Abstract
The purpose of this study was to select strains of lactic acid bacteria (LAB) by their in vitro adhesive and immunomodulatory properties for potential use as probiotics. In this study, 16 randomly selected LAB strains from fermented vegetables (sauerkraut, bean and cabbage) were first screened for their tolerance to acid, bile salts, pepsin and pancreatin, bacterial inhibitory activities and abilities to adherence to Caco-2 cells. Then, 4 strains with the highest adhesion abilities were selected for further studies of their immunomodulatory properties and inhibitory effects against Salmonella adhesion and invasion to Caco-2 cells in vitro. The results showed that these 16 LAB strains effectively survived in simulated gastrointestinal condition and inhibited growth of six tested pathogens. Lactobacillus rhamnosus P1, Lactobacillus plantarum P2, Lactobacillus rhamnosus P3 and Lactobacillus casei P4 had the highest abilities to adhere to Caco-2 cells. Furthermore, L. plantarum P2 strain showed higher abilities to induce expression of tumor necrosis factor-α and interleukin-12 by splenic monocytes and strongly inhibited the adhesion and invasion of S. enteritidis ATCC13076 to Caco-2 cells. These results suggest that Lactobacillus strains P2 could be used as a probiotic candidate in food against Salmonella infection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Probiotic bacteria, mostly isolated from cultural components of gut microbiota, play important roles in modulation of immunological and gastrointestinal functions (Behnsen et al. 2013). These bacteria can function against pathogens by competing for binding sites (Wang et al. 2014) and interact with mucosal immune cells or epithelial cells lining the mucosa to modulate specific functions of the mucosal immune system (Wells 2011).
Most published bacterial strains as potential probiotics are those from human or animal gastrointestinal tracts, considering that strains of these origins would be better adapted and colonized in human/animal gastrointestinal tracts. Other sources of potential probiotics include dairy products (Carafa et al. 2015), fermented meat products (Rubio et al. 2014), wine (Garcia-Ruiz et al. 2014) and, foods of plant origin (Wang et al. 2014). For example, Liu et al. (2013) isolated LAB strains from fermented pickles as potential probiotics. Lactic acid bacteria were also isolated and investigated from indigenous pickled vegetables for their functional characteristics in vitro as potential new probiotic strains (Kumar et al. 2012). However, characterization of those isolated bacteria from fermented vegetables is limited, most focusing on inhibiting growth of pathogenic bacteria and fungi in vitro. Only a few studies investigated the effect against Salmonella adhesion and invasion of epithelial cell in vitro for LAB strains (Chiu et al. 2008) and none studied the immunomodulation of isolated bacteria from fermented vegetables. It has been reported that animal-derived LAB strains stimulated co-cultured macrophage cells to secrete TNF-α (Lin et al. 2011; Tsai et al. 2011) and IL-12 (Chen et al. 2013). These two cytokines are key factors to examine LAB’s immunoenhancing activities (Hirose et al. 2010) and they could further regulate innate and adaptive immune responses (Chen et al. 2013). However, no attempts have ever been made to explore changes of cytokines at the protein level for bacteria from fermented food in vitro. Here, we hypothesized that LAB strains from fermented food also had immunomodulatory properties in vitro.
The aim of this paper was to assess the probiotic potential of LAB from fermentation vegetables. For that, selected LAB strains were subjected to a series of in vitro analyses to evaluate their resistance to acid and bile salt, their resistance to pathogens and adhesion to intestinal cells, and their effects on the adhesion and invasion of pathogen bacteria to cultured human intestinal cells. With availability of specific antibodies in our laboratory, this study also investigated expression levels of TNF-α and IL-12 at the protein level by immune cells in vitro in the presence of selected LAB from fermented vegetables.
Materials and methods
Isolation and 16S rRNA gene sequencing of LAB
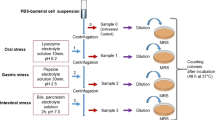
Lactic acid bacteria were isolated from fermented vegetables according to the method described by Wang et al. (2010). Briefly, fermented vegetables (sauerkraut, bean and cabbage) were purchased from local markets in Yangling, China. Each sample (10 g) was diluted in 50 ml of 0.85 % NaCl solution and plated onto the DeMan, Rogosa and Sharpe (MRS) agar (Difco, Sparks, MD, USA) and incubated at 37 °C under anaerobic conditions for 48 h for selection of lactic acid bacteria (Ni et al. 2015). Individual colonies were selected and subcultured twice. Sixteen strains were randomly selected for further analyses.
