Abstract
The effects of solid content (10–80 g/L) on the bioleaching of heavy metals (HMs) from pig manure (PM) were investigated using indigenous sulfur-oxidizing bacteria. The results showed that an increase in solid content increased the PM buffering capacity, which slowed the rates of pH reduction, ORP increase, and sulfur oxidation and decreased the solubilization efficiency of HMs from PM. Approximately 75–99% of Cu, 76–99% of Zn, and 55–88% of Mn were leached from PM with solid contents of 10–80 g/L after 28 days of bioleaching. However, the content of HMs in bioleached manure did not meet the requirement for agricultural application when the solid content was ≥ 60 g/L after 28 days of bioleaching. The solubilization of HMs from PM was well-described by a kinetic equation. Regression analysis showed that Cu solubilization was primarily controlled by ORP, and pH seemed to be the sole factor responsible for the solubilization of Zn and Mn. Additionally, nutrient (N, P, K, and organic matter) loss significantly increased when PM solid content decreased from 40 to 20 g/L. Therefore, the recommended solid content for the bioleaching of HMs from PM is 40 g/L.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The rapid development of intensive pig farming has caused a sharp increase in the yield of pig manure (PM) in recent years (Li et al. 2010; Maccari et al. 2016; Quan et al. 2016; Zhou et al. 2012). PM has been widely applied as an important organic fertilizer source in agriculture, since it is rich in nitrogen, phosphorus, and organic matter, which can improve soil physical and chemical properties and plant nutrient status (Qureshi et al. 2008). However, to promote weight increase and prevent animal diseases, feed additives and animal medicines have been added to the animal feed (Mccarthy et al. 2013). As a result, a variety of environmental problems, such as heavy metal pollution of soil and ground water, have emerged (Buelna et al. 2008; Tigini et al. 2016; Xu et al. 2013).
Although compost and anaerobic digestion methods are commonly applied to treat PM, they have some limitations; heavy metals (HMs) of PM can only be fixed, but the total amount cannot be reduced (Lu et al. 2015; Quan et al. 2016; Wang et al. 2016). A bioleaching technique has been successfully applied in biohydrometallurgy to extract metals from sulfide minerals over the last decade (Brierley and Brierley 2001; Ehrlich 2001). Because of its characteristics of simplicity, cost effectiveness, and convenience for application, the bioleaching process has also been developed as a promising method to remove HMs from sludge, sediments, and soils (Yang et al. 2015; Yang et al. 2016; Zhang et al. 2008). Sreekrishnan and Tyagi (1996) reported that the bioleaching process is less costly for the removal of HMs compared to the conventional aerobic digestion and acid leaching process, especially for small-scale operations and those that produce high solid content. In addition, the adverse effects on the environment are considerably decreased. Therefore, it is possible that bioleaching with sulfur-oxidizing bacteria may be one of the most promising methods for the removal of HMs from PM.
In the bioleaching process, the solubilization of metals from PM is mainly achieved either directly by the metabolism of bioleaching bacteria or indirectly by the products of metabolism (Zhou et al. 2012). The high efficiency of metal solubilization is usually obtained by using acclimated indigenous microorganisms as inoculums in the bioleaching process (Zeng et al. 2016). The performance of the bioleaching process can be affected by various physical, chemical, and biological parameters (Chen et al. 2011; Chen and Pan 2010; Chen et al. 2015), among which the solid contents play a significant role in controlling the treatment cost and determining the reactor capacity (Sreekrishnan and Tyagi 1996). Generally, a low solid content increases the rate of HM solubilization, while a large reactor volume is needed. A high solid content elevates the treatment capacity, but it also results in a low rate of metal solubilization and a long lag phase. In addition, solid content is of great significance for nutrient supply and pH regulation which ultimately influences bacterial activity and metal solubilization (Liu et al. 2008; Zhang et al. 2008). Thus, a good understanding of this parameter is important for optimizing the bioleaching process.
The objectives of this study were (1) to develop a bioleaching process for solubilizing HMs from PM using indigenous sulfur-oxidizing bacteria; (2) to investigate the effects of solid contents on this bioleaching process; and (3) to evaluate the effects of bioleaching on the fertility (N, P, K, and organic matter) of PM.
