Abstract
To investigate the potential pollution risk of permeable brick paving systems in areas with high groundwater levels, a system was constructed by using ceramic permeable bricks as the surface and a Chinese character “well”-shaped frame as the base on the top of a 1.0 m clay layer. The concentrations of total suspended solids (TSS), total phosphorus (TP), ammonia nitrogen (NH4+-N), total nitrogen (TN), chemical oxygen demand (COD), and heavy metals (Zn, Cu, and Pb) at different underground depths were measured, the potential pollution of the groundwater was assessed, and the effectiveness of the fillers inside the frame for improving the quality of the groundwater was discussed. The results showed that NH4+-N and COD concentrations detected at the depth of 0.6 m were higher than that of the national standard for groundwater (GBT14848-2017), these two pollutants had the potential pollution risk. The pollution risk by heavy metals was comparatively low because most of the heavy metals were likely retained in the surface soil by adsorption, complexation, and precipitation, while the pollution risk by TSS and TP was negligible due to the good purification ability in the clay layer. The results suggest that the removal rates of TSS, TP, TN, COD, and heavy metals can be improved by appropriate fillers’ adjustment, such as iron filings, coal slag, or volcanic rocks. This research offers a new perspective on the potential risk of pollution and the governance of groundwater.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
In recent years, permeable pavements have become one of the most frequently used low-impact development (LID) techniques (Dreelin et al. 2006; Ahiablame et al. 2012; Khan et al. 2012). This infiltration-based technology is composed of structural layers with a relatively high porosity to allow rainwater to pass through the surface and underlying layers (Chu and Fwa 2019), including a permeable pavement surface, aggregate subbases, geotextiles, and underdrains. The rainwater eventually infiltrates into the natural soil or is discharged into a drainage system (Drake et al. 2014).
The permeable brick paving system (PPS) was found to possess excellent hydrological properties and contribute to a reduction of rainwater pollution (Roseen et al. 2009; Jiang et al. 2015; Eck et al. 2015). The most significant environmental target of the PPS is to reduce the volume of rainwater runoff and to recharge the groundwater (Collins et al. 2008; Brown and Borst 2014). Naturally, it is ideal that the groundwater is directly recharged after the rainwater has been purified to acceptable standards by permeable brick paving, but the removal of pollutants in the rainwater runoff was not sufficient for a safe recharging of groundwater (Jin et al. 2017; Scholz 2013). However, the amount of water infiltration and the load intensity of pollution are large due to the serious non-point source pollution of urban rainwater runoff (Revitt et al. 2014), which may increase the amount of groundwater and pollutants at the same time, leading to a potential risk for contamination (Jin et al. 2017; Lin et al. 2020). Thus, the risk of groundwater contamination has become an emerging concern since the permeable brick paving system is not mainly designed for pollutants’ retention. For instance, in a study on the effect of permeable pavements on groundwater quality, it was found the effluent water quality of three permeable pavements was worse than that of the in situ groundwater level, resulting in a risk of groundwater pollution, especially by the heavy metals Cr and Pb (Jin et al. 2017).
The distance between the top surface of the soil and the groundwater level in China should be greater than 1.0 m in accordance with the standard of the technical specifications for water permeable brick pavements (CJJ/T 188-2012) (Ministry of Housing and Urban-Rural Development of the People’s Republic of China 2012); otherwise, extra pavement drainage facilities should be added, and this will obviously increase the cost of the PPS (Jin et al. 2017).
To date, gravel or cement concrete blocks have mainly been used as the base layer for PPSs (Jiang et al. 2015; Eck et al. 2015; Niu et al. 2016; Chu and Fwa 2019; Liu et al. 2019). Some researchers found that the base layer accounted for approximately 2/3 of the volume of the permeable pavement, but its contribution to runoff pollutant removal was only 10–25% (Sannudo-Fontaneda et al. 2014). This poor mitigation effectiveness indicated that the base layer had not been fully utilized. Therefore, it was essential to improve the base layer, mainly because it had an important influence on the removal rate of pollutants. To increase the removal rate by this layer and further improve effluent quality, a frame base layer with a Chinese character “well” shape was introduced into the permeable brick paving system, inside which different fillers was used to improve the permeable brick paving system. There are three main advantages to adopt this strategy: the first is to reduce the use of cement concrete and its negative impacts on water quality; the second is to adjust the flow rate by changing the diameter of the filler; and the third is to remove the contaminants by the filtration, retention, and adsorption of the fillers.
In the south of Jiangsu province, China, the groundwater level is relatively high, resulting in a shorter distance between the bottom of the PPS and the groundwater. The absence of extra drainage facilities for the PPS and the impact of the rainfall in the rainy season could aggravate the groundwater pollution problem in this region. In this study, a permeable brick paving system was constructed using ceramic permeable bricks as the surface and a Chinese character “well”-shaped frame as the base on the top of a 1.0 m clay layer. The effluent quality in the clay layers at different underground depths was measured, and the role the fillers played in improving the groundwater quality and its effectiveness were further discussed. The objectives and novelties of this research are to explore potential groundwater pollutants, which is a prerequisite for improving the effectiveness of the PPS, and to investigate the effectiveness of specific fillers in the removal of these characteristic pollutants.
