Abstract
The risk of heavy metal contamination of infiltrated water and underground soil on a permeable brick paving system was investigated. The paving system was constructed as a frame structure base on top of a 1.0-m-thick clay layer with permeable ceramic brick at the surface. The concentrations of heavy metals (Zn, Cu, and Pb) in infiltrated water and soil at different underground depths under the paving system were measured. Speciation rates of Zn, Cu, and Pb at different clay depths were further determined to ascertain the probability of downward migration of the unstable forms. The results showed reduced risk of infiltrated water pollution by heavy metals due to underground soil acting as an effective trap. However, topsoil was more susceptible to heavy metal pollution, with the different pollution soil depths of Cu, Zn, and Pb mainly attributed to the different binding abilities between the heavy metals and soil. Soil Cu and Zn remained relatively stable, whereas there was a potentially high risk of Pb migration. The study found that topsoil could accumulate non-degradable heavy metals to unacceptable levels over a period of 30 years and that topsoil should therefore be replaced after 30 years to reduce the risk of soil pollution. This study fills a knowledge gap by both determining the risks of heavy metal pollution to underground soil and infiltrated water and exploring effective ways to reduce heavy metal pollution.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rainfall runoff in urban areas has been identified as a major non-point source of pollutants such as nutrients, organics, sediments, and heavy metals (Ma et al. 2018). Low impact development technologies (LIDs) currently being employed to address urban rainwater challenges have proven to be effective in controlling rainwater runoff (Bai et al. 2019).
LID facilities such as constructed wetlands (Li et al. 2017a), rain gardens (de Macedo et al. 2019), grass swales (Gavrić et al. 2019), and permeable pavement (Brattebo and Booth 2003) seek to reduce the volume of runoff by increasing the permeability of the existing soil. These infiltration practices promote groundwater recharge and reduce runoff peak flows and volumes, with a secondary benefit being a reduction in the transport of non-point source pollutants. However, an emerging concern is whether these systems are resulting in the infiltration of pollutants present in rainwater to groundwater and underground soil (Li et al. 2019). Pitt et al. (1999) in a review of the potential for rainwater infiltration practices to contaminate groundwater found that nitrate had a low to moderate groundwater contamination potential, whereas heavy metals had moderate to high groundwater contamination potentials. Dechesne et al. (2005) in a long-term study of the evolution of soil pollution in four infiltration basins found a high degree of pollution of the topsoil layer, with the main pollutants being heavy metals and hydrocarbons. A research reported that the concentrations of a range of metals detected from urban road runoff near residential area and parking lot were Cu 0.27–0.5 mg/L, Zn 0.38–1.27 mg/L, and Pb 0.23–0.25 mg/L, respectively (Herngren et al. 2006). The concentrations were relatively high compared with the natural water.
The heavy metals have properties that distinguish them from other potential pollutants. First, even though there are some natural background concentrations for some heavy metals, much higher concentrations of heavy metals in the environment result from human activities such as industry and mining (Liu et al. 2018). Second, heavy metals do not degrade easily in the soil, resulting in accumulation over time (Aryal et al. 2006). Third, accumulated metals in the topsoil will migrate downward under the influence of hydraulic load and may eventually pollute groundwater and underground soil (Mikkelsen et al. 1997; Barraud 1999). Narain (2014) showed that heavy metals were more likely to enter groundwater from infiltration systems. Jin et al. (2017) in a study of the effect of permeable pavements on groundwater quality identified a risk of groundwater pollution, especially by the heavy metals Cr and Pb. Winiarski et al. (2006) reported high soil concentrations of heavy metals at a depth of 1.5 m due to the infiltration of rainwater-runoff originating from an industrial area.
