Abstract
The present study was the first trial to use the adsorptive capacity of the rice husk to reduce the toxicological impacts of the iron and aluminum oxides nanoparticles on Oreochromis niloticus. The fish groups were subjected to a sub-lethal concentration (10 mg/l) of both metal oxides nanoparticles (in single and combined doses) with and without rice husk water treatment for 7 days. The bioaccumulation of iron and aluminum metals showed a significant increase (p < 0.05) compared with the control groups. The results revealed a tissue-specific distribution pattern as following: liver > kidney > gills > skin > muscles for iron and liver > gills > kidney > skin > muscles for aluminum. Moreover, the bioaccumulation potency of iron was greater than that of aluminum in all studied tissues. Both studied nanoparticles caused a decrease in the red blood cells count, hemoglobin content, hematocrit values, and mean corpuscular hemoglobin concentration, with an obvious increase in mean corpuscular volume and mean corpuscular hemoglobin. While all those parameters were restored more or less to that of control groups after rice husk water treatment. The histological studies of the gills, liver, and kidneys showed different histopathological alterations ranging from compensatory histological changes in the rice husk–treated groups to severe histopathological damage in the untreated groups. Based on the all studied biomarkers, the rice husk is a good absorbent for both studied nanoparticles individually or combined.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The special characteristics of nanoparticles (NPs) are the major reason for entering them in many industries and increase their existence in the aquatic environment (Kaviani et al. 2019). Based on their novel characteristics and small sizes, the possible adverse and toxicological impacts of NPs are still a matter of investigation (Abdel-Khalek 2016). Metal oxide NPs are the most commonly used NPs due to their various properties (Wang et al. 2016). Iron oxide (Fe2O3) and aluminum oxide (Al2O3) NPs, for example, have been used up in various industries like biomedical, bioengineering, and clinical applications in addition to the ceramics, cosmetics, electronics, high performance paints, ultrafiltration membranes, and jet fuel industries (Lewis et al. 2010; Kadar et al. 2011; Chen et al. 2012). The high surface areas, tiny sizes, and enhanced reactivity of metal oxides NPs are the key factors of their toxicity. Therefore, the current emergence of manufactured metal oxides NPs that released into aquatic environments and increased the biological exposure to those NPs has enhanced the need to reduce NPs adverse impacts. Reducing the toxic effects of nanoparticles by reducing their discharges has become a difficult solution due to the continuous and growing increase of nanotechnology. Thus, the proper treatment of NPs before their discharge into the aquatic environment is a suitable solution. Various methods depended on agricultural or industrial by-products were developed to generate effective bio-adsorbents for purifying water and reducing the level of many environmental pollutants (Krishnani and Ayyappan 2006). Some of these adsorbents were rice husk, corncobs, peanut shells, tree leaves, sawdust, coir dust, and wheat bran (Sobhanardakani et al. 2013; Lata and Samadder 2014). Rice husk (RH) has an effective adsorbent capacity to remove various metals in their ordinary sizes from groundwater and surface water (Lata and Samadder 2014). The present study is the first trial to use RH as a low-cost, lingo-cellulosic, eco-friendly bio-adsorbent for treating both Fe2O3 and Al2O3 NPs (separately and combined) from water. To evaluate the efficiency of the RH water treatment, many biomarkers can be used to recognize the health status of fish and to identify any improvement after the treatment process. Hematological endpoints are frequently used to follow up the toxic NPs in the aquatic environment (Abdel-Khalek et al. 2016a). Furthermore, metallic NPs may be easily absorbed into the blood through gills then bioaccumulated in different tissues. Accordingly, the quantitative measuring of NPs in various tissues is an important biomarker too. The over-accumulation of NPs in certain tissues could induce several histopathological alterations; therefore, the histological evaluation of vital tissues is perceived as a highly relevant methodology since it reflects the true health state of the organism (Abdel-Khalek et al. 2016b). Therefore, the aim of this study was to evaluate the adsorptive capacity of RH toward Fe2O3 and Al2O3 NPs in single and combined doses using hematological, bioaccumulation, and histological biomarkers.
2 Materials and Methods
2.1 Preparation and Characterization of Nanoparticles
The nano-powders of Fe2O3 and Al2O3 were purchased from Sigma-Aldrich, St. Louis, MO, USA. As provided by the manufacturer, the molecular weight of Fe2O3 NPs is 159.69 with a surface area range of 50–245 m2/g and particle size less than 50 nm, while the molecular weight of Al2O3 NPs is 101.96 with surface area > 40 m2/g and the particle size less than 50 nm. To check the information of the manufacturer, some structural studies were done as summarized in Table 1. The used concentration (10 mg/l) of both NPs were prepared by dissolving the dry nano-powder into the dechlorinated water (pH 7.4), then ultrasonicated for 60 min (100 W, 40 kHz) using ultrasonic homogenizer (BioLogics, Inc., Manassas, VA, USA), to increase their dispersion.
2.2 Acclimatization of the Experimental Fish
Sixty-four of the experimental fish, Oreochromis niloticus, were purchased from an uncontaminated fish farm located in El-Ismailia governorate, Egypt. The total body length ranged from 10.5 to 13 cm while the bodyweight of the studied fish was 30–39.2 g. The transportation process of the fish was done in a large plastic container to the ecology laboratory, Faculty of Science, Cairo University with a good aeration condition. Fish were maintained for 14 days in glass aquaria (40 × 70 × 26 cm) with 40 l of aerated, dechlorinated tap water with 8 fish per aquarium. The temperature of the water was kept at 25 ± 1 °C, while the dissolved oxygen and pH were 6.5–7.8 mg l−1 and 7.1–7.3, respectively. Fish were fed once daily during the acclimatization period with commercial pellet food (20% crude protein, 4% crude fat, 5% crude fiber, 12% crude ash, and 10% crude moisture).