2 Materials and Methods
2.1 Experimental Setup
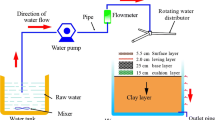
The test device for the PPS is shown in Fig. 1. The dimensions of the device were 0.8 m × 0.8 m × 1.5 m. A total of 16 permeable bricks measuring 20 cm × 20 cm × 5.0 cm were placed on the 0.8 m × 0.8 m cross section of the device. A total of six 10 mm-diameter outlet pipes were set, from the top to the bottom, at depths of 0 cm, 20 cm, 40 cm, 60 cm, 80 cm, and 100 cm in the clay layer. An overflow pipe with a diameter of 10 mm was set at the top of the device.
The prepared rainwater was lifted by a water pump and evenly distributed by a rotating water distributor. The rainwater was sampled after passing through the ceramic permeable brick and the paving system. Samples were taken every 10 min for a total of 2 h, while the outlet pipe of the pavement had a stable outflow. The water samples were collected using a 500 mL polyethylene sampling bottle, and all samples were measured within 48 h.
2.2 Used Materials
2.2.1 Permeable Brick
The ceramic permeable bricks measured 20 cm × 20 cm × 5.5 cm and were purchased from Youbang (Yixing, China) Building Materials Co., Ltd. The main properties of the ceramic bricks (Table 1), such as the splitting tensile strength, permeability coefficient, frost resistance, slip resistance, and porosity, all met the requirements of the Chinese national standard for permeable paving bricks and permeable paving flags (GB/T 25993-2010).
As shown in Fig. 2, the ceramic permeable bricks, in cross section, had a higher proportion of pores in the 0.5–1.5 mm size than impervious bricks, while the ceramic permeable bricks and impervious bricks had porosities of 24.82% and 9.75%, respectively. The permeability coefficient of the ceramic permeable bricks was 3.1 × 10−2 cm/s, which was higher than the national standard of 2.0 × 10−2 cm/s.
Besides, according to the Chinese Technical code for rainwater management and utilization of building and sub-district (GB 50400-2016), coarse sand with diameter 0.5–1.0 mm was used for the leveling layer which was 2.0-cm thick and gravel with diameter 15–20 mm for the cushion layer which was 15-cm thick.
2.2.2 Base Material
The base layer employed a well-shaped frame structure with a symmetrical shape (Fig. 3), length × breadth × height measuring 40 cm × 40 cm × 25 cm with an open ratio of approximately 40%, which contained the fillers. Volcanic rock (VC), coal slag (CS), iron filings (IF), and gravel (G) were selected as fillers to determine their effect on the reduction of the groundwater contamination in the test device (Fig. 4). The systems containing the four fillers are referred to as the VC, CS, IF, and G facilities, respectively.
2.2.3 Clay Layer
The silty clay was taken from the campus of Nanjing Forestry University. All the clays retained their original properties before being filled into the equipment. After the clays had been added, they were subjected to a compaction treatment layer by layer to make their permeability coefficient consistent with that of the soil. The permeability coefficient in the sampling test was approximately 10−6 cm/s. Furthermore, a blank test was carried out on the clay layer using clean water to eliminate the influence of the soil background on the water quality. The experiment indicated that up to 3.51 mg/L of TN was leached from the soil, but other pollutants were not detected.
2.3 Simulated Rainfall
This test was based on a 2-h rainfall duration and a 20-year return period of the precipitation in Nanjing as the experimental rainfall conditions. The rainfall intensity formula in Nanjing was as follows:
where i is the rainfall intensity in mm/min; P is the return period of the rainfall in years; and t is the rainfall duration in min.
According to the existing monitoring data, the concentrations of the various pollutants in the artificially prepared rainwater are shown in Table 2.
2.4 Test Methods
According to the Chinese National Standard Methods (SEPA of China 2002), TSS was determined by the gravimetric method (GB 11901-89); COD was determined by the fast digestion-spectrophotometric method (HJ/T 399-2007); concentrations of NH3-N, TN, and total phosphorus (TP) were determined by a spectrophotometric method using an UV spectrophotometer (L5S, China); and concentrations of Zn, Cu, and Pb were determined by atomic absorption spectrophotometry using an atomic absorption spectrophotometer (TAS-990, China). Specific surface area and pore structures of the fillers were measured using a surface area and porosimetry analyzer (V-Sorb 2800, China). Scanning electron microscope (SEM) and the main elemental composition was determined by an energy-dispersive spectrometer (FEI Quanta 200, The Netherlands). The water used in the experiment was ultra-pure water generated by an ultra-pure water machine (Biosafer-20TAB, China). The analyses were carried out three times and the average value was taken as the test results to make sure the value was typical.
3 Results and Discussion
3.1 Characterization of the Fillers
SEM micrographs of the fillers are shown in Fig. 5 at 300 times magnification. Based on these micrographs, the volcanic rock had a homogeneous distribution of grains and a well-developed porous structure with a small pore size. The coal slag had a heterogeneous distribution of grains and a well-developed porous structure with different pore sizes, where small pores were attributed to the evaporation of organic matter due to the combustion of the coal. The iron filings had a flat surface without any pores, and silicate crystals could be observed on the surface of the gravel.
The specific surface area and pore structure of the fillers were analyzed based on nitrogen adsorption, as shown in Table 3. The iron filings exhibited a poor adsorption of pollutants because of the lack of micropore volume, which plays an important role in the adsorption process. Moreover, the gravel fillers exhibited little affinity for surface adsorption because of their small specific surface area.