Among the LID technologies, permeable brick pavement system (PPS) has poor pollutant removal efficiency compared to bioretention or grass swales due to the lack of plant uptake (Hager et al. 2019). This infiltration-based technology comprises structural layers of relatively high porosity to allow infiltration of rainwater to the underlying soil and eventually to a drainage system. Among the LID technologies, PPS is therefore more likely to result in the pollution of groundwater and soil due to its poor pollutant removal efficiency. For pavements constructed of water-permeable brick, China’s technical regulations specify either a minimum distance between the top surface of the soil and the groundwater level of 1.0 m (CJJ/T 188–2012) or the construction of additional road drainage facilities, which obviously reduce the practicality of PPS. Groundwater levels are relatively high in the southern part of Jiangsu, China, resulting in a shorter distance between the bottom of the PPS and groundwater. The absence of extra road drainage facilities for PPS combined with extreme rainfall during the rainy season could aggravate groundwater and soil pollution. Pb, Zn, and Cu are the primary heavy metals of concern in urban rainwater runoff (Weiss et al. 2006), and the risk of these heavy metals infiltrating groundwater and soil cannot be ignored. Therefore, the study of the contamination of groundwater and soil by these three heavy metals is of great importance.
In the present study, a permeable brick paving system was constructed, with the surface layer comprising permeable ceramic brick and the base comprising a well-shaped frame of typical Chinese characteristics on top of a 1.0-m-thick clay layer. To investigate the potential risk of heavy metals infiltrating groundwater, the concentrations of Cu, Zn, and Pb in infiltrated water at different underground depths were measured. The risk to soil was investigated by measuring the concentrations and speciation of heavy metals at different clay depths. Measures to improve the pollutant retaining features of PPS were investigated such as adjusting the types of fillers used inside the frame structure, and potential pollution period was estimated based on the experimental results. The present study fills a knowledge gap by both determining the risks of heavy metal pollution to underground soil and infiltrated water within the use of PPS and exploring effective ways to reduce heavy metal pollution.
Materials and methods
Experimental setup
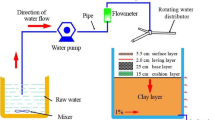
The PPS test device is shown in Fig. 1. The dimensions of the device were 0.8 m × 0.8 m × 1.5 m. A total of 16 permeable bricks measuring 20 cm × 20 cm × 5.0 cm were placed in close proximity. Leving layer was coarse sand with diameter 0.5–1.0 mm, base layer was a frame structure while cusion layer was gravel with diameter 15–20 mm. A total of six 10-mm diameter outlet pipes were set, from top to bottom, at heights of 0 cm, 20 cm, 40 cm, 60 cm, 80 cm, and 100 cm from the top of the clay layer. An overflow pipe with a diameter of 10 mm was set at the top of the device.
The prepared rainwater was pulled upward by a water pump and evenly distributed over the top surface area of the PPS by a rotating water distributor. The rainwater was sampled after passing through the ceramic permeable brick and the paving system. In addition, during the period of a stable outflow from the outlet pipe of the pavement, samples were taken every 10 min for a total of 2 h. Water samples were collected using sterilized 500-mL polyethylene sampling bottles and all samples were measured within 48 h.
Materials
Pervious brick
Ceramic permeable bricks measuring 20 cm × 20 cm × 5.5 cm were purchased from Youbang (Yi xing, China) Building Materials Co., Ltd. The main properties of the ceramic bricks, including splitting tensile strength, permeability coefficient, frost resistance, slip resistance, and porosity, met the requirements of the Chinese national standard for permeable paving bricks and permeable paving flags (GB/T 25993-2010). The main properties and chemical composition of the bricks are shown in Table 1 and Table 2, respectively.
Base layer
The base layer employed a well-shaped frame structure with a symmetrical shape measuring 40 cm × 40 cm × 25 cm with a spare ratio of approximately 40%, which contained the fillers with diameters of 3–5 cm. Volcanic rock, coal slag, iron filings, and gravel (Fig. 2) were selected as fillers to determine their effect on reducing the contamination of infiltrated water in the test device, and in the current study, the systems containing the four fillers are referred to as the VC, CS, IF, and G facilities, respectively.
Clay layer
Silty clay was collected from the campus of Nanjing Forestry University. All clays used retained their original properties during storage prior to use in the experiment. The clays were subject to compaction during addition to the test device to ensure a permeability coefficient consistent with that of soil of approximately 10−6 cm s−1. Furthermore, a blank test was carried out on the clay layer using clean water to eliminate the influence of soil background concentrations of heavy metals. The experiment indicated that up to 3.51 mg L−1 of total nitrogen (TN) was dissolved from the soil whereas other pollutants were not detected.