2.3 Preparation of Rice Husk
The RH from rice mills in Kafr El-Sheikh was chopped into tiny pieces, washed by deionized water, and left in the oven for 24 h at 80 °C. After complete drying, the RH was ground and passed through a 70-mesh sieve (< 210 μm) to be ready for use. Rice husk has entered the aquaria with a concentration of 50 mg/l (5 times the NPs concentration). To avoid eating of RH by fish, RH was isolated by a porous mesh that allows passage of the used NPs to the RH without escaping of RH to the water.
2.4 Experimental Design
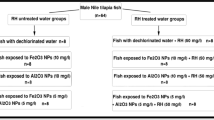
After the acclimatization period, fish were randomly divided into 8 groups as shown in Fig. 1. The sub-lethal concentration of our selected metal oxide NPs was according to Saravanan et al. (2015); Murali et al. (2017); and Canli et al. (2018). The conditions of the experiment were as of that of the acclimatization period, and water was daily checked for pH, temperature, and dissolved oxygen.
2.5 Fish Sampling
After the end of the experiment, blood samples from the caudal vein were withdrawn using heparin as an anticoagulant, and then the vital tissues (liver, gills, kidneys, skin, and muscle) were isolated for further investigations.
2.6 Hematological Studies
2.6.1 Hematological Parameters
Blood samples were diluted with saline solution (0.7% NaCl). Using the improved Neubauer hemocytometer, the red blood cells (RBCs) were counted according to Dacie and Lewis (1991). The determination of hemoglobin (Hb) concentrations was done by Drabkin (1964) method, as cyanomethemoglobin (equivalent to Hb concentration) was measured spectrophotomerically at a wavelength of 540 nm. The hematocrit (Hct) values carried out in small-heparinized capillary tubes using Hct centrifuge at 3000 rpm for 20 min, and then the percentage volume of the RBCs to total blood volume was estimated.
2.7 Calculated Blood Indices
All blood indices were calculated according to Gupta (1977) as below:
2.8 Bioaccumulation of the Studied Metal NPs in the Tissues
Iron and aluminum bioaccumulation levels in the liver, gills, kidneys, skin, and muscle tissues of the studied fish were measured using inductively coupled plasma (ICP-AES), Thermo Sci, model: iCAP6000 series. The measuring procedures were done according to APHA (2005). According to the dry ashing method, the tissues were acid digested (by concentrated HCl) after 8 h of oven drying at 80 °C. Then the mixture was diluted to known volume (25 ml) using deionized water. The detection limits are 1 μg/l for Fe and 0.1 μg/l for Al. All over the measuring process, blank samples were prepared perfectly in the same way as the samples to correct the background absorption. Before the aspiration of the tissue samples, different concentrations of working standard solutions (for each metal) were aspirated to get a straightline standard curve. The percentage recoveries of the measured metals were in the range of 95–110% (using Lake Superior fish 1946NIST, National Institute of Standards and Technology, USA). The measured metal concentrations were expressed as mg/kg dry weight of tissues
2.9 Histological Studies
The gills, liver, and posterior kidney were washed using saline solution then preserved in Bouin’s fixative. According to Bernet et al. (1999), tissues were processed, sectioned at 4 μm, then stained using hematoxylin and eosin. Eight specimens of each tissue were sectioned per group (one slide for each tissue/fish), and the histological alterations were recorded by light microscopy.
2.10 Statistical Analysis
The results were expressed as mean ± standard error. Data were statistically analyzed using the Student t test to estimate the significant difference between RH treated and untreated groups. Duncan’s multiple ranges were used to examine the homogeneity among the experimental groups as indicated by different case letters in the descending order A, B, C, and D at p < 0.05 using Statistical Processor Systems Support, SPSS software, version 16.0, IBM, Chicago, IL, USA.
3 Results
3.1 Blood Parameters and Blood Indices
3.1.1 Hematological Parameters
The single and combined effects of Fe2O3 and Al2O3 NPs on some hematological parameters (RBCs, Hb, and Hct) in Oreochromis niloticus with and without RH water treatment were recorded in Fig. 2. The one-way analysis of variance (ANOVA) showed a significant difference at P < 0.05. Compared with the control group, the different RH untreated metal oxide NPs exposed groups showed a remarkable decrease in all hematological parameters (Duncan’s test capital letters). Comparing these data with the hematological results of RH-treated water groups that exposed to the same metal oxide NPs (t test) showed that the recorded blood parameters were started to increase after the RH treatment, and in some cases (like Hb of Al2O3 NPs and Hct of mixed group), the results became insignificantly with the control group.
Single and combined effects of Fe2O3 and Al2O3 NPs on some blood parameters (RBCs, Hb, and Hct) in Oreochromis niloticus after rice husk (RH) water treatment. aData are represented as means of eight samples in each group ± SE. bThe small letters represent the Duncan’s test (p < 0.05) between rice husk–treated and untreated groups. Rows with same letters are not significantly different; otherwise, they do. cThe capital letters represent Duncan’s test (p < 0.05) between different NP-exposed groups compared with control groups. Columns with same letters are not significantly different; otherwise, they do. dNS = not significant; N.D. = not detected
3.2 Calculated Blood Indices
The calculated blood indices including MCV, MCH, and MCHC of all experimental fish groups were recorded in Fig. 3. Regarding the MCV and MCH of RH-untreated water groups, there were significant increases among the different exposed NPs groups compared with the control group with the highest values in the Fe2O3 NPs exposed group. Whereas the values of those indices became more or less close to the control group after the RH water treatment. In comparison with control groups, the MCHC index was significantly decreased in all studied fish groups.