Genomic DNA was extracted from LAB which were grown at 37 °C for 24 h in MRS broth, using a E.Z.N.A.® Bacteria DNA Kit (Omega, USA). The 16S rRNA gene sequence was amplified in a thermal cycler using the prokaryotic 16S ribosomal DNA universal primers 27F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1492R (5′-GGTTACCTTGTTACGACTT-3′). PCR products were then sequenced using the Sanger sequencing method on an ABI3730 DNA Analyzer. The identities of LAB were determined by blasting the sequences, using the Basic Local Alignment Search Tool (BLAST) program (Zhang et al. 2000).
Survival under conditions simulating the human GI tract
To evaluate the resistance to acid, pepsin and pancreatin of isolated LAB strains, the methods described by Yu et al. (2013) were used. Effects of bile salts on the growth of LAB cells were investigated by a method described by Garcia-Ruiz et al. (2014). For the acid and pepsin resistance, 150 μl of each culture containing about 108–109 c.f.u. ml−1 of LAB suspension was added to 4.85 ml MRS that had been adjusted to pH 2.5 by 0.1 mol l−1 HCl or 4.85 ml MRS broth with 3 mg ml−1 pepsin (pH 2.5) (Sigma, Saint Louis, Missouri, USA). Each mixture was incubated at 37 °C for 3 h, reflecting the time spent by food in the stomach. For the pancreatin resistance and the effect of bile salts, the same volume of LAB suspension was added to 4.85 ml MRS broth with 1 mg ml−1 pancreatin (pH 8.0) (Sigma, USA) or 4.85 ml MRS broth with 0.06, 0.125, 0.25, 0.5 and 1 % bile salts (Sigma, USA). Each mixture was incubated at 37 °C for 4 h, reflecting the time spent by food in the small intestine. After incubation, viable bacterial counts were determined by plating serial dilutions (with PBS, pH 7.2) on the MRS agar under anaerobic conditions at 37 °C for 48 h. The assays were performed in triplicate and repeated three times.
Antimicrobial activity assay
The antibacterial activities of LAB strains were studied using an agar diffusion test. Strains of LAB were grown over-night (20 h) in the MRS broth at 37 °C. Pathogenic bacterial strains including Staphylococcus aureus ATCC 29213, Escherichia coli K88, 25922 and 1569, Salmonella enteritidis ATCC 13076 and Salmonella typhimurium ATCC 14082 were used. Each pathogen was grown overnight and inoculated (0.1 % v/v) into Luria–Bertani (LB) agar. Then, wells were hollowed out of the LB plates by an Oxford cup and 200 μl of the spent culture suspension (LAB-SCS) was added into each well. The cultures were incubated at 37 °C for 14 h before determination of the antimicrobial activities. LAB strains with inhibition zones <11, 11–16, 17–22 and >23 mm were classified as strains of no -; mild +; strong ++; and very strong +++ inhibition, respectively (Tsai et al. 2011). The assays were performed in triplicate and repeated three times.
Adhesion of LAB to intestinal cell line and effect of LAB strains on Salmonella adhesion and invasion of Caco-2 cells
Caco-2 cells (ATCC HTB-37), a human colon adenocarcinoma cell line, were maintained in a Dulbecco’s modified Eagle’s medium (DMEM)-F12 medium (all from Gibco BRL) supplemented with 10 % (v/v) inactivated (30 min, 56 °C) fetal bovine serum, 20 U ml−1 penicillin and 100 μg ml−1 streptomycin. Cells were cultured at 37 °C in a 5 % CO2/95 % air atmosphere using a humidified CO2 incubator. Cells were used at post-confluence after 21 days of culture. For the adhesion assay as well as the inhibition of intestinal cell adhesion and invasion by Salmonella, monolayers of Caco-2 cells were prepared in 24-well tissue culture plates (Costar 3524, Corning Incorporated, NY, USA). Cells were seeded at a concentration of 5 × 104 cells ml−1 by the DMEM-F12 medium without penicillin and streptomycin.