Simulated rainfall
As the experimental rainfall condition, the test replicated the 2-h rainfall duration and 5-year repetition period in Nanjing, as shown in Fig. 3.
According to the existing monitoring rainwater data aside an urban road, the concentrations of the heavy metals Cu, Zn, and Pb in the artificially prepared rainwater were 0.4 mg L−1, 0.6 mg L−1, and 0.5 mg L−1. Concentrations of the other pollutants in the rainwater were TSS 370 mg/L, pH 7.22, COD 250 mg L−1, TN 11.4 mg L−1, and TP 1.71 mg L−1, respectively.
Test methods
A continuous experiment was conducted at the frequency of weekly rainfall for a total duration of 1.5 years, during which time the quality of water infiltrating the different clay layers was measured. At the end of the experiment, soil samples were collected from the different clay layers for the measurement of the concentrations and distribution of heavy metals. The background concentrations of heavy metals in the original soil samples were also measured to determine the changes in the concentration of heavy metals over the experiment duration.
Concentrations of Zn, Cu, and Pb in infiltrated water were determined as according to the Chinese National Standard Methods by atomic absorption spectrophotometry .
Crude soil samples were freeze-dried, finely ground, homogenized, and sieved (100 mesh). The prepared sample (1 g) was digested with mixed acid (9 mL HCl, 3 mL HNO3) in a microwave digester. Then, the exchangeable (S1), carbonate-bound (S2), iron-manganese oxide-bound (S3), organic-bound (S4), and residual-bound fraction (S5) solution of heavy metals were acquired step by step using Tessier method, and the concentrations of Zn, Cu, and Pb were detected by atomic absorption spectrophotometry.
Scanning electron microscope (SEM) analyses were examined on a Quanta 200 scanning electron microscope at an accelerating voltage of 20 kV; energy-dispersive X-ray spectroscopy (EDS) analyses were conducted using TN-5502 X-ray energy dispersive spectrum.
Results and discussion
Characterization of the fillers
Scanning electron microscope (SEM) micrographs of the fillers are shown in Fig. 4 at 300 times magnification. Figure 4 shows a homogeneous distribution of grain and a well-developed porous structure. The sizes and distributions of pores varied among the different fillers. The volcanic rock contained smaller pores. Coal slag contained a well-developed pore structure with pores of varying size in a heterogeneous distribution, where small pores were attributed to the evaporation of organic matter due to the combustion of coal. The iron filings had a flat surface absent of pores, while silicate crystal could be seen on the surface of the gravel.
Table 3 shows the results of the energy-dispersive X-ray spectroscopy (EDS) analysis. The results show that the main elements of volcanic rock and coal slag are oxygen, aluminum, silicon, and calcium. The main elements of iron filing are oxygen and iron. Calcium, magnesium, and aluminum in the fillers existed predominantly in the form of oxides whereas iron existed as elemental iron.
Removal of heavy metals from infiltrated water at different depths
The concentrations and removal rates of heavy metals (Cu, Zn, and Pb) by the PPS filled by gravel at different clay depths are shown in Fig. 5.
As shown in Fig. 5, the heavy metal contents of effluents in the paving system filled by the gravel at a depth of 0 m complied with the V-type surface water standard except for Pb. The system showed a high heavy metal removal efficiency. Although heavy metals were present in rainwater in the dissolved phase, a large fraction of most metals were typically bound to suspended solids (Davis and McCuen 2005). Many studies had found the removal efficiency of total suspended solids (TSS) by PPS to be approximately 90.0% due to the physical interception by the permeable brick (Li et al. 2017b). Thus, the higher removal rates of heavy metals might result from the tendency of Zn, Cu, and Pb to absorb to particles in rainwater. In this experiment, the concentration ofTSS was 370 mg L−1, so it was inferred that a large fraction of heavy metals were combined with it, thus improving the removal efficiency of heavy metals (Yang et al. 2019). The effluent concentrations of Zn and Cu met the surface water standard of type II, indicating that the water is of sufficiently good quality to be released to the primary protection area of surface source water. However, the effluent concentration of Pb exceeded the very strict 0.1 mg L−1 surface water standard of type V, and therefore, there remains a risk of Pb pollution in receiving surface water.