Single and combined effects of Fe2O3 and Al2O3 NPs on calculated blood indices (MCV, MCH, and MCHC) in Oreochromis niloticus after rice husk (RH) water treatment. aData are represented as means of eight samples in each group ± S.E. bThe small letters represent the Duncan’s test (p < 0.05) between rice husk (RH)–treated and untreated water within the same group. Columns with same letters are not significantly different; otherwise, they do. cThe capital letters represent the Duncan’s test (p < 0.05) between different metal oxide NPs exposed groups compared with control groups of each rice husk (RH)–treated and untreated water groups. Columns with same letters are not significantly different; otherwise, they do
3.3 Bioaccumulation of the Studied Metal NPs
Bioaccumulation potency of iron and aluminum NPs (in single and combined doses) in the liver, kidney, gills, skin, and muscles of Oreochromis niloticus with and without RH water treatment were recorded in Table 2. Regarding the bioaccumulation potency of both metals in studied tissues, there was a significant difference (P < 0.05) among all studied groups. Compared with the control groups, the Fe and Al contents in the studied tissues showed a significant increase in all studied groups with maximum elevation in untreated single metal oxides NPs exposed groups (Duncan’s test). Comparing the RH untreated and treated water groups that exposed to the same metal NPs (t test), the concentration of metal oxides NPs were recorded always significantly higher in RH-untreated water groups than treated water groups.
3.4 Histopathological Examination
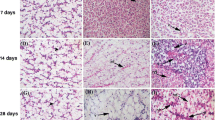
Single and combined effects of Fe2O3 and Al2O3 NPs on histological structures of gills, liver, and kidneys of Oreochromis niloticus with and without RH water treatment were shown in Figs. 4, 5, and 6.
Single and combined effects of Fe2O3 and Al2O3 NPs on histopathological sections in gills of Nile tilapia; Oreochromis niloticus after rice husk (RH) water treatment. PL, primary lamellae; SL, secondary lamellae; PLET, primary lamellar epithelial thickening; SHP, severe hyperplasia; F, fusion in secondary lamellae; A, aneurysm; CN, cellular necrosis; SE, severe edema, EL, epithelial lifting at the base of secondary lamellae; MCHP, mucosal cell hyperplasia; T, telangiectases at the tip of secondary lamellae; CO, congestion in the lamellar blood vessels; HP, hyperplasia; MCS, mucosal cell secretion. Scale bar = 100 μm
Single and combined effects of Fe2O3 and Al2O3 NPs on histopathological sections in the liver of Nile tilapia; Oreochromis niloticus after rice husk (RH) water treatment. HC, hepatic cells; S, sinusoids; HP, hepatopancreas; INF, infiltration of blood cells; CO, congestion in blood vessel; N, necrosis; FD, fatty degeneration and V, cytoplasmic vacuolation. Scale bar = 100 μm
Single and combined effects of Fe2O3 and Al2O3 NPs on histopathological sections in kidneys of Nile tilapia; Oreochromis niloticus after rice husk (RH) water treatment. RT, renal tubules; RC, renal corpuscle; G, glomerulus; DRT, degeneration of renal tubules architecture; CD, cellular degeneration; INF, infiltration of fibroblast; RRC, rupture of renal corpuscle and N, necrosis
3.5 Histopathological Studies on Gills
Gills of the control groups (Fig. 4a, b) showed well-structured primary filaments and secondary lamellae with flat epithelial cells and chloride cells found at the bases of the 2ry lamellae. Gills sections of fish that exposed to Fe2O3 and Al2O3 NPs (separately and in mixture) without RH water treatment (Fig. 4c, d, f, g, e, and j) showed primary lamellar epithelial thickening (PLET); severe hyperplasia (SHP) with fusion in secondary lamellae (F); aneurysm [outward bulging caused by an abnormal weak spot on a blood vessel wall] (A); cellular necrosis (CN); severe edema (SE); epithelial lifting at the base of secondary lamellae (EL); mucosal cell hyperplasia (MCHP); telangiectases [small dilated blood vessels near the surface of the mucous membranes] at the tip of secondary lamellae (T); congestion in the lamellar blood vessels (CO). While, gills sections of RH-treated groups (Fig. 4e, h, k) showed less noticeable alterations as hyperplasia (HP); congestion (CO); epithelial lifting (EL); mucosal cell secretion (MCS).
3.6 Histopathological Studies on Liver Tissues
The control groups (Fig. 5a, b) showed normal architecture with densely arranged polygonal hepatocytes showing a homogenous cytoplasm, and a large central or sub-central sphere-shaped nuclei and sinusoids were distributed randomly all over the hepatocytes without any histopathological abnormalities. While, liver sections of fish that exposed to Fe2O3 and Al2O3 NPs without RH water treatment (Fig. 5c, e, and g) showed a marked deterioration in liver histoarchitecture as infiltration of blood cells (INF); blood congestion (CO); necrosis (N). Whereas RH-treated group (Fig. 5d, f, and h) showed some fatty degeneration (FD) and cytoplasmic vacuolation (V).
3.7 Histopathological Studies on Kidney Tissues
Kidney sections of the control groups (Fig. 6a, b) showed regularly formed tubules, and the interstices of the tubules have normal hematopoietic tissues. It was composed also of numerous renal corpuscles with well-developed glomeruli. While kidney sections of fish that exposed to Fe2O3 and Al2O3 NPs (separately and in mixture) without RH water treatment (Fig. 6c, e, and g) showed progressive damage with degeneration of renal tubules architecture (DRT); cellular degeneration (CD); infiltration of fibroblast (INF); rupture of renal corpuscle (RRC); necrosis (N). However, fish that exposed to Fe2O3 and Al2O3 NPs with RH water treatment showed almost the same alternation but with less deformation of renal tubules and relatively good maintenance of renal architecture (Fig. 6d, f, and h). Generally, the severity of the alterations more pronounced in the fish groups that exposed to Fe2O3 and Al2O3 NPs (separately and combined) without RH water treatment especially in the Fe2O3-exposed group.
