in Fig. 3a, TFs showed no statistical difference between air and 5 % CO2 prior to 144 h. Subsequently, a rapid increase of TFs driven by 5 % CO2 was observed, which was sharper and appeared earlier than that with air. Similar trend was observed in the evolution of MF although the difference between the two conditions seemed less dramatic.
Fluorescence images of stained cells in the two conditions were captured via confocal laser scanning microscope (CLSM) to visualize intracellular lipid droplets (Fig. 4). Compared to the stained cells with air (Fig. 4a), more intracellular lipid droplets with their corresponding brilliant green fluorescence and stronger fluorescence were observed in the stained cells with 5 % CO2 (Fig. 4b).
Nitrate concentration was monitored throughout the cultivation (Fig. 3b). A constant decrease of nitrate concentration in the media was observed in both conditions, while the average nitrate uptake rate (0.78 mmol L−1 day−1) with 5 % CO2 was higher than that 0.62 mmol L−1 day−1 with air. A sharper decrease was observed with 5 % CO2 after 144 h, since when the neutral lipid biosynthesis was enhanced by the additional CO2 (reflected by TF and MF). It has been extensively reported that lipid accumulation can be triggered in microalgae by nutrient limitation (e.g., nitrogen deficiency) (Guschina and Harwood 2006). In the present study, neutral lipid accumulation occurred when the nitrate was not significantly depleted in the medium. One possible reason for this phenomenon is that CO2 supplementation not only provided adequate carbon source leading to a high C/N ratio to regulate carbon metabolic flux, but also enhanced photosynthesis to generate more reducing power (NADH and NADPH) and energy (ATP) for FAs and lipid biosynthesis.
Biochemical analysis
As one of the major components in C. vulgaris, carbohydrate content is approximately 12–17 % DW under common condition (Spolaore et al. 2006), which is generally lower than that of protein or lipid. As shown in Table 1, 5 % CO2 drove cells to accumulate carbohydrate up to 36.77 % DW, which was significantly higher (p < 0.05) than that with air (20.30 %). Carbohydrate productivity was 0.05 and 0.02 g L−1 day−1 with 5 % CO2 and air, respectively, which presents a marked increase of 150 %.
Lipid content and lipid productivity are the two vital indicators to assess the performance of lipid production from microalgae (Griffiths and Harrison 2009). In this study, due to the increase of biomass productivity (0.10 to 0.13 g L−1 day−1) and lipid content (21.69 to 28.07 % DW) (Table 1), total lipid productivity with 5 % CO2 (0.04 g L−1 day−1) was 100 % higher than that with air (0.02 g L−1 day−1).
The biomass in the late growth phase at 216, 264, and 312 h was used for total fatty acid (TFA) profiling by GC-MS. The result shown in Fig. 5 that the contents of TFA continuously increased in both conditions and the highest content of TFA reached at 312 h and presented a significant difference (p < 0.05) between 5 % CO2 (22.56 % DW) and air (14.34 % DW), which accounted for 80.4 and 66.1 % of total lipid, respectively. C16 and C18 FAs were the main FA components in biomass, which accounted for more than 90 % of TFA and were favorable for biodiesel production (Miao et al. 2009). The remarkable changes associated with CO2 concentration were observed in C18:2 and C18:3. As depicted in Fig. 5, 5 % CO2 was preferable for C18:2 formation, while C18:3 was much higher with air. This result was in accordance with Tsuzuki et al. (1990). Consequently, the ratio of C18:2/C18:3 led to a decline of DLU from 1.92▽/mole to 1.74▽/mole with the increase of CO2 level. One possible reason is that FAs in the membrane system (both thylakoid and plasma membranes) changed their unsaturated degree in order to adapt varied CO2 concentrations in the environment (Tsuzuki et al. 1990).