Fortunately, we identified an approach to further improve the quality of effluent in the pavement system. The concentrations of heavy metals could be effectively reduced by changing the fillers inside the frame structure.
The effect of the fillers on the quality of effluent produced by the PPS was determined, with gravel, coal slag, volcanic rock, and iron filing fillers investigated. As can be seen from Fig. 6, the VC, CS, and IF systems all achieved better effluent quality at a depth of 0 m compared to the gravel system, thereby also improving the quality of the influent into the soil and enhancing infiltrated water quality at different depths.
The rank of the different facilities in removing Zn was VC > CS > IF > G. The effectiveness of VC could be ascribed to its rougher surface with a natural honeycomb texture and denser voids and gaps between particles, which was more conducive to the interception of suspended solids (SS) bounded to heavy metals.
The rank of the different facilities in removing Cu was IF > VC > CS > G. IF performed the best because zero-valent iron reduces Cu2+ to Cu, i.e., Fe0 + Cu2+ → Fe2+ + Cu0 (Cheng et al. 2007; Reddy et al. 2014; Statham et al. 2015). Furthermore, the formation of iron-based flocculants through the aforementioned reduction reaction is characterized by a strong adsorption capacity which was beneficial for the removal of SS and Cu (Reddy et al. 2014; Statham et al. 2015).
As can be seen from Fig. 6 c, the effluent produced by CS, VC, and IF at a depth of 0 m showed a good improvement and complied with the V-type surface water standard for Pb. The rank of the different facilities in removing Pb was VC > IF > CS > G. Similar to the case for Cu, the reduction potential of IF was the reason for its good performance: Fe0 + Pb2+ → Fe2+ + Pb0 (Cheng et al. 2007; Reddy et al. 2014; Statham et al. 2015). The effectiveness of fillers in removing Cu, Zn, and Pb was different. This result may be due to their different binding potentials to TSS in the runoff (Lin et al. 2019).
Zn concentrations of infiltrated water at depths > 0.4 m were within the 0.05 mg L−1 groundwater type I standard. Infiltrated water concentrations of Cu at depths > 0.4 m were within the 0.5 mg L−1 type II standard. Therefore, in terms of Zn and Cu, the infiltrated water was suitable for a variety of applications. Pb concentration at a depth of 0.2 m was within the 0.1 mg L−1 type IV standard, making this water suitable for application to agricultural and industry. In general, infiltration water at different depths in the clay complied with the type V standards for all the heavy metals investigated. This is because most of the heavy metals were trapped by the surface soil through adsorption, complexation, and precipitation once the heavy metals entered the soil. This demonstrates the reduction of the risk of contamination of infiltrated water by heavy metals. However, the high removal efficiency of heavy metals in soils makes it necessary to explore heavy metal pollution of the underlying soil in the system.
The variation in heavy metal soil content at different depths
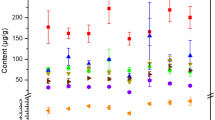
Figure 7 shows underground soil contents of Zn, Cu, and Pb at different depths. Zn, Cu, and Pb decreased with increasing depth, most likely due to the shallower clay layers trapping heavy metals from the infiltrated water before the water infiltrated to the deeper clay layers. The results show that the underground soil effectively removed heavy metals from the groundwater. Therefore, the accumulation of heavy metals in the soil was inevitable.
The Pb concentration of underground soil at a depth of 0.2 m was very close to the soil background value, demonstrating the effectiveness of Pb removal from the upper 0–20-cm soil layer. However, the concentrations of Cu and Zn were closer to their background values at greater depths of 80 cm and 100 cm, respectively, indicating that following an initial accumulation of the metals in the upper soil, they tended to migrate downward with water flow (Ghayoraneh and Qishlaqi 2017). These results show that removal efficiencies in underground soil differed for the three metals, with the rank of removal being Pb > Cu > Zn. This result was closely related to the binding potentials of the different heavy metals to soil. The results of Pitt et al. (1995) support the outcomes of the current study, where it was found the ranking of adsorption potential for the heavy metals to soil particles is Pb > Cu > Ni > Co > Zn > Cd.