The energy-dispersive X-ray spectroscopy (EDS) analysis results are shown in Table 4. We inferred from these analyses that the main elements of the volcanic rock and coal slag were oxygen, aluminum, silicon, and calcium, and the main elements of the iron filings were oxygen and iron. The elements of calcium, magnesium, and aluminum in the fillers mainly existed in the form of oxides, and the iron existed as elemental iron.
3.2 The Removal of TSS
The concentrations and removal rates of TSS at different clay depths are shown in Fig. 6. The TSS concentration in the effluent of the systems with different fillers at the clay depth of 0 m was low, with a good removal rate higher than 80%. The TSS concentration was stable with a removal rate of nearly 100% at the clay depth of 0.6 m because of the good filtration due to a low permeability coefficient at this depth. The amount of TSS in the seepage water at the clay depth of 1.0 m was negligible and would not cause any damage to the groundwater quality.
The removal efficiency of TSS by different materials inside the frame structure was found to follow the order volcanic rock > iron filings > coal slag > gravel. The filling of volcanic rock, iron filings, or coal slag into the frame effectively improved the TSS effluent quality of the system, which was shown by the reduction in TSS quality at the clay depth of 0 m. In addition to the physical interception by the permeable brick at the surface layer, the fillers inside the frame structure also had interception and adsorption effects on the TSS (Jin et al. 2017; Niu et al. 2016; Kamali et al. 2017). The weak adsorption of the gravel might mainly be responsible for the low removal rate of TSS. In contrast, the rough and porous surfaces with a higher specific surface areas of the volcanic rock and coal slag had a good adsorption effect, while the iron filings were likely converted to Fe2+ or Fe3+ (Cheng et al. 2007; Reddy et al. 2014; Statham et al. 2015), which exhibited a certain adsorption-flocculation activity.
The permeable brick paving system exhibited a good decontamination performance for the TSS, and the removal of TSS mainly depended on the interception by the surface permeable brick in the paving system. Research found that the average removal efficiency of TSS was approximately 90.0% for six common surface materials (porous asphalt, porous concrete, cement brick, ceramic brick, sand base brick, and shale brick) due to physical interception (Li et al. 2017; Roseen et al. 2012; Zhang et al. 2018). TSS were effectively removed via straining by the system. Because TSS pose little health risk, suspended solids are mainly a concern because they might clog the paving system. The ceramic brick in our study could be divided into two layers in a common configuration in terms of the distribution of the pores, with small pores lying on the top and large pores being on the bottom (Fig. 2). This made the surface layer function as a sieve that prevented the downward movement of particles, which was beneficial to the removal of TSS and the prevention of blockage.
3.3 The Removal of TP
The concentrations and removal rates of TP at different clay depths are shown in Fig. 7. The removal rates of TP in the permeable brick paving system at the clay depth of 0 m with different fillers were all above 75%. TP was easily adsorbed on the suspended particles and could be removed with the TSS by the filtration of the permeable brick (Jiang et al. 2015; Eck et al. 2015; Cates et al. 2009). The ranking of the removal efficiency of TP was volcanic rock > iron filings > coal slag > gravel, and the reason for this order might be that the TP was removed along with TSS. Filling volcanic rock, iron filings, or coal slag instead of gravel into the frame effectively improved the TP effluent quality of the system, particularly at a depth of 0 m.
The TP concentration of the effluent in the paving system at the clay depth of 0 m filled with gravel was higher than the 0.4 mg/L limit for the V level (0.4 mg/L) in the Chinese Standard (Environmental Quality Standard for Surface Water) (GB 3838-2002), which was caused by the relatively low removal efficiency of TP in this system. The removal rates of TP for all the systems were nearly 100% at a clay depth of 0.6 m, which was likely due to the good purification ability for TP and TSS in the clay layer (Luk 2013; Jiang et al. 2015; Risto et al. 2001; Mcdowell and sharpley 2003; Chittoori and puppala 2011). The TP concentrations of all the systems in the seepage water at a clay depth of 1.0 m were negligible, indicating that TP will not pollute the groundwater.
Orthophosphate (PO43−) is the most common form of phosphorus occurring in rainwater, and there was basically no environmental problem in the treatment of orthophosphate by the permeable pavement system. First, the system had a high removal efficiency. Once in the soil media, PO43− could be transferred from rainwater via precipitation or chemical adsorption to the surface of soil particles, and easily chemically reacts with a large amount of Ca2+, Fe3+, and Al3+ ions present in the soil (Muhammetoglu et al. 2002), followed by a conversion of the sorbed phosphorus into minerals. The downward movement of phosphorus in different soils was found to be directly related to the reactivity index measured for each soil (Pitt et al. 1999). However, the investigated depth in previous studies was shallow and controllable, and most of the phosphorus remained within the top 5–7.5 cm of the surface when phosphate fertilizer was applied (Lauer 1988).
3.4 The Removal of NH4+-N
The concentrations and removal rates of NH4+-N at different clay depths are shown in Fig. 8. The removal efficiency of NH4+-N in the paving system was significantly lower than that of TSS and TP, and the efficiency of removing NH4+-N was similar in the four paving systems. The NH3-N effluent concentrations in four systems were all higher than 2.0 mg/L, which is the limit for the V level in the Chinese Environmental Quality Standard for Surface Water (GB 3838-2002). The weak adsorption of dissolved ammonia ions by the permeable bricks was likely responsible for the low NH4+-N removal rate.