The adhesion studies of LAB strains were performed following the procedures described by Bianchi et al. (2004) and Tsai et al. (2011). LAB strains were stained with Fluorescein isothiocyanate (FITC, Sigma) and kept in dark at 37 °C for 2 h, then washed three times with an antibiotic-free PBS solution (pH 7.2) to remove the unlabeled FITC and resuspended in PBS. 100 μl of each suspension (5 × 108 c.f.u. ml−1) was transferred to the 24-well multidish containing the Caco-2 cells and incubated at 37 °C for 2 h. After incubation, non-adherent bacteria were removed by washing three times with PBS. 200 μl trypsin/EDTA-Na2 was used to digest the cells and adherent bacteria for 5 min before 600 μl of PBS was added into the wells. After mixing, 200 μl mixture containing cells and bacteria were added to 96 well. This fraction contained lysed bacteria attached to or within Caco-2 cells and it was reported as the adherent fraction. The fluorescence was read on a Perkin-Elmer LS55 Spectrophotometer (λex = 492 nm; λem = 517 nm). Six independent experiments were performed for each LAB strain. The results of adhesion assays were expressed as the adhesion percentage, namely the ratio between adherent bacteria and added bacteria per well.
The impact of adhesion and invasion of S. enteritidis by LAB strains was examined as previously described by Garcia-Ruiz et al. (2014) with minor modifications. For the exclusion assay, LAB strains (5 × 107 c.f.u. per well) were first added to the monolayer of Caco-2 cells and incubated for 2 h. Non-adherent bacteria were removed by washing and FITC labeled Salmonella (5 × 107 c.f.u. per well) were added to the wells and the mixture was incubated for additional 2 h. The competitiveness was tested by adding LAB strains and FITC labeled Salmonella simultaneously (in an initial ration of 1:1, 5 × 107 c.f.u. per well, respectively) to the Caco-2 cells followed by incubation for 2 h. The abilities of the LAB strains to displace previously adhered and invaded Salmonella were assessed as follows. FITC labeled Salmonella (5 × 107 c.f.u. per well) were first added to Caco-2 cells and incubated for 2 h. Non-adherent Salmonella bacteria were removed by washing before adding LAB strains (5 × 107 c.f.u. per well). The mixture was incubated for additional 2 h.
In all assays, monolayers were washed three times with PBS after incubation. To determine the inhibition of adhesion of Salmonella to Caco-2 cells by LAB strains, one 24-well multidish was added with 200 μl trypsin to digest the cells and adherent bacteria for 5 min before adding 600 μl of PBS. The inhibition of the adhesion of S. enteritidis ATCC13076 was expressed as a percentage using the following formula: Inhibition of adhesion = 100 × (1 − A1/A2), where A1 and A2 were the percentages of adhesion by S. enteritidis ATCC13076 in the presence and absence of LAB strains, respectively. To determine the inhibition of invasion of S. enteritidis ATCC13076 to Caco-2 cells by LAB strains, another 24-well multidish was lysed with 200 μl of 1 % Triton X-100 for 10 min. The blank control used 200 μl trypsin to digest the cells and adherent bacteria. 600 μl of PBS was added into both groups. After mixing, 200 μl mixture containing cells and bacteria were added to 96-well for measuring the strength of fluorescence. Six independent experiments were performed for each LAB strain. The inhibition of invasion of S. enteritidis ATCC13076 was expressed as the percentage of invasion by S. enteritidis ATCC13076 in the presence and absence of LAB strains, as described above.
TNF-α and IL-12 production by spleen cells after stimulation with LAB strains
Spleens were harvested from four adult chickens (Brisbin et al. 2011). The spleens were rinsed in 1 × Hanks’ balanced salt solution (HBSS) and then minced with sterile scalpels. The tissue was further disrupted with the flat end of a 10-ml syringe plunger and filtered through a 40-μm nylon cell strainer to obtain a single-cell suspension. The suspension was then overlaid onto a Histopaque-1077 (Sigma, Oakville, ON, USA) density gradient and centrifuged at 400×g for 30 min. Mononuclear cells at the interface were collected and washed twice in 1 × HBSS and then suspended in RPMI 1640 containing 10 % fetal bovine serum, 2 % chicken serum, 0.146 g l-glutamine, 1.6 mM 2-mercaptoethanol. Cells were counted by the trypan blue dye exclusion assay before being resuspended in RPMI 1640.