The average background values of Cu, Zn, and Pb at different depths are shown in Table 4. The background values for Cu and Pb within the campus grounds were higher than those of Jiangsu province. Since Cu mainly originates from dry deposition in the atmosphere (Nicholson et al. 2003) and because of the higher degree of air pollution in the provincial capital of Nanjing, it is not surprising that the Cu background value within the campus was higher than the average value of Jiangsu province. The higher background value of Pb can be explained by the soil being collected from close to a highway. Zn mainly originates from rainfall (Liu et al. 2018), and the background concentration on campus was consistent with the average value for the province.
The Cu, Zn, and Pb soil contents all met the requirements of national standards [soil environmental quality-risk control standard for soil contamination of agricultural land (GB15618-2018)], indicating that the risk of soil contamination was within the scope of control for application within agriculture. However, soil accumulation of Cu, Zn, and Pb continued, demonstrating the potential risk of pollution due to the non-degradability of heavy metals. Previous research into prolonged deposition (20 years) of heavy metals demonstrated a high potential for leaching of heavy metals to the underlying soil (Aryal et al. 2006). The leaching of accumulated heavy metals to the underlying soil is likely a threshold event as the finite sorption capacities of the soil media is exceeded. Moreover, it is generally accepted that the risk and degree of soil contamination are determined by the chemical forms of metals rather than by their total concentrations. Since the stabilities of the different forms of heavy metals are different, it is necessary to analyze the distribution of different chemical species of heavy metals in soil.
Variation of different heavy metal species at different soil depths
Figure 8 shows the exchangeable (S1), carbonate-bound (S2), iron-manganese oxide-bound (S3), organic-bound (S4), and residual-bound (S5) fractions of heavy metals at different depths. Heavy metals in the soil are generally categorized into five forms, among which the exchangeable- and carbonate-bound forms are unstable, and therefore could be transported downward with water in the soil.
Cu existed mainly in the form of the residual-bound fraction (S5), with the proportions of the five forms (S1–S5) being 5.7–9.5%, 10.2–13.7%, 17.9–21.9%, 7.3–10.4%, and 44.9–53.6% in the depth direction, respectively, with the rank among the five chemical forms being S5 > S3 > S2 > S4 > S1. The high percentage of S5 in Cu is likely due the easy absorption of Cu to clay minerals, and Cu also adsorbs to organic matter as it forms strong associations with oxygen and sulfur atoms at the organic matter surface (Evans 1989). The proportion of exchangeable and carbonate-bound forms accounted for 16.7–23.2%, demonstrating the relative stability of Cu among the heavy metals, which reduces the possibility of downward migration of Cu in soil.
Zn existed mainly in the residual-bound fraction (S5), with the proportions of the five forms (S1–S5) being 4.9–9.1%, 2.8–5.5%, 15.9–22.9%, 5.9–10.9%, and 59.3–63.3% in the depth direction, respectively, and the rank among the forms being S5 > S3 > S4 > S1 > S2. Several studies have concluded that Zn sorption to Fe-Mn oxides is likely the most important mechanism controlling the behavior of this element in soil (Mahanta and Bhattacharyya 2011). However, the high proportion of S5 suggests that this residual-bound form originated from a natural source. The proportion of exchangeable and carbonate-bound forms accounted for 9.5–12.8%, demonstrating the relative stability of Zn among the heavy metals and the low risk of downward migration of Zn in soil.