4 Discussion
Many scientists ratify that NPs are one of the main basics of the developing technologies in the twenty-first century (Zhu et al. 2019), and therefore, a growing number of NPs can enter the aquatic environment through different pathways (Adam and Nowack 2017). Therefore, the present study aimed to use sensitive and effective biomarkers to evaluate a new treatment method of two commonly used metal oxide NPs to reduce their toxicological impacts. The toxic NPs can cause variations in many hematological parameters, either by increasing their number or concentration by boosting their biosynthetic activities or by decreasing their number or concentration by quelling their biosynthetic sites (Khabbazi et al. 2015). The recorded reduction in RBCs count, Hb content and Hct of Oreochromis niloticus after the exposure to Fe2O3 and Al2O3 NPs was in accordance with Karthikeyeni et al. (2013) who found same results in Oreochromis mossambicus that exposed to different concentrations of Fe2O3 NPs. Moreover, this reduction was noticed in Labeo rohita after the treatment with different metal NPs like Ni, Ag, Cr3O4, and Co3O4NPs (Kanwal et al. 2019). The reduction in RBCs count may be due to the ability of NPs to penetrate the erythrocyte membranes and increase their permeability in addition to the ability of NPS to impair the hemopoietic processes may increase the fragility of RBCs and accelerating the degeneration of their membranes (Shaluei et al. 2013). Dar and Borana (2014) suggested that the overproduction of many reactive oxygen species (ROS) reduces RBCs count via inhibition of DNA synthesis in RBCs production or impaired intestinal absorption of iron. Moreover, lysing of erythrocytes due to NPs exposure may consequently lead to a reduction in Hb content and Hct values in exposed fish (Abdel-Khalek et al. 2016a). The hypoxic condition resulted from reduced RBCs may inhibit the aerobic glycolysis and consequently decrease the energy required for Hb synthesis (Joshi et al. 2002). Furthermore, Kori-Siakpere and Ubogu (2008) referred the reduction of Hct values to the hemodilution and disturbed osmoregulation process that may occur due to gill destruction (confirmed by the present histopathological examination). The MCV, MCH, and MCHC values are exactly calculated based on RBCs count, Hb content, and Hct changes, so any change in those values will lead to change in the blood indices values. The present study showed a significant increase in values of MCV, MCH with a decrease in MCHC after the exposure to both metal oxides NPs when compared with control groups. These results were in accordance with Karthikeyeni et al. (2013) who found same findings in Oreochromis mossambicus exposed to different concentrations of Fe2O3 NPs. Jahanbakhshi et al. (2015) referred the elevation in MCV and MCH values to the decreased RBCs number induced by respiratory and hemopoietic processes disorders. Also, Lavanya et al. (2011) suggested that the elevation in MCV might be attributed to the swelling of RBCs that are associated with intracellular osmotic disorders due to the toxic stress of NPs. The elevation of MCV accompanied by MCH increase more than the control group indicated a sign of the macrocytic hyperchromic anemic condition. The decreased values of MCHC, which is the ratio of Hb concentration as opposed to the Hct, considered as an indication of RBCs swelling and/or a decrease in hemoglobin biosynthesis. After RH water treatment, an obvious improvement in the hematological conditions of the fish exposed to the same NPs was observed. Based on our studied parameters and indices, some of those hematological endpoints (ex. Hb and MCV of Al2O3 NPs and mixture exposed groups) improved to be insignificant with the control group.
Accumulation of nanometals refers to metals reserved by the organism that is neither excreted nor egested. Fish can absorb metals from water and sediments, then accumulate it in various tissues in amounts above those found in their environment, and these cause more adverse biological effects (Abdel-Khalek 2015, 2018). The obtained results showed a tissue-specific increase of Fe and Al contents in different vital tissues (liver, kidney, gills, skin, and muscles) of fish after metal oxides NPs exposure. This was in agreement with Benavides et al. (2016) who observed that Zn and Al contents could effectively increase in liver and gills of Carassius auratus exposed to ZnO and Al2O3 NPs in single or combined doses. Also, the elevation in iron content in the liver, gills, kidney, and muscles was observed also by Ates et al. (2016) when Oreochromis niloticus was exposed to sub-lethal concentrations of Fe2O3 NPs. Metals may be distributed uniformly within the body tissues of fish but accumulate in dissimilar form. As in our study, the highest Fe or Al contents, in general, were detected in the liver tissue followed by the kidney in case of Fe and followed by gills in case of Al than other tissues. In our previous work, the maximum Cu content also was observed in the liver followed by kidney then gills when Oreochromis niloticus was exposed to CuO NPs for 30 days (Abdel-Khalek et al. 2016a). The high hepatic accumulation of metal NPs may be due to the high production rate of metallothioneins (MTs) which ultimately bind the metals in order to avoid the possible harm (Abbas et al. 2018). Moreover, the liver is a specialized organ for metal detoxification through metal sulfur protein formation (Abdel-Khalek 2015). The excess Fe is stored in the form of heme protein and ferritin for various metabolic activities, and this may explain the high iron content in the liver too (Javed et al. 2016). The kidney is also deeming as good metal accumulator tissue due to its role in the re-absorption and excretion processes (Barbier et al. 2005). The tendency of Fe to bind with intracellular proteins for keeping Fe in a soluble bioavailable non-toxic form in the cytoplasm until its excretion is the major cause of the high renal Fe concentration (Arosio and Levi, 2010). According to Sivakumar et al. (2012), the kidney usually accumulated Al in large quantities because the excess amounts of Al are rapidly eliminated from the body through the kidney mainly in detoxification mechanism during the excretion process. The accumulation of NPs in gills according to Hao et al. (2013) might be due to the adsorption of NPs directly on the surface of the gills with subsequent penetration across the gill membrane. This observation was confirmed in our study as many NPs aggregates were seen on the surface of the gills during the dissection process. The higher skin and lower muscle Fe and Al contents in the present study were in accordance with Abdel-Khalek et al. (2016a) who observed same results in Oreochromis niloticus after 30 days of CuO NPs exposure. The higher metal oxides in the skin especially Fe2O3 NPs (observed as brown particles) may be due to the tendency of metallic-NPs to adhere to skin. The lowest concentration of metal oxide NPs in the muscle may be due to the low