The concentrations of NH4+-N in the clay layer greatly decreased with increasing depth, which indicates that some of the NH4+-N had been purified or transformed in the upper soil. Although the soil itself had a good performance in removing NH4+-N, the NH4+-N in the seepage water at the clay depth of 0.6 m still exceeded the national standard for groundwater of 1.5 mg/L. For the NH4+-N concentrations meeting the national standard, the seepage water needed to reach a depth of 0.8–1.0 m. Therefore, NH4+-N had the potential of polluting the groundwater.
NH4+-N was the most toxic form to aquatic life in the rainwater, but most of the NH4+-N was adsorbed and transformed by the upper soil below the permeable system. The high adsorption rate was likely caused by the attraction between the positively charged ammonia ions and the negatively charged clay (Ingvertsen et al. 2012). It is also possible that some of the NH4+-N had changed to NO2−-N or NO3−-N because there was more air and enough oxygen in the upper surface soil, which could promote the nitrification by microorganisms in the soil (Wang et al. 2019).
3.5 The Removal of TN
The concentration and removal rates of TN at different clay depths are shown in Fig. 9. The concentrations of TN at different depths all exceeded that of the national standard. It is important to emphasize that with the increasing depth, the concentration rate of TN increased and the removal rate decreased respectively.
There are three main reasons for the decrease in the TN removal rate with depth in the clay layer. First, the reduction in NH4+-N (Fig. 8) might have been mainly caused by the nitrification of ammonia in the clay layer (Schipper et al. 2004; Huang et al. 1998), which would increase the nitrate load. Second, nitrate is more difficult to adsorb due to its negative charge, which is repelled by the negative charges of the clay particles (Davis et al. 2001). Third, TN itself was easily leached from the soil layer that was rinsed with clean water (up to 3.51 mg/L).
As shown in Fig. 9, the permeable brick paving system filled with iron filings had the lowest TN concentration with the highest removal efficiency, which was mainly due to the reduction reaction of Fe with NO3−-N (Zhang et al. 2010). These results provide a feasible technical idea for improving the removal efficiency of TN; that is, the removal efficiency of TN from rainwater can be further improved by optimizing the composition of iron filings and the other functional fillers to remove nitrate and ammonia.
Considering the nitrification and adsorption of the soil, TN was basically composed of nitrate. A related research found over a constant 15-year infiltration by wastewater, a quarter of the NH4+ in raw sewage would oxidize to NO3− while some 60% would be adsorbed by the soil, and the groundwater would be polluted by NO3− (Foppen 2002). The nitrification of ammonia made the nitrates become a potential risk of groundwater pollution. Nitrates present in drinking water could pose a health concern to fetuses and infants. Most studies indicated that nitrate was poorly retained in infiltration devices due to a high solubility (Bhatnagar and Sillanpaa 2011). This was the reason why nitrate was suggested to be one of the most frequently encountered contaminants in groundwater (Pitt et al. 1995; Nolan and Weber 2015). The nitrates could travel toward the groundwater with some being removed by the soil via processes that involve a complex array of variables. The movement of nitrate in the soil was dependent on the rate and volume of infiltration, horizontal and vertical groundwater flow, depth to the groundwater table, and preferential flow paths (Ferguson et al. 1990). Hence, the factors responsible for the concentration of nitrates in groundwater and soil need to be further studied in permeable paving systems.
3.6 The Removal of COD
As shown in Fig. 10, the effluent COD concentrations at the clay depth of 0 m from the four systems all satisfied the standard for surface water quality (40 mg/L). Moreover, the systems filled with volcanic rock and coal slag had better removal rates of COD compared with those filled with iron filings and gravel, mainly due to the high content of Fe2O3 in the volcanic rock (Koupai et al. 2016) and coal slag, which has a high affinity of adsorbing the COD. Thus, the potential pollution risk by COD could be decreased by filling coal slag or volcanic rock into the frame structure.
The COD in the seepage water at the different clay depths exceeded the national standard for groundwater quality, which is 10 mg/L, although the clay layer was capable of degrading a portion of the COD. Still, it had the potential of polluting the groundwater.
The removal rate between the volcanic rocks and gravel differed by 25.58%, indicating that the adsorption properties of the materials played a key role in COD removal. The addition of fillers with good adsorption properties, such as volcanic rock and coal slag, was shown to help remove dissolved organic matter, which was a composition part of COD.
The COD represented the amount of reductive substances in the rainwater, which were great differences in the composition of organic compounds from different environments. A research found most organics were removed by adsorption, degradation, and decomposition during the soil percolation (Jensen et al. 2017; Newman et al. 2014). A considerable amount of organic pollutants in groundwater would be previously degraded in the soil, thus, improving the quality of the groundwater. Several organic compounds found in runoff were retained in the soil, with only one priority pollutant being detected in the aquifer (German 1989). Therefore, it is necessary to discuss the degradation of specific organic pollutants in the clay layer according to the different applications of the permeable pavement system. For example, emerging organic contaminants (EOCs) detected in groundwater have a potential threat on human health and aquatic ecosystem, making the contamination of groundwater by EOCs a growing concern (Lapworth et al. 2012); therefore, it is meaningful to study the pollution of EOCs in the permeable brick system.