2 Materials and Methods
2.1 Pig Manure Samples
The PM used throughout this study was obtained from a local pig farm in Tianjin, China. The fresh PM was collected in plastic drum and stored at 4 °C for further use. Before the bioleaching experiments, each batch of PM samples was characterized. The solid content was measured based on weight loss by oven-drying at 105 °C. The pH of the PM was measured using 1:10 (dry PM:distilled water) pig slurry. The dried PM samples were, first, digested according to Standard Methods (APAH 1995) and, then, measured for the total metal content, total nitrogen (TN), total phosphorus (TP), and total potassium (TK), in the digested residues. Organic matter was measured in terms of total organic carbon (TOC) by a TOC analyzer (multi N/C3100, Germany). The measured physicochemical characteristics of the PM samples are listed in Table 1.
2.2 Enrichment and Culturing of Indigenous Sulfur-Oxidizing Bacteria
Fresh PM obtained from the pig farm was used as seed inoculum to enrich and culture the indigenous sulfur-oxidizing bacteria. Approximately 100 mL of pig slurry with 20 g/L solid contents and 10 g/L elemental sulfur were added to a 250 mL Erlenmeyer flask and agitated on a gyratory shaker (ZQLY-180F, China) at 180 r/min and 28 °C. The pH of the pig slurry was monitored. When the pH dropped below 2.0, 10 mL of the acidified slurry was transferred to 100 mL fresh pig slurry with 10 g/L elemental sulfur, and the process was repeated under the same conditions. After four cultivations and inoculations, the indigenous sulfur-oxidizing bacteria in the pig slurry achieved the highest acidification rate. The inoculum for the bioleaching experiment was obtained.
2.3 Bioleaching Experiments
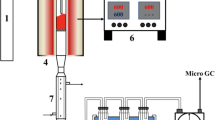
The buffering capacity of PM with different solid contents (10, 20, 40, 60, 80, and 100 g/L) was measured according to the method reported by Chen and Lin (2000). The bioleaching experiments were performed in a 500 mL Erlenmeyer flask with a 200 mL pig slurry sample, and the solid contents were 10, 20, 40, 60, and 80 g/L. With the addition of 10% (v/v) previously activated indigenous sulfur-oxidizing bacteria, the pig slurry was agitated at 180 r/min and 28 °C on a gyrator shaker (ZQLY-180F, China). Then, 10 g/L of the elemental sulfur was added to the flasks. During the bioleaching process, water loss via evaporation was compensated by adding distilled water based on weight loss. All experiments were performed in triplicate.
2.4 Chemical Analysis
During the bioleaching experiment, the pH and oxidation-reduction potential (ORP) values were measured using a pH/ORP meter (HANNA HI 8424, Italy). Binding fractions (exchangeable, carbonate-bound, Fe/Mn oxides, organic/sulfates, and residual) of HMs (Cu, Zn, Mn) in PM were extracted by the sequential extraction procedure from Tessier et al. (1979). Ultimate analysis of PM was carried out with a LEEMAN EA300 elemental analyzer. In this study, the SMT extraction protocol was used to analyze phosphorus fractions in the PM before and after bioleaching (Medeiros et al. 2005). The slurry sample taken from the flasks at two-day intervals was centrifuged at a speed of 12,000 r/min for 15 min and, then, filtered through a 0.45-μm filter membrane. The filtrate was analyzed for sulfate concentration according to the Standard Methods (APAH 1995). Heavy metal analysis was carried out using a flame/graphite atomic absorption spectrophotometer (TAS-990, China).
3 Results and Discussion
3.1 Quantification of Buffering Capacity of Pig Manure
As shown in Fig. 1, the quantity of acid required to achieve the same pH value increased with increasing PM solid content. This result implied that the change in pH was dependent on the buffering capacity of PM. It was also found that a large amount of acid was required to alter the pH of the pig slurry at a lower pH and a small amount influenced the pH at a higher pH. Therefore, a proper pH value should be chosen to calculate the buffering capacity of PM. The buffering capacity index (BCI) was first reported by Sreekrishnan et al. (1993), which was defined as the quantity of sulfate required to change the pH by one unit at a pH of 4.0.
During the bioleaching process, however, sulfate production consists of oxidation of both sulfides and elemental sulfur. Sulfate cannot account for all of the acid production. Therefore, Chen and Lin (2000) suggested using hydrogen ion concentrations to replace sulfate. The buffering capacity was then defined as the quantity of hydrogen ions required to change the pH by one unit at a pH of 4.0.
The buffering capacities of the pig slurry with different solid contents are shown in Fig. 2. The buffering capacity varied linearly with the PM solid content. As the solid content increased from 10 to 100 g/L, the corresponding buffering capacity increased from 1.08 to 9.4 mmol H+.