Pb existed mainly in the exchangeable fraction (S1), with the proportions of the five forms (S1–S5) being 30.1–49.8%, 14.8–27.2%, 7.1–12.2%, 8.5–15.5%, and 17.6–21.5% in the depth direction, respectively, with the ranking among the forms being S1 > S5 > S2 > S4 > S3. It has often been argued that external sources of Pb result in Pb in soil existing initially in unstable chemical forms, with precipitates forming after continued pollutant accumulation (Lee 2006). The results of the present study coincidentally show the content of unstable forms of Pb being significantly higher than that of steady-state forms within the morphological distribution of Pb. This indicates that the soil has been polluted by the Pb originating from the groundwater. The proportion of exchangeable and carbonate-bound forms accounted for 54.0–64.5%, suggesting that Pb is relatively unstable among the heavy metals and therefore has a high probability of downward migration. Fortunately, since the potential for absorption of Pb to soil particles is relatively high, it is possible to control Pb pollution.
In summary, considering both the content of heavy metals and the distribution of their chemical species at different depths, the risk of Cu and Zn pollution is high but the risk of their downward migration is low, whereas the risk of Pb pollution is low but the risk of its downward migration is high.
Figure 9 shows the vertical distribution of steady and unsteady heavy metals in soil. The concentrations of the unsteady forms of Cu and Zn remained generally stable in the vertical direction at 5–10 mg kg−1, whereas Pb remained stable in a relatively large concentration range of 27.5–37.5 mg kg−1. The mobilities of heavy metals have been found to be influenced by the nature of soils, such as pH and organic matter content (Kabala and Singh 2001). However, since these factors vary with depth, it is difficult to fully understand the vertical distribution of unsteady forms of heavy metals. In addition, many studies have demonstrated that vertical migration of metals in soil cannot be fully understood given our limited knowledge of soil properties and limited data for mineralogical transformation processes (Chrastný et al. 2012). In the current study, it is speculated that the concentration range of 5–10 mg kg−1 might represent the soil saturation threshold for the unsteady forms of Cu and Zn since the metals had migrated to soil depths of 0.8–1.0 m, whereas the concentrations of the unsteady forms remained stable. The unsteady distribution of Pb may be related to the original content of Pb in the soil since Pb affected only the 0–20 cm layer of topsoil. The concentrations of the steady forms of the three metals all showed the same trend of decreasing with increasing depth; therefore, it is speculated that the distribution of the steady form may be related to the nature of the original soil since the three forms were relatively stable. Alternatively, it is possible that the unsteady forms of the metals in the upper layer were transformed to the steady forms, thereby explaining the decrease in the unsteady forms and the corresponding decrease in the total content of heavy metals. Regardless, further investigation of the changes in the morphologies of heavy metals with depth are required, which can be achieved by extending the experimental period used in the current study.
Determination of potential pollution period
The strictest soil standard for Cu in the soil environmental quality-risk control standard was used to evaluate the potential pollution period. After 1.5 years of continuous weekly rainfall experiments, the concentration of Cu in the soil increased from 33.75 to 46.83 mg kg−1, an increase of approximately 40%. At this rate (13 mg kg−1 every 1.5 years), only 6 years would be required to reach the national standard of 100 mg kg−1. However, given the frequency and intensity of rainfall, this estimate requires further correction.
With the average annual precipitation of Nanjing City calculated as 1110 mm and with the Chinese requirement of sponge cities absorbing 70% of precipitation in situ, approximately 770 mm precipitation filtrated into the soils, indicating about 10 similar rainfall events in a normal year. In the present study, the annual rainfall was approximately 5 times that of a normal year. Therefore, it would require approximately 30 years for the concentration of Cu to reach the national limit. This indicates that upper soil should be replaced every 30 years to reduce the risk of soil pollution due to accumulation of non-degradable heavy metals.
Conclusions
A permeable brick paving system was constructed using ceramic permeable brick as the surface layer and a well-shaped frame as the base layer with a 1.0-m clay layer placed at the bottom of the system. The potential risk of contamination of the infiltrated water and soil by Cu, Zn, and Pb was studied. Based on the results from the current study, the following conclusions could be drawn.
- (1)
The concentrations of Cu, Zn, and Pb in infiltration water at different clay depths generally were within the groundwater quality standards. The risk of heavy metal pollution in infiltrated water was lower due to the good retention of the underground soil. The quality of infiltration water in the system and of groundwater can be improved by changing the types of additives, thereby further reducing the risk of pollution.