3.7 The Removal of Heavy Metals
The concentrations and removal rates of heavy metals (Zn, Cu, and Pb) at different clay depths are shown in Fig. 11. In general, the concentrations of heavy metals in the effluent from the four systems basically met the V-type surface water standard, except for Pb. Heavy metals are present in rainwater in dissolved phases, but a large fraction of most metals is usually bound to suspended solids (Davis and McCuen 2005). The high removal rates in the present study are attributed to the easy attachment of Zn, Cu, and Pb to suspended particles in rainwater (Jiang et al. 2015; Zuo et al. 2011; Nie et al. 2008). The quality of the seepage water at different depths in the clay was higher than that of the groundwater of type V because most of the heavy metals were likely retained in the surface soil by adsorption, complexation, and precipitation once the heavy metals entered the soil (Nikonov et al. 2001; Soon 1994; Rodriguez-Rubio et al. 2010; Banerjee et al. 2017). As a result, the risk of groundwater contamination by heavy metals was relatively low.
The concentration of Zn in the effluent from all the systems met the surface water standard of type V, and some concentrations were even lower than the 0.05 mg/L level of type I, which meant that the water could be applicable as source water and in national nature reserves. The Zn concentrations in the groundwater below the depth of 0.4 m were also lower than the 0.05 mg/L level of the groundwater standard of type I, indicating that the groundwater could be used for various purposes.
For the heavy metal Cu, the concentrations in the effluent from all systems were lower than the 1.0 mg/L level of the surface water standard of type II, and the water could be applicable to primary protection areas of surface source water. The concentration of Zn in the groundwater below the depth of 0.4 m was lower than the 0.5 mg/L level of the groundwater standard of type II, indicating that the groundwater could be used for various purposes as well.
For the heavy metal Pb, the concentration in the effluent from all systems, except for the gravel filler, was lower than the 0.1 mg/L level of the surface water standard of type V, and the water could be applied in agriculture and landscape implementations. The Pb concentration in the groundwater below the depth of 0.2 m was lower than the 0.1 mg/L level of the groundwater standard of type IV, indicating that the groundwater could be used in agriculture and industrial applications.
Heavy metals are generally not an important groundwater contaminant because the metals were easily removed along with TSS and extra affinity to soils. Previous studies have demonstrated that metals are retained in the upper soil layers via adsorption to solid particles (Robert Pitt et al. 1999). The water quality monitoring data also showed that the concentrations of Zn, Cu, and Pb in the effluent decreased with increasing depth, indicating that the heavy metals were effectively retained in the soil. Research on the prolonged deposition of heavy metals in infiltration facilities has shown the probable leaching of heavy metals to the underlying soil (Aryal et al. 2006). This process may occur due to the finite sorption capacities of the soil when the accumulation of heavy metals exceed a certain threshold. Periodic replacement of the upper soil layer within paving systems may be a good method for preventing a possible groundwater contamination and maintaining low heavy metal concentrations. Our continuous experiments for 1 year showed that the effluent samples had a lower concentration at the depth of 0.4 m, indicating that the heavy metals might be retained in the upper 0.4 m. Thus, we propose that replacing the upper 0.4 m of the clay layer would be sufficient for environmental safety for the complete permeable pavement’s life span.
3.8 The Effectiveness of the Fillers
The effectiveness of the fillers inside the frame in improving the water quality can be inferred specifically from the effluent quality of the system, which can be reflected by the improvement in the water quality at the clay depth of 0 m. Although the fillers cannot directly improve the quality of the groundwater, they can, however, improve the effluent quality of the PPS system. Thus, the fillers play an indirect role in improving the quality of the groundwater by reducing its pollution load.
Filling volcanic rock, iron filings, or coal slag instead of gravel into the frame effectively reduced the TSS, TP, and heavy metal loads in the effluent of the system, particularly at the depth of 0 m. Iron filings were beneficial to the degradation of TN due to the reduction reaction of Fe with NO3−-N. The effluent load of COD can be reduced by filling coal slag or volcanic rock into the frame structure due to the good adsorption of COD to the fillers. In addition, filling the synthetic materials with high adsorption capacity should also be very helpful in improving the removal of heavy metals (Samuel et al. 2018a, 2018b, 2018c, 2018d; Samuel et al. 2019; Samuel et al. 2020).
The frame structure provides an environmental benefit to the paving system. First, the fillers inside the frame structure efficiently removed pollutants from the rainwater. Second, the frame structure is conducive to the presence of oxygen and is helpful for the reproduction and survival of denitrifying bacteria, which is beneficial to the degradation of TN. Third, a paving system using a frame structure will not have a reduced bearing capacity, and the use of a frame structure has no effect on the life cycle of the paving system.
4 Conclusions
A permeable brick paving system was constructed using ceramic permeable bricks as the surface layer and a well-shaped frame as the base layer with a 1.0 m clay layer at the bottom of the system. The fillers’ effectiveness in improving the quality of the effluent was studied, and the effluent quality of clay layers at different belowground depths was detected. Based on our results, the following conclusions could be drawn:
-
1.
TSS and TP were well removed by the permeable paving systems, and the infiltration water at different clay depths met the requirements of the Chinese groundwater quality standards. TSS and TP had no potential for polluting the groundwater.
-
2.