level of metal-binding protein (for example MT) in the muscle and because of the large mass with low metabolic activity of muscular tissues (Murugan et al. 2008; Abdel-Khalek et al. 2016b, 2018). The elevated NPs accumulation, especially iron, when it was singly dosed may be due to its high surface adsorption tendency which is due to its shape, size, aggregation, and magnetic properties. This surface adsorption mechanism may alter when Fe NPs combined with other NPs. The spherical-shaped Fe2O3 NPs could attach to rod-shaped of Al2O3 NPs, thereby changing the direct surface contact area for both NPs which agreed with Hua et al. (2016) who found the attachment of TiO2 NPs (spherical shape) to ZnO NPs (rod shape) caused a change in their surface areas. This attachment may be occurring in water, and the new particle formed may be a reason for decreasing the uptake of Fe when it combined with Al NPs. Moreover, the higher accumulation of Fe compared with Al may be due to the high ability of Fe2O3 NPs to adsorb through gills and enter the circulation (observed as red aggregates during the present work). Chen et al. (2012) stated that there is a high adhesion tendency of iron NPs to the surfaces of the gills that could damage the epithelial cell and facilitate its entry into the fish body. In addition, NPs can be rapidly cleared through the kidneys when the particle size is small, but it is efficiently trapped by cells when the particle size is large (Feng et al. 2018). Therefore, the clearance of Fe2O3 NPs may be less than Al2O3NPs, so it is more accumulated in tissues. After RH treatment, the bioaccumulation data corroborated that RH has the ability to adsorb both metals NPs from water in all studied groups. As we found that the concentrations of Fe were decreased by 74%, 79%, 74%, 77%, and 32% in the single Fe2O3 NPs dosed group and by 74%, 85%, 67%, 62%, and 36% in combined NPs dosed group in liver, kidney, gills, skin, and muscle tissues, respectively. While the concentrations of Al were decreased by 56%, 83%, 46%, 46%, and 27% in the single Al2O3 NPs dosed group and by 61%, 74%, 65%, 65%, and 49% in combined NPs dosed group in liver, kidney, gills, skin, and muscle tissues, respectively.Histopathological investigations show initial signs of lesions and alterations to evaluate the toxicity of many pollutants (Khosravi-Katuli et al. 2018). External stressors are directly affecting gills as gills, owing to their anatomical position and function, are in direct and continuous contact with external medium (Capaldo et al. 2019). Similar to the present study, many histological alternations in gills such as aneurism, hyperplasia, edema, epithelial lifting, congestion, and cellular necrosis were observed in Oreochromis mossambicus exposed to sub-lethal concentrations of Al2O3 NPs (Murali et al. 2018). Moreover, the exposure to Fe3O4 NPs for 96 h and 60 days resulted in mucous deposition, vacuolization, aneurysm, hyperplasia, absence of secondary lamellae, and blebbing of epithelium in Oreochromis mossambicus (Vidya and Chitra 2019). The histological alterations like epithelial lifting, hyperplasia, and the partial or total fusion of some secondary lamellae are examples of resistance mechanisms to increase the distance between the external pollutants and the blood, thus act as an obstruction to the entrance of contaminants (Hadi and Alwan 2012). Although these alterations act as defense mechanisms, it causes a reduction in the respiratory surface and oxygen uptake of fish (Antunes et al. 2017). Hypoxia-induced by histopathological lesions was confirmed in Oryzias latipes after 14 days of Ag NPs exposure (Wu and Zhou 2013). The reduction of O2 uptake can also lead to the weakness of blood vessels, disturbances in blood flow, congestion, and aneurysm (Murali et al. 2018). Increased permeability of capillary walls and vessel dilatation at the site of toxic damage could be responsible for the observed lamellar edema. Marked deteriorations in liver histoarchitecture including infiltration of blood cells, blood congestion, and necrotic degeneration were observed in the current study in all RH-untreated water groups. These changes were in agreement with Vidya and Chitra (2019) who found that liver tissue showed notable lesions such as segmentation of hepatocytes, spindle-shaped nucleus, and severe necrosis when exposed to Fe3O4 NPs for a short-term duration and 60 days. Hepatic histopathological changes including vacuolization, blood congestion, and necrosis were previously observed also in the Oreochromis mossambicus after sub-lethal exposure to the Al2O3 NPs (Murali et al. 2017). Thophon et al. (2003) referred the hepatic histopathological changes to high hepatocytes metabolic activity in response to metal toxicity. Vacuolization of the hepatocytes is a result of the imbalance between the rate of the synthesis and release of materials produced by the hepatocytes and/or the excessive deposits of fat in the cytoplasm (Ciji and Nandan 2014). Degeneration of hepatocytes and necrotic degeneration is a common result of cell membrane damages, disorders in proteins, and carbohydrates metabolism (Mela et al. 2007). According to Wang et al. (2015), hepatocyte damage and necrosis may result from excessive accumulation of iron metal; the high hepatic metal concentrations in the present study confirmed this observation. Blood congestion and RBCs infiltration indicated an impaired venous outflow and weakened hepatic blood vessels. The kidneys play a critical role to preserve the osmoregulatory mechanism and renal filtration rate; therefore, renal lesions could be a good indicator of external stress (Gupta et al. 2016). The progressive damage of renal tissues recorded in the present study was associated with degeneration, and deformation of renal tubules architecture was in accordance with Chupani et al. (2018) who observed renal alterations including deformations in the epithelia of renal tubules and necrosis in renal tissues of Cyprinus carpio after ZnO NPs exposure. This degeneration of tubular epithelial cells and necrosis may be due to the accumulation of inflammatory cells associated with NPs toxicity. The high metal bioaccumulation levels in kidney and the free radicals produced from metals ions also contribute substantially to renal injury and tissue damage (Hermenean et al. 2015; Abdel-Khalek et al. 2018). Generally, the histopathological examination showed that the severity of the recorded alterations was more pronounced in the tissues of fish that exposed to NPs metals without any treatment of water. While the recorded histological changes in the tissues of fish that exposed to the same NPs with RH water treatment were less severe. These observations may be due to the ability of RH to absorb NPs and decrease their accumulation level in fish tissues. These were in agreement with Kaur et al. (2018) who suggested that the high accumulation of metals in tissues would probably dysfunction the detoxification mechanism and cause severe histopathological abnormalities in it.