Spleen cells were cultured in triplicate, at a density of 1 × 106 cells ml−1 of RPMI-1640 medium without Penicillin–Streptomycin, in 24-well tissue culture plates. LAB cells were centrifuged at 8, 000g for 5 min and the pellets were resuspended in RPMI-1640 medium containing spleen cells to a final concentration from 108 to 109 c.f.u. ml−1. Lipopolysaccharide (10 μg ml−1) from E. coli O26:B6 (Sigma, USA) was used as a positive control. After 24 h and 48 h incubation, TNF-α and IL-12 produced in the culture supernatants were analyzed.
Cytokines were measured using an enzyme-linked immunosorbent assay (ELISA) method. Ninety-six-well Immuno-Maxisorp plates (Nunc) were coated with polyclonal antibodies for TNF-α and IL-12 (1: 1, 000) in coating buffer (0.05 M Carbonate Buffer, pH 9.6) over-night at 4 °C. Plates were blocked and washed. Culture medium was added to the plates and they were incubated at 37 °C for 2 h. Plates were then washed again and biotinylated anti-rabbit and horseradish peroxidase-conjugated streptavidin (1: 20, 000) were added, followed by incubation at 37 °C for 1 h. The chromogenic reactions were developed with 3, 3′, 5, 5′-tetramethylbenzidine substrate at 37 °C for 30 min. The reactions were terminated with 50 μl 2 mol l−1 H2SO4 and the absorbance at A450 nm was measured. Equivalent levels of TNF-α and IL-12 were calculated by comparison with reference curves generated using TNF-α and IL-12 standards. The results were expressed as the concentration of the cytokines in culture medium (ng ml−1).
Statistical analyses
All results were expressed as mean ± SD from at least three independent experiments. Statistical analysis was done using the SPSS for Windows version 17.0 (Chicago, IL, USA). Data were subjected to one-way ANOVA and, where appropriate, the Scheffe test was used for comparison of means. Differences were considered to be statistically significant when the P value was <0.05.
Results
Resistance of LAB to simulated GI conditions
All randomly selected 16 strains were able to survive following exposure to pH 2.5 alone, or pH 2.5 and pepsin (3 mg ml−1) for 3 h, with negligibly reduced viable counts (Table 1). Similarly, all 16 LAB strains survived in the presence of pancreatin (1 mg ml−1, pH 8.0) for 4 h (Table 1). While all 16 LAB strains survived oxgall up to the 0.5 % level, c.f.u. ml−1 of L. casei LP1, L. rhamnosus P1, L. rhamnosus P3, L. casei P4, and L. rhamnosus P5 were significantly decreased by about 3 log scales after exposure to 1.0 % bile salts for 4 h (P < 0.05) (Table 2).
Antimicrobial activity and in vitro adhesion assay to Caco-2 cells
As shown in Table 3, all the isolated 16 LAB strains had strong inhibition against E. coli ATCC K88, ATCC 1569 and ATCC 25922, Staphylococcus aureus ATCC 29213, Salmonella enteritidis ATCC 13076 and Salmonella typhimurium ATCC 14082 except LP1 and P4 which exhibited mild inhibition against Salmonella typhimurium.
All 16 tested LAB strains were able to adhere to Caco-2 cells with different adhesion activities, ranging from 0.45 to 12.27 % (Fig. 1). L. plantarum P1, L. rhamnosus P2, L. rhamnosus P3 and L. casei P4 showed the strongest adherence to Caco-2 cells (from 7. 98 to 12.27 %). Because of their high adhesive capacities, L. rhamnosus P1, L. plantarum P2, L. rhamnosus P3 and L. casei P4 were selected for further studies.