The contents of other biochemical components in biomass harvested at 312 h are shown in Table 1. The soluble protein content decreased with 5 % CO2 but presented no significant difference with air (p > 0.05). The content of chlorophylls significantly decreased from 5.65 to 4.00 % DW (p < 0.05) with 5 % CO2. This might be due to light limitation and nutrient deficiency as the biomass concentration increased rapidly with 5 % CO2, for depletion of Mg2+, K+, and Ca2+ could contribute to the decrease of chlorophyll (Gu et al. 2012; Sydney et al. 2010). It is similar to the results of Gardner (2013) on Chlamydomonas reinhardtii. Additionally, CO2-prompted N-deficiency in the late period was another possible reason since it could decrease the total chlorophyll content, which eventually compromised the yield of the photochemistry of photosystem II (PS II) and photosynthetic efficiency (Sayed 1998).
The biosynthesis of protein, carbohydrate, and lipid originates from the assimilated carbon through photosynthesis in microalgae. In terms of carbon flux, metabolic pathways can be affected by availability of carbon source. According to the results in this work, carbohydrate biosynthesis was enhanced dominantly over lipid with 5 % CO2 since the increase in carbohydrate content (81.1 %) was obviously greater than that (29.4 %) in lipid content, while the protein content seemed to be comparatively constant. These results suggest that the carbon metabolic flux partition more into carbohydrate biosynthesis pathway in C. vulgaris CS-42 in carbon-enriched environment. During stress conditions like N-deficiency, the biosynthesis of lipid and carbohydrate is activated in many microalgae, which are two competing pathways of photosynthetic carbon storage (Siaut et al. 2011). However, the cell growth could be compromised at the same time since nitrogen is indispensible for amino acid and nucleotide synthesis. In the present study, 5 % CO2 boosted microalgal growth and increased the C/N ratio in the medium, consequently enhancing the FA, total lipid, and carbohydrate productivity from C. vulgaris CS-42.
Comparison of the present work with other studies
In Table 2, the present results are compared with other studies which focused on the performance of Chlorella in response to CO2 levels. The comparison shows that higher CO2 concentration has positive effects on the total performance of microalgae. Therefore, CO2 supplementation seems to be one of the most efficient methods for fast and massive cultivation of microalgae.
It is well known that biomass productivity as well as CO2 fixation can be greatly affected by many factors such as culture system configuration, nutrients, gas transfer, pH, temperature, etc. (Kumar et al. 2010). For example, Yun et al. (1997), who applied gradual increase of CO2 concentrations from 5 to 15 % and cultured the C. vulgaris UTEX 259 autotrophically in wastewater, achieved higher levels of biomass productivity and CO2 fixation than the present study. Hirata et al. (1996) obtained even higher results using Roux flask for cultivation with fluorescent lamps as the light source. However, in terms of lipid productivity, the results of the present study had higher levels than other data shown in Table 2, and this was probably due to the strain capacity as well as the intensive and continuous illumination supplied throughout the cultivation. Therefore, as compared with other studies, C. vulgaris CS-42 exhibited favorable features and great potential for efficient CO2 biofixation and lipid production.
In conclusion. compared to the results with air, 5 % CO2 supplementation was found to increase CO2 fixation rate, nitrate uptake rate, and biomass production during the cultivation of C. vulgaris CS-42 in PBRs. Cytomic analysis revealed that the biosynthesis of intracellular neutral lipid was enhanced by N-deficiency resulted from CO2 supplementation. Biochemical compounds were significantly affected by the CO2 concentration, among which the carbohydrate content was enhanced dominantly over total FAs. The present results show that 5 % CO2 supplementation is an effective way to prompt cell growth and rapid depletion of nitrate in the media, consequently enhancing the lipid, carbohydrate, and FA production from C. vulgaris CS-42 by increasing the C/N ratio.