3.2 Effects of Solid Contents on pH, ORP, and Sulfate During Bioleaching
The solid content was directly related to the buffering capacity (Fig. 1). Higher solid content led to a greater difficulty of overcoming the pig slurry buffer and, hence, to a higher final slurry pH. In this study, acid was a byproduct of the metabolic activity of sulfur-oxidizing bacteria, such that changes in pig slurry pH over time can be used to represent the degree of bioleaching. Variations in pH observed during the bioleaching process with different PM solid contents are presented in Fig. 3a. After 28 days of bioleaching, the pig slurry pH decreased to 1.66–2.46. The final steady pH values were as follows: 1.66, 1.75, 2.07, 2.23, and 2.46 in solid contents of 10, 20, 40, 60, and 80 g/L, respectively. The results herein also showed that the slurry pH at higher solid contents (60–80 g/L) did not decrease at the initial stage and even slightly increased, which was perhaps attributed to the absence of bioactive sulfur-oxidizing bacteria and the release of buffering components (Chen and Lin 2004b; Tsai et al. 2003). Moreover, it took only 8 days for the pig slurry pH to drop below 2.0 with a solid content of 10 g/L, whereas 20 days was required to achieve a pH below 2.5 with a solid content of 80 g/L, confirming that solid content affected the acidification rate of PM during bioleaching.
In addition to the acidification of slurry, the oxidation of sulfur to sulfate also led to an increase in the pig slurry ORP during the bioleaching process. The change in ORP was indicative of the activity of sulfur-oxidizing bacteria (Liu et al. 2008). The variations in ORP during bioleaching at different solid contents are presented in Fig. 3b. The variations of ORP were strongly related to the changes in pH. The increase in ORP followed the same trend as the decrease in pH. After 28 days of bioleaching, the ORP increased to 416, 394, 350, 324, and 238 mV for solid contents of 10, 20, 40, 60, and 80 g/L, respectively. A higher solid content in pig slurry induced a lower increase rate in ORP. This result was consistent with the results reported by Chen and Lin (2004b) using the heavy metal-contaminated sediment.
The sulfate production of pig slurry with different solid contents during the bioleaching process is shown in Fig. 3c. The sulfate generation in the slurry with solid contents of 60 and 80 g/L was 4–6 days behind those with lower solid contents. This can be explained by the fact that higher solid contents led to poor efficiency of oxygen transportation which finally resulted in the poor growth of bacteria (Chen and Lin 2000; Zhou et al. 2009). However, after 28 days of PM bioleaching, the final production of sulfate in the system increased with increasing solid content. Sulfur oxidation in solid contents of 10, 20, 40, 60, and 80 g/L was 16.8, 22.6, 30.1, 38.4, and 46.6%, respectively (Table 2). According to the study of Sreekrishnan et al. (1993), a higher solid content implied a higher concentration of nutrients. This signified that higher solid contents were able to supply sufficient micronutrients and trace metals that were essential for bacterial growth, hence resulting in better bacterial growth and sulfate production. It was also observed that the slurry with lower solid contents reached the maximum sulfate production rate earlier than those with higher solid contents. This may be due to the sharp drop in the pH of slurry with lower solid contents, which seriously inhibited bacterial growth (Sreekrishnan et al. 1993).
The results in Table 2 also show that about 53.3–83.2% of the sulfur added in the system was not oxidized during PM bioleaching. It is necessary to remove or recycle the remaining sulfur from the bioleached manure before its final disposal. In fact, some recoverable forms of sulfur from the bioleaching of sewage sludge have been reported by Chen and Lin (2004a). In addition, Zhou et al. (2008) reported that the co-addition of Fe2+ and sulfur as substrates can not only achieve a sulfur oxidation rate of 98% but also reduce the sulfur addition from 5–10 to 2 g/L during sewage sludge bioleaching. These methods can also be applied to PM bioleaching in future studies to eliminate the risk of remaining sulfur.
3.3 Solubilization of Heavy Metals During Bioleaching
The effects of solid content on the solubilization of Cu, Zn, and Mn from PM during bioleaching are shown in Fig. 4. The solubilization patterns of Cu, Zn, and Mn were similar. HMs had a long lag phase before solubilization from pig slurry with higher solid contents, and it required more time to reach maximum solubilization. The solubilization of Cu, Zn, and Mn began on the 2nd day for a solid content of 10 g/L and reached 96.2% for Cu on day 12 and 95.9% and 87.0% for Zn and Mn, respectively, on day 6, and, then, continued to increase slightly. The leaching time for Zn and Mn to achieve maximum solubilization increased from 6 days at a solid content of 10 g/L to 20 days at a solid content of 80 g/L. For Cu, the time was increased from 12 days to more than 28 days. Table 3 summarizes the final leaching efficiencies of HMs and their content in bioleached PM. The solubilization of Cu, Zn, and Mn varied greatly for different solid contents. For example, the leaching efficiency of Cu achieved higher than 90% solubilization with solid contents lower than 20 g/L, at 99 and 91% for solid contents of 10 and 20 g/L, respectively. The solubilization decreased to less than 80% with solid contents higher than 60 g/L, at 78 and 75% for solid contents of 60 and 80 g/L, respectively. The solubilization of Zn and Mn showed a similar variation trend to Cu solubilization. Thus, the solubilization of HMs was significantly decreased with increasing solid content. This could result from the lower ORP and higher pH obtained in the system with higher solid content (Fig. 3).