5 Conclusions
It can be recognized that both Fe2O3 and Al2O3 NPs in single and combined doses could accumulate in fish tissues and induce hematological and histological alterations to Oreochromis niloticus. This is the first attempt to display the adsorptive properties of RH to metal NPs, and the results of the present study showed that RH had adsorptive capacity to both studied NPs and could improve the health status of the exposed fish. More studies are needed using different organisms and alternative NPs to improve RH removal efficiency.
References
Abbas, S., Javed, M., Ahmad Khan, H., & Rahman, K. (2018). Toxicity And Bioaccumulation Of Metals (Al And Co) In Three Economically Important Carnivorous Fish Species Of Pakistan. International Journal Of Agriculture And Biology, 20(5), 1123–1128.
Abdel-Khalek, A. A. (2015). Antioxidant Responses And Nuclear Deformations In Freshwater Fish, Oreochromis Niloticus, Facing Degraded Environmental Conditions. Bulletin Of Environmental Contamination And Toxicology, 94(6), 701–708.
Abdel-Khalek, A. A. (2016). Comparative Evaluation Of Genotoxic Effects Induced By Cuo Bulk And Nano-Particles In Nile Tilapia, Oreochromis Niloticus. Water, Air And Soil Pollution, 227, 35.
Abdel-Khalek, A. A. (2018). Chronic Exposure To Water Of Lake Qaroun Induced Metal-Related Testicular Damage And Endocrine Disruption In Male Fish. Biological Trace Element Research, 185(1), 197–204.
Abdel-Khalek, A. A., Badran, S. R., & Marie, M.-A. S. (2016a). Toxicity Evaluation Of Copper Oxide Bulk And Nanoparticles In Nile Tilapia, Oreochromis Niloticus, Using Hematological, Bioaccumulation And Histological Biomarkers. Fish Physiology And Biochemistry, 42, 1225–1236.
Abdel-Khalek, A. A., Hamed, A., & Marie, M.-A. S. (2016b). The Accumulation Potency Of Bulk And Nano Zinc Metal And Their Impacts On The Hematological And Histological Perturbations Of Oreochromis Niloticus. Water, Air And Soil Pollution, 227, 206.
Abdel-Khalek, A. A., Elhaddad, E., Mamdouh, S., & Marie, M. S. (2018). The Chronic Exposure To Discharges Of Sabal Drain Induces Oxidative Stress And Histopathological Alterations In Oreochromis Niloticus. Bulletin Of Environmental Contamination And Toxicology, 101(1), 92–98.
Adam, V., & Nowack, B. (2017). European country-specific probabilistic assessment of nanomaterial flows towards landfilling, incineration and recycling. Environmental Science: Nano, 4(10), 1961–1973.
Antunes, A. M., Rocha, T. L., Pires, F. S., de Freitas, M. A., Leite, V. R. M. C., Arana, S., Moreira, P. C., & Sabóia-Morais, S. M. T. (2017). Gender-specific histopathological response in guppies Poecilia reticulata exposed to glyphosate or its metabolite aminomethylphosphonic acid. Journal of Applied Toxicology, 37(9), 1098–1107.
American Public 439 Health Association (APHA). (2005). Standard methods for the examination of water and wastewater. New York: American Water Works Association.
Arosio, P., & Levi, S. (2010). Cytosolic and mitochondrial ferritins in the regulation of cellular iron homeostasis and oxidative damage. Biochimica et Biophysica Acta, 1800, 783–792.
Ates, M., Demir, V., Arslan, Z., Kaya, H., Yılmaz, S., & Camas, M. (2016). Chronic exposure of tilapia (Oreochromis niloticus) to iron oxide nanoparticles: effects of particle morphology on accumulation, elimination, hematology and immune responses. Aquatic Toxicology, 177, 22–32.
Barbier, O., Jacquillet, G., Tauc, M., Cougnon, M., & Poujeol, P. (2005). Effect of heavy metals on, and handling by, the kidney. Nephron Physiology, 99, 105–110.
Benavides, M., Fernández-Lodeiro, J., Coelho, P., Lodeiro, C., & Diniz, M. S. (2016). Single and combined effects of aluminum (Al2O3) and zinc (ZnO) oxide nanoparticles in a freshwater fish, Carassius auratus. Environmental Science and Pollution Research, 23, 24578–24591.
Bernet, D., Schmidt, H., Meier, W., Burkhardt-Holm, P., & Wahli, T. (1999). Histopathology in fish: proposal for a protocol to assess aquatic pollution. Journal of Fish Diseases, 22(1), 25–34.
Canli, E. G., Dogan, A., & Canli, M. (2018). Serum biomarker levels alter following nanoparticle (Al2O3, CuO, TiO2) exposures in freshwater fish (Oreochromis niloticus). Environmental Toxicology and Pharmacology, 62, 181–187.
Capaldo, A., Gay, F., & Laforgia, V. (2019). Changes in the gills of the European eel (Anguilla anguilla) after chronic exposure to environmental cocaine concentration. Ecotoxicology and Environmental Safety, 169, 112–119.
Chen, P. J., Tan, S. W., & Wu, W. L. (2012). Stabilization or oxidation of nanoscale zerovalent iron at environmentally relevant exposure changes bioavailability and toxicity in medaka fish. Environmental Science and Technology, 46(15), 8431–8439.