References
Bradford MM (1976) Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brennan L, Blanco Fernández A, Mostaert AS, Owende P (2012) Enhancement of BODIPY 505/515 lipid fluorescence method for applications in biofuel-directed microalgae production. J Microbiol Methods 90:137–143
Burja AM, Armenta RE, Radianingtyas H, Barrow CJ (2007) Evaluation of fatty acid extraction methods for Thraustochytrium sp. ONC-T18. J Agric Food Chem 55:4795–4801
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25:294–306
Chisti Y (2008) Biodiesel from microalgae beats bioethanol. Trends Biotechnol 26:126–131
Chiu S, Kao C, Chen C, Kuan T, Ong S, Lin C (2008) Reduction of CO2 by a high-density culture of Chlorella sp. in a semicontinuous photobioreactor. Bioresour Technol 99:3389–3396
De Morais MG, Costa JAV (2007a) Biofixation of carbon dioxide by Spirulina sp. and Scenedesmus obliquus cultivated in a three-stage serial tubular photobioreactor. J Biotechnol 129:439–445
De Morais MG, Costa JAV (2007b) Carbon dioxide fixation by Chlorella kessleri, C. vulgaris, Scenedesmus obliquus and Spirulina sp. cultivated in flasks and vertical tubular photobioreactors. Biotechnol Lett 29:1349–1352
Doucha J, Straka F, Lívanský K (2005) Utilization of flue gas for cultivation of microalgae Chlorella sp. in an outdoor open thin-layer photobioreactor. J Appl Phycol 17:403–412
Dubois M, Gilles KA, Hamilton JK, Rebers P, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Francisco ÉC, Neves DB, Jacob Lopes E, Franco TT (2010) Microalgae as feedstock for biodiesel production: carbon dioxide sequestration, lipid production and biofuel quality. J Chem Technol Biotechnol 85:395–403
Gardner RD (2013) Comparison of CO2 and bicarbonate as inorganic carbon sources for triacylglycerol and starch accumulation in Chlamydomonas reinhardtii. Biotechnol Bioeng 110:87–96
Govender T, Ramanna L, Rawat I, Bux F (2012) BODIPY staining, an alternative to the Nile Red fluorescence method for the evaluation of intracellular lipids in microalgae. Bioresour Technol 114:507–511
Griffiths MJ, Harrison ST (2009) Lipid productivity as a key characteristic for choosing algal species for biodiesel production. J Appl Phycol 21:493–507
Gu N, Lin Q, Li G, Tan YH, Huang LM, Lin JD (2012) Effect of salinity on growth, biochemical composition, and lipid productivity of Nannochloropsis oculata CS 179. Eng Life Sci 12:631–637
Guschina IA, Harwood JL (2006) Lipids and lipid metabolism in eukaryotic algae. Prog Lipid Res 45:160–186
Hecht U, Mohr H (1990) Factors controlling nitrate and ammonium accumulation in mustard (Sinapis alba) seedlings. Physiol Plant 78:379–387
Hirata S, Hayashitani M, Taya M, Tone S (1996) Carbon dioxide fixation in batch culture of Chlorella sp. using a photobioreactor with a sunlight-collection device. J Biosci Bioeng 81:470–472
Huang G, Chen F, Wei D, Zhang X, Chen G (2010) Biodiesel production by microalgal biotechnology. Appl Energy 87:38–46
Kates M, Baxter R (1962) Lipid composition of mesophilic and psychrophilic yeasts (Candida species) as influenced by environmental temperature. Can J Biochem Physiol 40:1213–1227
Kumar A, Ergas S, Yuan X, Sahu A, Zhang Q, Dewulf J, Malcata FX, Van Langenhove H (2010) Enhanced CO2 fixation and biofuel production via microalgae: recent developments and future directions. Trends Biotechnol 28:371–380
Lu N, Wei D, Jiang X, Chen F, Yang S (2012) Fatty acids profiling and biomarker identification in snow alga Chlamydomonas nivalis by NaCl stress using GC/MS and multivariate statistical analysis. Anal Lett 45:1172–1183
Malcata FX (2011) Microalgae and biofuels: a promising partnership? Trends Biotechnol 29:542–549
Matsuda Y, Nakajima K, Tachibana M (2011) Recent progresses on the genetic basis of the regulation of CO2 acquisition systems in response to CO2 concentration. Photosynth Res 109:191–203
Miao X, Li R, Yao H (2009) Effective acid-catalyzed transesterification for biodiesel production. Energy Convers Manag 50:2680–2684