TNF-α and IL-12 production by spleen mononuclear cells in response to recall antigen stimulation
L. plantarum P2 had the strongest ability to enhance TNF-α and IL-12 production (P < 0.05) (Fig. 2), with 20.1 and 15.4 ng ml−1 for TNF-α and 50.5 and 41.6 ng ml−1 for IL-12 at 24 h and 48 h post-treatment, respectively. The other three LAB strains had similar and still higher abilities to enhance TNF-α and IL-12 production, in comparison with the positive control group (P < 0.05) (Fig. 2). Expression of TNF-α and IL-12 of L. plantarum P2 were decreased from 24 to 48 h, while the remaining 3 lactic acid bacteria were increased during that period.
a TNF-α, b IL-12 production from chicken spleen mononuclear cells after stimulation for 24 and 48 h with different LAB strains. A negative control (RPMI-1640) and a positive control with LPS (10 μg ml−1) were also included. Each value represents the mean value ± SD from six experiments. Different letters above bars indicate significant differences between treatments (P < 0.05)
Inhibition of adhesion and invasion of S. enteritidis by Lactobacillus strains
As shown in Fig. 3, S. enteritidis ATCC13076 adhesion and invasion were reduced significantly by the four Lactobacillus strains in all assays. The levels of adhesion and invasion inhibition in exclusion assays were higher than those in competition and displacement assays (P < 0.05) except L. plantarum P2 which had no significant difference of invasion inhibition among exclusion, competition and displacement assays (Fig. 3b).
a Anti-adhesion (exclusion, competition and displacement) and b anti-invasion assays (exclusion, competition and displacement) of S. enteritidis ATCC 13076 by L. rhamnosus P1, L. plantarum P2, L. rhamnosus P3 and L. casei P4. Each value represents the mean value ± SD from six experiments. Different letters above bars indicate significant differences between treatments for a same bacterium (P < 0.05)
Discussion
It has been reported that some plant-derived LAB strains are as resistant as (or even more resistant than) animal-derived LAB strains to artificial gastric juices and bile (Higashikawa et al. 2010). Research on lactic acid bacteria isolated from fermented products, especially fermented vegetables is increasing. Peres et al. (2012) has attributed this increase to the followings: (1) traditional fermented vegetables are a plentiful source of microorganisms and some of them show probiotic characteristics; (2) their cell wall is forced to become more solid and thicker in order to permit adaptation to the harsh environmental conditions prevailing in plant matrices-high osmotic pressure, poor nutrient profile, and presence of antibacterial compounds.
In this study, tolerance to acid, bile salts and the enzymes was demonstrated in all the 16 isolated LAB strains, suggesting that these 16 LAB strains could pass through the gastrointestinal tract and be functional effectively. Similar to our results, Argyri et al. (2013) reported that 9 LAB strains showed consistent tolerance of pH 2.5 for 3 h, which could be used as a criteria to select the LAB strains in vitro (Lin et al. 2011). In addition, all the selected 16 LAB strains in this study were able to inhibit the growth of six pathogenic bacteria. Probiotics have been shown to play a protective role by directly competing with intestinal pathogens through the release of antibacterial substances such as bacteriocins (Cotter et al. 2005; Messaoudi et al. 2013) or metabolites such as acetic acid and lactic acid (Servin 2004) and proteinaceous substances (Liu et al. 2013), which enable them to become established and to dominate their environment. Furthermore, the amounts of the metabolites of lactic acid bacteria are related to their ability to inhibit the growth of pathogenic bacteria in vitro (Van Coillie et al. 2007).
The ability of LAB strains to adhere to epithelial cells is another important selection criterion for potential probiotic microorganisms. This ability provides beneficial effects, including exclusion of pathogens by competing or blocking of their binding sites in the mucosa (Wang et al. 2014) and host immunomodulation (Behnsen et al. 2013). Adhesion of probiotic bacteria to mucosal surfaces is a complex process and may be associated with exopolysaccharide (Garcia-Ruiz et al. 2014), surface proteins and fatty acids (Polak-Berecka et al. 2014). Similar with other studies, the abilities to adhere to Caco-2 cells by the selected LAB strains in this study varied significantly, ranging from 0.45 to 12.27 %, possibly due to differences in their cell surface structures.