The concentrations of NH3 and COD at the depth of 0.6 m exceeded the standard limits for groundwater, and there was a risk of groundwater pollution, especially for areas with high groundwater levels.
-
3.
The nitrification of ammonia made the nitrates become a potential risk of groundwater pollution. The pollution risk by heavy metals was comparatively low due to their effective retention in the soil; therefore, regular replacement of the top soil was a good way to prevent heavy metal pollution.
-
4.
The removal rates of TSS, TP, TN, COD, and heavy metals could be increased by appropriately adjusting the fillers, such as iron filings, volcanic rock, or other similar functional fillers, in the framework structure, reducing the pollution load and the potential pollution risk of the groundwater.
-
5.
Optimizing the type and proportion of fillers in the framework to reduce the potential risk of groundwater pollution is the focus for further attempts. Also, changes in underground soil concentrations under long-term operating conditions should be investigated to examine the risk of groundwater contamination from the aspects of subsurface soil.
References
Ahiablame, L. M., Engel, B. A., & Chaubey, I. (2012). Effectiveness of low impact development practices: literature review and suggestions for future research. Water, Air, and Soil Pollution, 223, 4253–4273.
Aryal, R. K., Murakami, M., Furumai, H., Nakajima, F., & Jinadasa, H. K. P. K. (2006). Prolonged deposition of heavy metals in infiltration facilities and its possible threat to groundwater contamination. Water Science and Technology, 4, 205–212.
Banerjee, U. S., Guo, Z. H., Zhou, K. G., & Chai, L. Y. (2017). Distribution and plant availability of Cd, Pb, Cu and As in different particle size soil fractions. Journal of the Indian Chemical Society, 94, 1029–1035.
Bhatnagar, A., & Sillanpaa, M. (2011). A review of emerging adsorbents or nitrate removal from water. Chemical Engineering Journal, 168, 493–504.
Brown, R. A., & Borst, M. (2014). Nutrient infiltrate concentrations from three permeable pavement types. Journal of Environmental Management, 164, 74–85.
Cates, E. L., Westphal, M. J., Cox, J. H., Calabria, J., & Patch, S. C. (2009). Field evaluation of a proprietary storm-water treatment system: removal efficiency and relationships to peak flow, season, and dry time. Journal of Environmental Engineering, 135, 511–517.
Cheng, H. F., Xu, W. P., Liu, J. L., Wang, H. J., He, Y. Q., & Chen, G. (2007). Pretreatment of wastewater from triazine manufacturing by coagulation, electrolysis, and internal microelectrolysis. Journal of Hazardous Materials, 146, 385–392.
Chittoori, B., & Puppala, A. J. (2011). Quantitative estimation of clay mineralogy in fine-grained soils. J Geotech Geoenviron, 137, 997–1008.
Chu, L., & Fwa, T. F. (2019). Evaluation of surface infiltration performance of permeable pavements. Journal of Environmental Management, 238, 136–143.
Collins, K. A., Hunt, W. F., & Hathaway, J. M. (2008). Hydrologic comparison of four types of permeable pavement and standard asphalt in Eastern North Carolina. Journal of Hydrologic Engineering, 13, 1146–1157.
Davis, A. P., & McCuen, R. (2005). Stormwater management for smart growth, 368 pp. New York: Springer.
Davis, A. P., Mohammad, S., Himanshu, S., & Minami, C. (2001). Laboratory study of biological retention for urban stormwater management. Water Environment Research, 73, 5–14.
Drake, J., Bradford, A., & Van, S. T. (2014). Winter effluent quality from partial-infiltration permeable pavement systems. Journal of Environmental Management, 140, 04014036.
Dreelin, E. A., Fowler, L., & Carroll, C. R. (2006). A test of porous pavement effectiveness on clay soils during natural storm events. Water Research, 40, 799–805.
Eck, B. J., Winston, R. J., Hunt, W. F., & Barrett, M. E. (2015). Water quality of drainage from permeable friction course. J Environ Eng, 138, 174–181.
Ferguson, R. B., Eisenbauer, D. E., Bockstadter, T. L., Krull, D. H., & Buttermore, G. (1990). Water and nitrogen management in Central Platte Valley of Nebraska. Journal of Irrigation and Drainage Engineering, 116, 557–565.
Foppen, J. W. A. (2002). Impact of high strength wastewater infiltration on groundwater quality and drinking water supply the case of Sana’a, Yemen. Journal of Hydrology, 263, 198–216.
German ER (1989). Quantity and quality of stormwater runoff recharged to the floridan aquifer system through two drainage wells in the Orlando, Florida, area. U.S. Geological Survey water-supply paper (USA).
Huang, C. P., Wang, H. W., & Chiu, P. C. (1998). Nitrate reduction by metallic iron. Water Research, 32, 2257–2264.
Ingvertsen, S. T., Cederkvist, K., Jensen, M. B., & Magid, J. (2012). Assessment of existing roadside swales with engineered filter soil: II. Treatment efficiency and in situ mobilization in soil columns. Journal of Environmental Quality, 41, 970–1981.
Jensen, J. L., Schjonning, P., Watts, C. W., Christensen, B. T., & Munkholm, L. J. (2017). Soil texture analysis revisited: removal of organic matter matters more than ever. PLoS One, 12, e0178039.