In addition, after 28 days of bioleaching, the contents of Cu, Zn, and Mn remaining in PM with different solid contents were 2–79, 28–512, and 77–293 mg/kg (Table 3). For Cu and Zn, the remaining content in bioleached manure with a solid content of 40 g/L was 52 and 253 mg/kg, which reached the standard for agriculture application (Verdonck 1998). However, with solid contents increasing to higher than 60 g/L, the contents of Cu and Zn remaining in PM were higher than 70 and 396 mg/kg, which was very close to the thresholds (Cu, 100 mg/kg, and Zn, 400 mg/kg) for agricultural application according to the German compost standard (Verdonck 1998), posing a risk to agricultural land. Although low solid contents (10 and 20 g/L) promoted metal solubilization, a large reactor volume was required, which increased the operation cost. Therefore, for practical consideration, a solid content of 40 g/L is the most appropriate for the bioleaching of HMs from PM.
The binding forms of HMs in PM affected not only the efficiency of metal solubilization but also the bioavailability of HMs after bioleaching (Zhou et al. 2012). HMs in exchangeable, carbonate-bound, and Fe/Mn oxide fractions are considered to be more mobile, dangerous, and bioavailable. The organic matter/sulfide-bound and residual fractions are stable and non-bioavailable (Liu et al. 2008). However, many studies have shown that HMs in organic and sulfate forms can also be partially solubilized (Chen et al. 2005). Figure 5 shows the proportion of heavy metal fractions in PM. It was observed that 67% of Zn and 75% of Mn existed in mobile forms. Most Cu (73%) existed in stable forms, of which 67% existed as organic/sulfide forms, and this form of Cu was efficiently oxidized by sulfur-oxidizing bacteria during the bioleaching process.
3.4 Kinetic Study
For the bioleaching process, a first-order kinetic model (Bayat and Sari 2010; Chen and Pan 2010) was employed; the equations of which are as follows:
and
where k is the rate constant for metal solubilization (d−1), Ms and M represent the initial mass (mg) of metal in PM and mass (mg) in the aqueous phase, respectively, and t is the bioleaching time. The fitting of the results from the bioleaching process for different solid contents is shown in Table 4. It was found that the metal solubilization can be approximated to the first-order kinetics. The relationship between the rate of metal solubilization and solid contents is shown in Fig. 6. The rate of metal solubilization decreased linearly with increasing solid contents. The coefficient of total solid (TS) reflects the influence degree of solid content on the rate of metal solubilization during bioleaching. The coefficient obtained for Zn and Cu showed that the influence of solid content on the rate of Zn solubilization was significant, but the effect on Cu was relatively small. This may be because the rate of metal solubilization was highly related to the initial metal contents in the PM (Chen and Lin 2004a).
3.5 Relationship Between Heavy Metal Solubilization and pH and ORP During Bioleaching
The relationship between HM solubilization and pig slurry pH and ORP as a function of solid content is shown in Fig. 7 and Fig. 8. The leaching pH for Zn and Mn was less than 5.5 and 6, respectively (Fig. 7). However, the leaching pattern for Cu was slightly different, and the pH value was less than 3. The fitting results shown in Fig. 8 indicated that the Cu solubilization percentage was highly correlated with ORP. The ORP value for Cu extraction was more than 120 mV. Moreover, the results in Table 5 also show that the solubilization of Cu from PM was mainly related to ORP. The relative impact force of ORP and pH was 98.1 and 1.9%, respectively. Thus, lowering the PM pH alone barely contributes to Cu solubilization during bioleaching. Increasing sulfur oxidation to sustain high ORP in the bioleaching system was necessary to achieve a higher leaching efficiency of Cu. However, in contrast to Cu, the relative impact force of ORP for Zn and Mn was 0 and 0.3, respectively, and pH seemed to be the sole factor responsible for Zn and Mn solubilization. The results shown in Fig. 4 prove that Zn and Mn were more easily solubilized from PM than Cu under the same acidic conditions. This might be attributed to the large amount of Zn and Mn in unstable forms (Fig. 5). However, although Cu (73%) mainly existed in stable forms, over 99% of Cu was leached out when the solid content was 10 g/L, which might have resulted from the PM digestion. Some data in the literature also confirmed that a highly oxidizing environment and low pH result in the digestion of organic matter and further contribute to the solubilization of HMs (Wong et al. 2002).