Chupani, L., Niksirat, H., Velíšek, J., Stará, A., Hradilová, Š., Kolařík, J., Panáček, A., & Zusková, E. (2018). Chronic dietary toxicity of zinc oxide nanoparticles in common carp (Cyprinus carpio L.): tissue accumulation and physiological responses. Ecotoxicology and Environmental Safety, 147, 110–116.
Ciji, P. P., & Nandan, S. B. (2014). Toxicity of copper and zinc to Puntius parrah (day, 1865). Marine Environmental Research, 93, 38–46.
Dacie, J. V. & Lewis, S. M. (1991). Practical hematology. chuchill. Livigstone, Chap.5, 79.
Dar, Z. A., & Borana, K. (2014). Effect of sublethal doses of copper sulphate on certain haematological parameters of common carp, Cyprinus carpio. International Journal of Recent Scientific Research, 5(2), 332–335.
Drabkin, D. L. (1964). Spectrophotometric studies: XIV. The crystallographic and optical properties of the hemoglobin of man in comparison with those of other species. Journal of Biological Chemistry, 164, 703–723.
Feng, Q., Liu, Y., Huang, J., Chen, K., Huang, J., & Xiao, K. (2018). Uptake, distribution, clearance, and toxicity of iron oxide nanoparticles with different sizes and coatings. Scientific Reports, 8(1), 2082–2094.
Gupta, P. K. (1977). Haematological Techniques. 4th edition Syndicate, India, pp.231.
Gupta, Y. R., Sellegounder, D., Kannan, M., Deepa, S., Senthilkumaran, B., & Basavaraju, Y. (2016). Effect of copper nanoparticles exposure in the physiology of the common carp (Cyprinus carpio): biochemical, histological and proteomic approaches. Aquaculture and Fisheries, 1, 15–23.
Hadi, A. A., & Alwan, S. F. (2012). Histopathological changes in gills, liver and kidney of fresh water fish, Tilapia zillii, exposed to aluminum. International Journal of Pharmaceutical and Life Sciences, 3(11), 2071–2081.
Hao, L., Chen, L., Hao, J., & Zhong, N. (2013). Bioaccumulation and sub-acute toxicity of zinc oxide nanoparticles in juvenile carp (Cyprinus carpio): a comparative study with its bulk counterparts. Ecotoxicology and Environmental Safety, 91, 52–60.
Hermenean, A., Damache, G., Albu, P., Ardelean, A., Ardelean, G., Ardelean, D. P., Horge, M., Nagy, T., Braun, M., Zsuga, M., & Kéki, S. (2015). Histopatological alterations and oxidative stress in liver and kidney of Leuciscus cephalus following exposure to heavy metals in the Tur River, North Western Romania. Ecotoxicology and Environmental Safety, 119, 198–205.
Hua, J., Peijnenburg, W. J., & Vijver, M. G. (2016). TiO2 nanoparticles reduce the effects of ZnO nanoparticles and Zn ions on zebra fish embryos (Danio rerio). NanoImpact, 2, 45–53.
Jahanbakhshi, A., Hedayati, A., & Pirbeigi, A. (2015). Determination of acute toxicity and the effects of sub-acute concentrations of CuO nanoparticles on blood parameters in Rutilus rutilus. Nanomedicine Journal, 2(3), 195–202.
Javed, M., Ahmad, I., Usmani, N., & Ahmad, M. (2016). Bioaccumulation, oxidative stress and genotoxicity in fish (Channa punctatus) exposed to a thermal power plant effluent. Ecotoxicology and Environmental Safety, 127, 163–169.
Joshi, P. K., Bose, M., & Harish, D. (2002). Changes in certain haematological parameters in a siluroid catfish Clarias batrachus (Linn) exposed to cadmium chloride. Pollution Research, 21(2), 129–131.
Kadar, E., Tarran, G. A., Jha, A. N., & Al-Subiai, S. N. (2011). Stabilization of engineered zero-valent nano iron with Na-acrylic copolymer enhances spermiotoxicity. Environmental Science and Technology, 45(8), 3245–3251.
Kanwal, Z., Raza, M. A., Manzoor, F., Riaz, S., Jabeen, G., Fatima, S., & Naseem, S. (2019). A comparative assessment of nanotoxicity induced by metal (silver, nickel) and metal oxide (cobalt, chromium) nanoparticles in Labeo rohita. Nanomaterials, 9, 309–329.
Karthikeyeni, S., Vijayakumar, T. S., Vasanth, S., Ganesh, A., Manimegalai, M., & Subramanian, P. (2013). Biosynthesis of iron oxide nanoparticles and its haematological effects on fresh water fish Oreochromis mossambicus. Journal of Academia and Industrial Research, 1(10), 645–649.
Kaur, S., Khera, K. S., & Kondal, J. K. (2018). Effect of water contaminated with heavy metals on histopathology of freshwater catfish, Clarias batrachus. International Journal of Chemical Studies, 6(4), 3103–3108.
Kaviani, E. F., Naeemi, A. S., & Salehzadeh, A. (2019). Influence of copper oxide nanoparticle on hematology and plasma biochemistry of Caspian trout (Salmo trutta caspius), following acute and chronic exposure. Pollution, 5(1), 225–234.
Khabbazi, M., Harsij, M., Hedayati, S. A. A., Gholipoor, H., Gerami, M. H., & Farsani, H. G. (2015). Effect of CuO nanoparticles on some hematological indices of rainbow trout Oncorhynchus mykiss and their potential toxicity. Nanomedicine Journal, 2, 67–73.
Khosravi-Katuli, K., Shabani, A., Paknejad, H., & Imanpoor, M. R. (2018). Comparative toxicity of silver nanoparticle and ionic silver in juvenile common carp (Cyprinus carpio): accumulation, physiology and histopathology. Journal of Hazardous Materials, 359, 373–381.