The immune system is the body’s most important defense system against pathogens violations. This system consists of immune organs, cells, and molecules. Upon infection by pathogenic bacteria, the body’s immune system will secrete immune factors, such as complement, immunoglobulins, interferons, interleukins, tumor necrosis factor and other cytokines, for resistance to pathogen invasion.
In this study, L. plantarum P2 strains with the highest adhesive capacities significantly induced splenic monocytes to secrete higher amounts of TNF-α and IL-12 than other three strains (Fig. 2), reflecting L. plantarum P2’s strong immunoenhancing activity in vitro. Results from others and our own have suggested that LAB strains with higher in vitro immunomodulatory properties have more probiotic properties in vivo (Chen et al. 2013).
All four Lactobacillus strains with the highest adhesion abilities also significantly inhibited S. enteritidis TACC13076 adhesion and invasion to Caco-2 cells in exclusion, competition and displacement assays (more than 50 %) in this study. Our results were in agreement with several previous reports which confirmed that adherent Lactobacillus effectively inhibit the enterocyte cell adhesion and invasion by pathogens (Chiu et al. 2008; Garcia-Ruiz et al. 2014). Furthermore, these four LAB strains showed negative hemolytic activities (data not shown), suggesting that they were biologically safe (Argyri et al. 2013). In conclusion, considering all the selection criterions, L. plantarum P2 strain is a good probiotic candidate.
References
Argyri AA, Zoumpopoulou G, Karatzas KAG, Tsakalidou E, Nychas GJE, Panagou EZ, Tassou CC (2013) Selection of potential probiotic lactic acid bacteria from fermented olives by in vitro tests. Food Microbiol 33:282–291
Behnsen J, Deriu E, Sassone-Corsi M, Raffatellu M (2013) Probiotics: properties, examples, and specific applications. Cold Spring Harb Perspect Med 3:a010074. doi:10.1101/cshperspect.a010074
Bianchi MA, Del Rio D, Pellegrini N, Sansebastiano G, Neviani E, Brighenti F (2004) A fluorescence-based method for the detection of adhesive properties of lactic acid bacteria to Caco-2 cells. Lett Appl Microbiol 39:301–305
Brisbin JT, Gong J, Orouji S, Esufali J, Mallick AI, Parvizi P, Shewen PE, Sharif S (2011) Oral treatment of chickens with lactobacilli influences elicitation of immune responses. Clin Vaccine Immunol 18:1447–1455
Carafa I, Nardin T, Larcher R, Viola R, Tuohy K, Franciosi E (2015) Identification and characterization of wild lactobacilli and pediococci from spontaneously fermented Mountain cheese. Food Microbiol 48:123–132
Chen CY, Tsen HY, Lin CL, Lin CK, Chuang LT, Chen CS, Chiang YC (2013) Enhancement of the immune response against Salmonella infection of mice by heat-killed multispecies combinations of lactic acid bacteria. J Med Microbiol 62:1657–1664
Chiu HH, Tsai CC, Hsih HY, Tsen HY (2008) Screening from pickled vegetables the potential probiotic strains of lactic acid bacteria able to inhibit the Salmonella invasion in mice. J Appl Microbiol 104:605–612
Cotter PD, Hill C, Ross RP (2005) Bacteriocins: developing innate immunity for food. Nat Rev Microbiol 3:777–788
Garcia-Ruiz A, de Llano DG, Esteban-Fernandez A, Requena T, Bartolome B, Moreno-Arribas MV (2014) Assessment of probiotic properties in lactic acid bacteria isolated from wine. Food Microbiol 44:220–225
Higashikawa F, Noda M, Awaya T, Nomura K, Oku H, Sugiyama M (2010) Improvement of constipation and liver function by plant-derived lactic acid bacteria: a double-blind, randomized trial. Nutrition 26:367–374
Hirose Y, Murosaki S, Fujiki T, Yamamoto Y, Yoshikai Y, Yamashita M (2010) Lipoteichoic acids on Lactobacillus plantarum cell surfaces correlate with induction of interleukin-12p40 production. Microbiol Immunol 54:143–151