Jiang, W., Sha, A. M., Xiao, J. J., Li, Y. L., & Huang, Y. (2015). Experimental study on filtration effect and mechanism of pavement runoff in permeable asphalt pavement. Construction and Building Materials, 100, 102–110.
Jin, J. R., Li, T., & Shi, Z. B. (2017). Performance of applying scale permeable pavements for control of runoff pollution in an area with high groundwater level. Environmental Sciences, 38, 2379–2384.
Kamali, M., Delkash, M., & Tajrishy, M. (2017). Evaluation of permeable pavement responses to urban surface runoff. Journal of Environmental Management, 187, 43–53.
Khan, U., Valeo, C., Chu, A., & van, D. B. (2012). Bioretention cell efficacy in cold climates: part 1: hydrologic performance. Canadian Journal of Civil Engineering, 39, 1210–1221.
Koupai, J. A., Nejad, S. S., Mostafazadeh-Fard, S., & Behfarnia, K. (2016). Reduction of urban storm-runoff pollution using porous concrete containing iron slag adsorbent. J Environ Eng, 142, 04015072.
Lapworth, D. J., Baran, N., Stuart, M., & Ward, R. S. (2012). Emerging organic contaminants in groundwater: a review of sources, fate and occurrence. Environmental Pollution, 163, 287–303.
Lauer, D. A. (1988). Vertical distribution in soil of unincorporated surface-applied phosphorus under sprinkler irrigation. Soil Science Society of America Journal, 52, 1685–1692.
Li, H., Li, Z., Zhang, X., Li, Z., Liu, D., Li, T., & Zhang, Z. (2017). The effect of different surface materials on runoff quality in permeable pavement systems. Environ Sci Pollut, 24, 21103–21110.
Lin, Z. Z., Chen, H. M., & Yang, H. (2020). Risk of contamination of infiltrated water and underground soil by heavy metals within a ceramic permeable brick paving system. Environemental Science and Pollution Research. https://doi.org/10.1007/s11356-020-08745-w.
Liu, J., Yan, H. X., Zhang, K., Schmidt, A. R., & Tao, T. (2019). Laboratory analysis on the surface runoff pollution reduction performance of permeable pavements. Sci Total Environ, 691, 1–8.
Luk, G. K. (2013). Removal of total phosphorus from domestic wastewater with clay-zeolite medium. Advances in Materials Research, 647, 753–757.
Mcdowell, R. W., & Sharpley, A. N. (2003). The effects of soil carbon on phosphorus and sediment loss from soil trays by overland flow. Journal of Environmental Quality, 321, 207–214.
Ministry of Housing and Urban-Rural Development of the People’s Republic of China. (2012). Technical specification for pavement of water permeable brick (CJJ/T 188–2012). Beijing: China Building Industry Press (in Chinese).
Muhammetoglu, H., Muhammetoglu, A., & Soyupak, S. (2002). Vulnerability of groundwater to pollution from agricultural diffuse sources: a case study. Water Science and Technology, 45, 1–7.
Newman, A. P., Puehmeier, T., Shuttleworth, A., & Pratt, C. J. (2014). Performance of an enhanced pervious pavement system loaded with large volumes of hydrocarbons. Water Science and Technology, 70, 835–842.
Nie, F. H., Li, T., Yao, H. F., Feng, M., & Zhang, G. K. (2008). Characteristics of suspended solids and particle-bound heavy metals in a first flush of highway runoff. Journal of Zhejiang University. Science. A, 11, 1567–1575 (in Chinese).
Nikonov, V., Goryainova, V., & Lukina, N. (2001). Ni and Cu migration and accumulation in forest ecosystems on the Kola Peninsula. Chemosphere., 42, 93–100.
Niu, Z. G., Lv, Z. W., Zhang, Y., & Cui, Z. Z. (2016). Stormwater infiltration and surface runoff pollution reduction performance of permeable pavement layers. Environmental Science and Pollution Research, 23, 2576–2587.
Nolan, J., & Weber, K. A. (2015). Natural uranium contamination in major US aquifers linked to nitrate. Environmental Science & Technology, 2, 215–220.
Pitt, R., Clark, S. M., Parmer, K., & Field, R. (1995). Groundwater contamination from stormwater Infiltration (pp. 127–132). Reston: American Society of Civil Engineers.
Pitt, R., Clark, S., & Field, R. (1999). Groundwater contamination potential from stormwater infiltration practices. Urban Water., 1, 217–236.
Reddy, K. R., Xie, T., & Dastgheibi, S. (2014). Adsorption of mixtures of nutrients and heavy metals in simulated urban stormwater by different filter materials. Journal of Environmental Science and Health, Part A, 49, 524–539.
Revitt, D. M., Lundy, L., Coulon, F., & Fairley, M. (2014). The sources, impact and management of car park runoff pollution: a review. Journal of Environmental Management, 146, 552–567.
Risto, U., Eila, T., Tommi, K., & Lilja, T. (2001). Particulate phosphorus and sediment in surface runoff and drain flow from clayey soils. Journal of Environmental Quality, 30, 589–595.
Rodriguez-Rubio, P., Morillo, E., Madrid, L., Undabeytia, T., & Maqueda, C. (2010). Retention of copper by a calcareous soil and its textural fractions: influence of amendment with two agroindustrial residues. European Journal of Soil Science, 54, 401–409.