Kori-Siakpere, O., & Ubogu, E. O. (2008). Sublethal haematological effects of zinc on the freshwater fish, Heteroclarias sp. (Osteichthyes: Clariidae). African Journal of Biotechnology, 7(12), 2068–2073.
Krishnani, K. K., & Ayyappan, S. (2006). Heavy metals remediation of water using plants and lignocellulosic agrowastes. Reviews of Environmental Contamination and Toxicology, 188, 59–84.
Lata, S., & Samadder, S. R. (2014). Removal of heavy metals using rice husk: a review. International Journal of Environmental Research and Development, 4(2), 165–170.
Lavanya, S., Ramesh, M., Kavitha, C., & Malarvizhi, A. (2011). Hematological, biochemical and ionoregulatory responses of Indian major carp Catla catla during chronic sublethal exposure to inorganic arsenic. Chemosphere, 82(7), 977–985.
Lewis, W. K., Harruff, B. A., Gord, J. R., Rosenberger, A. T., Sexton, T. M., Guliants, E. A., & Bunker, C. E. (2010). Chemical dynamics of aluminum nanoparticles in ammonium nitrate and ammonium perchlorate matrices: enhanced reactivity of organically capped aluminum. Journal of Physical Chemistry C, 115, 70–77.
Mela, M., Randi, M. A. F., Ventura, D. F., Carvalho, C. E. V., Pelletier, E., & Ribeiro, C. O. (2007). Effects of dietary methyl mercury on liver and kidney histology in the neotrophical fish Hoplias malabaricus. Ecotoxicology and Environmental Safety, 68, 426–435.
Murali, M., Suganthi, P., Athif, P., Bukhari, A. S., Mohamed, S. H. E., Basu, H., & Singhal, R. K. (2017). Histological alterations in the hepatic tissues of Al2O3 nanoparticles exposed freshwater fish Oreochromis mossambicus. Journal of Trace Elements in Medicine and Biology, 44, 125–131.
Murali, M., Athif, P., Suganthi, P., Bukhari, A. S., Mohamed, H. S., Basu, H., & Singhal, R. K. (2018). Toxicological effect of Al2O3 nanoparticles on histoarchitecture of the freshwater fish Oreochromis mossambicus. Environmental Toxicology and Pharmacology, 59, 74–81.
Murugan, S. S., Karuppasamy, R., Poongodi, K., & Puvaneswari, S. (2008). Bioaccumulation pattern of zinc in freshwater fish Channa punctatus (Bloch) after chronic exposure. Turkish Journal of Fisheries and Aquatic Sciences, 8, 55–59.
Saravanan, M., Suganya, R., Ramesh, M., Poopal, R. K., Gopalan, N., & Ponpandian, N. (2015). Iron oxide nanoparticles induced alterations in haematological, biochemical and ionoregulatory responses of an Indian major carp Labeo rohita. Journal of Nanoparticle Research, 17, 274–285.
Shaluei, F., Hedayati, A., Jahanbakhshi, A., Kolangi, H., & Fotovat, M. (2013). Effect of subacute exposure to silver nanoparticle on some hematological and plasma biochemical indices in silver carp (Hypophthalmichthys molitrix). Human and Experimental Toxicology, 32(12), 1270–1277.
Sivakumar, S., Khatiwada, C. P., & Sivasubramanian, J. (2012). Bioaccumulations of aluminum and the effects of chelating agents on different organs of Cirrhinus mrigala. Environmental Toxicology and Pharmacology, 34(3), 791–800.
Sobhanardakani, S., Parvizimosaed, H., & Olyaie, E. (2013). Heavy metals removal from wastewaters using organic solid waste-rice husk. Environmental Science and Pollution Research International, 20(8), 5265–5271.
Thophon, S., Kruatrachue, M., Upatham, E. S., Pokethitiyook, P., Sahaphong, S., & Jaritkhuan, S. (2003). Histopathological alterations of white sea bass, Later calcarifer, in acute and subchronic cadmium exposure. Environmental Pollution, 121, 307–320.
Vidya, P. V., & Chitra, K. C. (2019). Irreversible histopathological modifications induced by iron oxide nanoparticles in the fish, Oreochromis mossambicus (Peters, 1852). Biological Forum Journal, 11(1), 01–06.
Wang, D., Lin, Z., Wang, T., Yao, Z., Qin, M., Zheng, S., & Lu, W. (2016). Where does the toxicity of metal oxide nanoparticles come from: the nanoparticles, the ions, or a combination of both? Journal of Hazardous Materials, 308, 328–334.
Wang, M., Liu, R. R., Wang, C. J., Kang, W., Yang, G. H., Zhong, W. N., & Lai, Y. R. (2015). Combined histological and hematological assessment of iron-induced organ damage in a gerbil model of iron overload. American Journal of Translational Research, 7(2), 385–392.
Wu, Y., & Zhou, Q. (2013). Silver nanoparticles cause oxidative damage and histological changes in medaka (Oryzias latipes) after 14 days of exposure. Environmental Toxicology and Chemistry, 32, 165–173.
Zhu, B., He, W., Hu, S., Kong, R., & Yang, L. (2019). The fate and oxidative stress of different sized SiO2 nanoparticles in zebrafish (Danio rerio) larvae. Chemosphere, 225, 705–712.
Acknowledgment
The authors extend their appreciation to the Faculty of Science, Cairo University, Egypt for supporting the current work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in the present study involving animals (fish) were approved (approval no. CUFS F ECO 4615) and were in accordance with the ethical standards of Faculty of Science, Cairo University, Institutional Animal Care and Use Committee (IACUC) at which the studies were conducted.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abdel-Khalek, A.A., Badran, S.R. & Marie, MA.S. The Efficient Role of Rice Husk in Reducing the Toxicity of Iron and Aluminum Oxides Nanoparticles in Oreochromis niloticus: Hematological, Bioaccumulation, and Histological Endpoints. Water Air Soil Pollut 231, 53 (2020). https://doi.org/10.1007/s11270-020-4424-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-020-4424-2