- (2)
Zn, Cu, and Pb concentrations in the soil decreased with increasing soil depth. This was most likely due to the metals absorbing to the soil, indicating that the topsoil was more susceptible to heavy metal pollution. The varying pollution depths of Cu, Zn, and Pb in topsoil were mainly attributed to the different binding abilities of the metals to soil.
- (3)
Cu and Zn in soil existed mainly in the form of the residual-bound fraction, with the proportions of this form among the two metals being 44.9–53.6% and 59.3–63.3%, respectively, suggesting that these metals remain relatively stable in soil. The proportion of exchangeable and carbonate-bound forms of Pb accounted for 54.0–64.5%, suggesting that Pb was relatively unstable among the heavy metals and has a high possibility of downward migration.
- (4)
After 1.5 years of continuous weekly rainfall experiments, the concentrations of Cu, Zn, and Pb in infiltrated water and underground soil did not exceed their respective standards, indicating that the risk of pollution was low. However, due to the accumulation of heavy metals and their non-degradable nature, the topsoil should be replaced every 30 years to reduce the risk of soil pollution.
References
Aryal RK, Murakami M, Furumai H, Nakajima F, Jinadasa HKPK (2006) Prolonged deposition of heavy metals in infiltration facilities and its possible threat to groundwater contamination. Water Sci Technol 54:205–212
Bai Y, Li Y, Zhang R, Zhao N, Zeng X (2019) Comprehensive performance evaluation system based on environmental and economic benefits for optimal allocation of LID facilities. Water-Sui 11:341
Barraud AGJPS (1999) The impact of intentional storm water infiltration on soil and groundwater. Water Sci Technol 39:185–192
Brattebo BO, Booth DB (2003) Long-term stormwater quantity and quality performance of permeable pavement systems. Water Res 37:4369–4376
Cheng HF, Xu WP, Liu JL, Wang HJ, He YQ, Chen G (2007) Pretreatment of wastewater from triazine manufacturing by coagulation, electrolysis, and internal microelectrolysis. J Hazard Mater 146:385–392
Chrastný V, Vanek A, Teper L, Cabala J, Procházka J, Pechar L (2012) Geochemical position of Pb, Zn and Cd in soils near the Olkusz mine/smelter, South Poland: effects of landuse, type of contamination and distance from pollution source. Environ Monit Assess 184:2517–2536
Davis AP, McCuen RH (2005) stormwater management for smart growth
De Macedo MB, do Lago CAF, Mendiondo EM (2019) Stormwater volume reduction and water quality improvement by bioretention: potentials and challenges for water security in a subtropical catchment. Sci Total Environ 647:923–931
Dechesne M, Barraud S, Bardin J (2005) Experimental assessment of stormwater infiltration basin evolution. J Environ Eng 131:1090–1098
Evans LJ (1989) Chemistry of metal retention by soils. Environ Sci Technol 23:1046–1056
Gavrić S, Leonhardt G, Marsalek J, Viklander M (2019) Processes improving urban stormwater quality in grass swales and filter strips: a review of research findings. Sci Total Environ 669:431–447
Ghayoraneh M, Qishlaqi A (2017) Concentration, distribution and speciation of toxic metals in soils along a transect around a Zn/Pb smelter in the northwest of Iran. J Geochem Explor 180:1–14
Hager J, Hu GJ, Hewage K, Sadiq R (2019) Performance of low impact development best management practices: a critical review. Environ Rev 27:17–42
Herngren L, Goonetilleke A, Ayoko GA (2006) Analysis of heavy metals in roaddeposited sediments. Anal Chim Acta 76:149–158
Jin JR, Li T, Shi ZB (2017) Performance of applying scale permeable pavements for control of runoff pollution in an area with high groundwater level. Environ Sci 38:2379–2384
Kabala C, Singh BR (2001) Fractionation and mobility of copper, lead, and zinc in soil profiles in the vicinity of a copper smelter. J Environ Qual 30:485–492