Muradyan EA, Klyachko-Gurvich GL, Tsoglin LN, Sergeyenko TV, Pronina NA (2004) Changes in lipid metabolism during adaptation of the Dunaliella salina photosynthetic apparatus to high CO2 concentration. Russ J Plant Physiol 51:53–62
Naik SN, Goud VV, Rout PK, Dalai AK (2010) Production of first and second generation biofuels: a comprehensive review. Renew Sust Energ Rev 14:578–597
Ota M, Kato Y, Watanabe H, Watanabe M, Sato Y, Smith RL, Inomata H (2009) Effect of inorganic carbon on photoautotrophic growth of microalga Chlorococcum littorale. Biotechnol Prog 25:492–498
Popovich CA, Damiani C, Constenla D, Martinez AM, Freije H, Giovanardi M, Pancaldi S, Leonardi PI (2012) Neochloris oleoabundans grown in enriched natural seawater for biodiesel feedstock: evaluation of its growth and biochemical composition. Bioresour Technol 114:287–293
Ritchie RJ (2006) Consistent sets of spectrophotometric chlorophyll equations for acetone, methanol and ethanol solvents. Photosynth Res 89:27–41
Sasi D, Mitra P, Vigueras A, Hill GA (2011) Growth kinetics and lipid production using Chlorella vulgaris in a circulating loop photobioreactor. J Chem Technol Biotechnol 86:875–880
Sayed OH (1998) Analysis of photosynthetic responses and adaptation to nitrogen starvation in Chlorella using in vivo chlorophyll fluorescence. Photosynthetica 35:611–619
Sforza E, Bertucco A, Morosinotto T, Giacometti GM (2010) Vegetal oil from microalgae: species selection and optimization growth parameters. Chem Eng Trans 2:199–204
Shi X, Chen F, Yuan J, Chen H (1997) Heterotrophic production of lutein by selected Chlorella strains. J Appl Phycol 9:445–450
Siaut M, Cuine S, Cagnon C, Fessler B, Nguyen M, Carrier P, Beyly A, Beisson F, Triantaphylides C, Li-Beisson Y, Peltier G (2011) Oil accumulation in the model green alga Chlamydomonas reinhardtii: characterization, variability between common laboratory strains and relationship with starch reserves. BMC Biotechnol 11:7
Spolaore P, Joannis-Cassan C, Duran E, Isambert A (2006) Commercial applications of microalgae. J Biosci Bioeng 101:87–96
Sydney EB, Sturm W, de Carvalho JC, Thomaz-Soccol V, Larroche C, Pandey A, Soccol CR (2010) Potential carbon dioxide fixation by industrially important microalgae. Bioresour Technol 101:5892–5896
Tang D, Han W, Li P, Miao X, Zhong J (2011a) CO2 biofixation and fatty acid composition of Scenedesmus obliquus and Chlorella pyrenoidosa in response to different CO2 levels. Bioresour Technol 102:3071–3076
Tang H, Chen M, Garcia M, Abunasser N, Ng KY, Salley SO (2011b) Culture of microalgae Chlorella minutissima for biodiesel feedstock production. Biotechnol Bioeng 108:2280–2287
Tsuzuki M, Ohnuma E, Sato N, Takaku T, Kawaguchi A (1990) Effects of CO2 concentration during growth on fatty acid composition in microalgae. Plant Physiol 93:851–856
Widjaja A, Chien C, Ju Y (2009) Study of increasing lipid production from fresh water microalgae Chlorella vulgaris. J Taiwan Inst Chem Eng 40:13–20
Yun YS, Lee SB, Park JM, Lee CI, Yang JW (1997) Carbon dioxide fixation by algal cultivation using wastewater nutrients. J Chem Technol Biotechnol 69:451–455
Zeng X, Danquah MK, Chen XD, Lu Y (2011) Microalgae bioengineering: from CO2 fixation to biofuel production. Renew Sust Energ Rev 15:3252–3260
Acknowledgments
This work was supported by the Major State Basic Research Development Program of China (973 Project) (Grant Nos. 2011CB200904 and 2011CB200901), National Hi-Tech Research and Development Program (863 Project) (Grant No. 2013AA065802), and National Sciences Foundation of China (Grant No. 31370383, 31270085).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Peng, H., Wei, D., Chen, F. et al. Regulation of carbon metabolic fluxes in response to CO2 supplementation in phototrophic Chlorella vulgaris: a cytomic and biochemical study. J Appl Phycol 28, 737–745 (2016). https://doi.org/10.1007/s10811-015-0542-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-015-0542-4