Kumar M, Ghosh M, Ganguli A (2012) Mitogenic response and probiotic characteristics of lactic acid bacteria isolated from indigenously pickled vegetables and fermented beverages. World J Microb Biot 28:703–711
Lin WH, Wu CR, Fang TJ, Lee MS, Lin KL, Chen HC, Huang SY, Hseu YC (2011) Adherent properties and macrophage activation ability of 3 strains of lactic acid bacteria. J Food Sci 76:M1–M7
Liu XM, Liu WY, Zhang QX, Tian FW, Wang G, Zhang H, Chen W (2013) Screening of lactobacilli with antagonistic activity against enteroinvasive Escherichia coli. Food Control 30:563–568
Messaoudi S, Manai M, Kergourlay G, Prevost H, Connil N, Chobert JM, Dousset X (2013) Lactobacillus salivarius: bacteriocin and probiotic activity. Food Microbiol 36:296–304
Ni K, Wang Y, Li D, Cai Y, Pang H (2015) Characterization, identification and application of lactic acid bacteria isolated from forage paddy rice silage. PLoS ONE 10:e0121967
Peres CM, Peres C, Hernandez-Mendoza A, Malcata FX (2012) Review on fermented plant materials as carriers and sources of potentially probiotic lactic acid bacteria - with an emphasis on table olives trends. Food Sci Tech 26:31–42
Polak-Berecka M, Wasko A, Paduch R, Skrzypek T, Sroka-Bartnicka A (2014) The effect of cell surface components on adhesion ability of Lactobacillus rhamnosus. Anton Leeuw Int J G 106:751–762
Rubio R, Jofre A, Martin B, Aymerich T, Garriga M (2014) Characterization of lactic acid bacteria isolated from infant faeces as potential probiotic starter cultures for fermented sausages. Food Microbiol 38:303–311
Servin AL (2004) Antagonistic activities of lactobacilli and bifidobacteria against microbial pathogens. FEMS Microbiol Rev 28:405–440
Tsai CC, Liang HW, Yu B, Hsieh CC, Hwang CF, Chen MH, Tsen HY (2011) The relative efficacy of different strain combinations of lactic acid bacteria in the reduction of populations of Salmonella enterica Typhimurium in the livers and spleens of mice. FEMS Immunol Med Microbiol 63:44–53
Van Coillie E, Goris J, Cleenwerck I, Grijspeerdt K, Botteldoorn N, Van Immerseel F, De Buck J, Vaccanneyt M, Swings J, Herman L, Heyndrickx M (2007) Identification of lactobacilli isolated from the cloaca and vagina of laying hens and characterization for potential use as probiotics to control Salmonella Enteritidis. J Appl Microbiol 102:1095–1106
Wang CY, Lin PR, Ng CC, Shyu YT (2010) Probiotic properties of Lactobacillus strains isolated from the feces of breast-fed infants and Taiwanese pickled cabbage. Anaerobe 16:578–585
Wang G, Zhao Y, Tian FW, Jin X, Chen HQ, Liu XM, Zhang QX, Zhao JX, Chen YQ, Zhang H, Chen W (2014) Screening of adhesive lactobacilli with antagonistic activity against Campylobacter jejuni. Food Control 44:49–57
Wells JM (2011) Immunomodulatory mechanisms of lactobacilli. Microb Cell Fact 10(Suppl 1):S17
Yu Z, Zhang X, Li S, Li C, Li D, Yang Z (2013) Evaluation of probiotic properties of Lactobacillus plantarum strains isolated from Chinese sauerkraut. World J Microbiol Biotechnol 29:489–498
Zhang Z, Schwartz S, Wagner L, Miller W (2000) A greedy algorithm for aligning DNA sequences. J Comput Biol 7:203–214
Acknowledgments
This work was supported by funding from a innovation project of science and technology plan project of Shaanxi Province, China (2014KTCL02-21) and a Ministry of Agriculture (No. 2013-S16), and the thousand talent program to X. Z. and Natural Science Foundation of China (No. 31402095) and Northwest A&F University PhD Research Start-up funds (No. 2012BSJJ094) to X. Y.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Feng, J., Liu, P., Yang, X. et al. Screening of immunomodulatory and adhesive Lactobacillus with antagonistic activities against Salmonella from fermented vegetables. World J Microbiol Biotechnol 31, 1947–1954 (2015). https://doi.org/10.1007/s11274-015-1939-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-015-1939-6