Lee S (2006) Geochemistry and partitioning of trace metals in paddy soils affected by metal mine tailings in Korea. Geoderma 135:26–37
Li YC, Zhang DQ, Wang M (2017a) Performance evaluation of a full-scale constructed wetland for treating stormwater runoff. CLEAN-Soil Air Water 45:1600740
Li H, Li Z, Zhang X, Li Z, Liu D, Li T, Zhang Z (2017b) The effect of different surface materials on runoff quality in permeable pavement systems. Environ Sci Pollut R 24:21103–21110
Li J, Li F, Li H, Guo C, Dong W (2019) Analysis of rainfall infiltration and its influence on groundwater in rain gardens. Environ Sci Pollut R 26:22641–22655
Lin ZZ, Yang H, Chen HM (2019) Influence of fillers on the removal of rainwater runoff pollutants by a permeable brick system with a frame structure base. Water Sci Technol 80:2231–2140
Liu A, Ma Y, Gunawardena JMA, Egodawatta P, Ayoko GA, Goonetilleke A (2018) Heavy metals transport pathways: the importance of atmospheric pollution contributing to stormwater pollution. Ecotox Environ Safe 164:696–703
Ma Y, Hao S, Zhao H, Fang J, Zhao J, Li X (2018) Pollutant transport analysis and source apportionment of the entire non-point source pollution process in separate sewer systems. Chemosphere 211:557–565
Mahanta MJ, Bhattacharyya KG (2011) Total concentrations, fractionation and mobility of heavy metals in soils of urban area of Guwahati, India. Environ Monit Assess 173:221–240
Mikkelsen PS, Häfligerb M, Ochs M, Jacobsen P, Tjell JC, Boller M (1997) Pollution of soil and groundwater from infiltration of highly contaminated stormwater - a case study. Water Sci Technol 36:325–330
Narain V (2014) Shifting the focus from women to gender relations: assessing the impacts of water supply interventions in the Morni-Shiwalik Hills of Northwest India. Mt Res Dev 34:208–213
Nicholson FA, Smith SR, Alloway BJ, Carlton-Smith C, Chambers BJ (2003) An inventory of heavy metals inputs to agricultural soils in England and Wales. Sci Total Environ 311:205–219
Pitt R, Field R, Lalor M, Brown M (1995) Urban stormwater toxic pollutants: assessment, soruces, and treatability. Water Environ Res 67:260–275
Pitt R, Clark SC, Field R (1999) Groundwater contamination potential from stormwater infltration practices. Urban Water 1:217–236
Reddy KR, Xie T, Dastgheibi S (2014) Adsorption of mixtures of nutrients and heavy metals in simulated urban stormwater by different filter materials. J Environ Sci Heal A 49:524–539
Statham TM, Mumford KA, Rayner JL, Geoffrey WS (2015) Removal of copper and zinc from ground water by granular zero-valent iron: a dynamic freeze–thaw permeable reactive barrier laboratory experiment. Cold Reg Sci Technol 110:120–128
Weiss J, Hondzo M, Biesboer D, Semmens M (2006) Laboratory study of heavy metal phytoremediation by three wetland macrophytes. Int J Phytoremediat 8:245–259
Winiarski T, Bedell JP, Delolme C, Perrodin Y (2006) The impact of stormwater on a soil profile in an infiltration basin. Hydrogeol J 14:1244–1251
Yang H, Lin ZZ, Huang X, Jia QS, Li YL, Liu YY, Chen HM (2019) Rainwater runoff pollutant removal experiment using a ceramic permeable brick system with a frame structure base. Fresenius Environ Bull 28:9924–9934
Funding
This study was supported by the Science and Technology Project of Jiangsu provincial Construction System (No. 2018ZD203), the National Natural Science Foundation of China (No. 51608272), and the Science and Technology Project of Nanjing Municipal Construction System (No. Ks1914) provided financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lin, Z., Chen, H. & Yang, H. Risk of contamination of infiltrated water and underground soil by heavy metals within a ceramic permeable brick paving system. Environ Sci Pollut Res 27, 22795–22805 (2020). https://doi.org/10.1007/s11356-020-08745-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-08745-w