Roseen, R. M., Ballestero, T. P., Houle, J. J., Avellaneda, P., Briggs, J., Fowler, G., & Wildey, R. (2009). Seasonal performance variations for storm-water management systems in cold climate conditions. Journal of Environmental Engineering, 135, 128–137.
Roseen, R. M., Ballestero, T. P., Houle, J. J., Avellaneda, P., Briggs, J. F., & Houle, K. M. (2012). Water quality and hydrologic performance of a porous asphalt pavement as a storm-water treatment strategy in a cold climate. Journal of Environmental Engineering, 138, 128–137.
Samuel, M. S., Sheriff Shah, S. K., Subramaniyan, V., Qureshi, T., Bhattacharya, J., & Pradeep Singh, N. D. (2018a). Preparation of graphene oxide/chitosan/ferrite nanocomposite for chromium (VI) removal from aqueous solution. Int J Biol Macromol, 119, 540–547.
Samuel, M. S., Subramaniyan, V., Bhattacharya, J., Chidambaram, R., Qureshi, T., & Pradeep Singh, N. D. (2018b). Ultrasonic-assisted synthesis of graphene oxide – fungal hyphae: an efficient and reclaimable adsorbent for chromium (VI) removal from aqueous solution. Ultrason Sonochem, 48, 412–417.
Samuel, M. S., Sk, S. S., Bhattacharya, J., Subramaniam, K., & Pradeep Singh, N. D. (2018c). Adsorption of Pb (II) from aqueous solution using a magnetic chitosan/graphene oxide composite and its toxicity studies. International Journal of Biological Macromolecules, 115, 1142–1150.
Samuel, M. S., Subramaniyan, V., Bhattacharya, J., Parthibanb, C., Chand, S., & Pradeep Singh, N. D. (2018d). A GO-CS@MOF [Zn (BDC)(DMF)] material for the adsorption of chromium (VI) ions from aqueous solution. Compos Part B-Eng., 152, 116–125.
Samuel, M. S., Bhattacharya, J., Raj, S., Santhanamb, N., Singh, H., & Pradeep Singh, N. D. (2019). Efficient removal of chromium (VI) fromaqueous solution using chitosan grafted graphene oxide (CS-GO) nanocomposite. International Journal of Biological Macromolecules, 121, 285–292.
Samuel, M. S., Selvarajanb, E., Subramaniamc, K., Mathimanid, T., Seethappane, S., & Pugazhendhif, A. (2020). Synthesized β-cyclodextrin modified graphene oxide (β-CD-GO) composite for adsorption of cadmium and their toxicity profile in cervical cancer (HeLa) cell lines. Process Biochemistry, 93, 28–35.
Sannudo-Fontaneda, L. A., Charlesworth, S. M., Castro-Fresno, D., Andres-Valeri, V. C. A., & Rodriguez-Hernandez, J. (2014). Water quality and quantity assessment of pervious pavements performance in experimental car park areas. Water Science and Technology, 69, 1526–1533.
Schipper, L. A., Barkle, G. F., Hadfield, J. C., Vojvodic-Vukovic, M., & Burgess, C. P. (2004). Hydraulic constraints on the performance of a groundwater denitrification wall for nitrate removal from shallow groundwater. Journal of Contaminant Hydrology, 69, 263–279.
Scholz, M. (2013). Water quality improvement performance of geotextiles within permeable pavement systems: a critical review. Water., 5, 462–479.
Soon, Y. K. (1994). Changes in forms of soil zinc after 23 years of cropping following cle. Canadian Journal of Soil Science, 74, 179–184.
Statham, T. M., Mumford, K. A., Rayner, J. L., & Geoffrey, W. S. (2015). Removal of copper and zinc from ground water by granular zero-valent iron: a dynamic freeze–thaw permeable reactive barrier laboratory experiment. Cold Regions Science and Technology, 110, 120–128.
Wang, Z. H., Cao, Y. Q., Wright, A. L., Shi, X. L., & Jiang, X. J. (2019). Different ammonia oxidizers are responsible for nitrification in two neutral paddy soils. Soil and Tillage Research, 195, 104433.
Zhang, J., Hao, Z., Zhang, Z., Yang, Y. P., & Xu, X. H. (2010). Kinetics of nitrate reductive denitrification by nanoscale zero-valent iron. Process Safety and Environmental Protechion., 88, 439–445.
Zhang, Z., Li, Z., Zhang, X. R., Liu, D. Q., Li, Z. R., & Li, H. Y. (2018). Systematically investigated the influences of permeable pavement materials on the water quality of runoff: batch and column experiments. Water, Air, and Soil Pollution, 229, 155.
Zuo, X. J., Fu, D. F., Li, H., & Singh, R. P. (2011). Distribution characteristics of pollutants and their mutual influence in highway runoff. Clean: Soil, Air, Water, 39, 956–963.
Funding
This study was supported by the Science and Technology Project of Jiangsu provincial Construction System (No. 2018ZD203), the National Natural Science Foundation of China (No. 51608272), and the Student’s Innovative Projects of Nanjing Forestry University (2018NFUSPITP769) for financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lin, ZZ., Chen, HM. & Yang, H. The Potential Pollution Risk of Groundwater by a Ceramic Permeable Brick Paving System. Water Air Soil Pollut 231, 356 (2020). https://doi.org/10.1007/s11270-020-04740-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-020-04740-6