Given our insight into this result, we also found that regardless of the solid content, the metal solubilization percentage was constant for a given pH and ORP of the pig slurry. In other words, the changes in pH and ORP lead to variations in the metal solubilization percentage. However, the quantity of acid and sulfur oxidation required to decrease the pH and increase the ORP to the same level was larger for the pig slurry with higher solid contents. This provided an explanation for the phenomenon of lower solubilization of HMs with higher solid contents shown in Fig. 4 and Fig. 6. This result also revealed the real mechanism for the solid contents influencing the metal solubilization, namely, the solid contents did not affect the metal solubilization directly but influenced the nature of pH reduction and ORP increase in the system by indirectly regulating the acid production required to overcome the buffering.
3.6 Variations of Nutrients
The analysis results of PM samples at different solid contents are shown in Table 6. Variations in the C, H, and N contents and H/C and N/C ratio demonstrated that the organic matter composition of PM was changed by the bioleaching process. The N/C ratio can be used to illustrate the polymerization degree of organic matter in PM. Organic matter containing more nitrogenous functional groups would be less polymerized (Chen et al. 2015; Gascó et al. 2005). Compared with the raw PM, the N/C and H/C ratios both decreased after bioleaching. In addition, the N/C ratio increased from 0.62 to 0.85 as the solid content increased from 10 to 80 g/L. These results indicated that the polymerization degree of bioleached PM decreased with increasing solid content, i.e., the dewaterability of bioleached PM decreased with increasing solid content after 28 days of bioleaching. Similar results were also observed in variations of the H/C ratio. According to Chen et al. (2015), the variations in the N/C and H/C ratios can result from the solubilization of light organic compounds during the bioleaching process.
The concentrations of TN, TP, TK, and organic matter (TOC) at different solid contents before and after bioleaching are shown in Fig. 9. The trend of nutrient leaching with respect to solid content was similar to that of HM solubilization with respect to solid content. The higher the solid contents, the higher the solubilization of HMs and dissolution of nutrients (N, P, K, and organic matter (TOC)). This may be because the increase in solid contents increased the buffering capacity of PM, which finally lowered the nutrient loss resulting from acidification and digestion. The contents of TN, TP, TK, and organic matter (TOC) remaining in PM at solid content of 40 g/L were 26.3 g/kg, 14.2 g/kg, 6.27 g/kg, and 445 mg/kg, respectively. When solid content was increased from 40 to 60 g/L, the nutrient content only slightly increased to 26.7 g/kg (TN), 15.5 g/kg (TP), 8.3 g/kg (TK), and 476 mg/kg (TOC). However, lowering the solid content to 20 g/L resulted in a sharp decrease in the nutrient content in the bioleached manure. The percentage of TN, TP, TK, and organic matter (TOC) released from PM increased from 15.7, 53, 58, and 24.4% at solid content of 40 g/L to 18.2, 62.1, 65.5, and 43.3% at solid content of 20 g/L, respectively. This result further supported that 40 g/L was the most appropriate solid content for PM bioleaching.
The analytical results of the phosphorus fraction in PM before and after bioleaching are shown in Table 7. The primary form of phosphorus in PM was inorganic, accounting for 91% of TP. In addition, OP was only about 9% of TP, indicating that OP was not directly related to the organic content in PM. The results also showed that the loss of TP, OP, IP, and AP decreased with increasing solid contents, while the loss of NAIP showed an opposite trend. Phosphorus has been proven to be essential for the growth of Acidithiobacillus thiooxidans (Zheng and Zhou 2011). NAIP is regarded as the most labile and bioavailable phosphorus fraction (Huang et al. 2015; Medeiros et al. 2005). The trend of NAIP loss with respect to solid content during bioleaching was the same as that of sulfur oxidation with respect to solid content as shown in Table 2. Therefore, the loss of AP may have been mainly caused by the acidification of PM, but the loss of NAIP was primarily attributed to biological degradation.
4 Conclusions
A bioleaching process with indigenous sulfur-oxidizing bacteria was developed to remove HMs from PM in this study. The performance of the bioleaching process was influenced by solid content. The higher the solid content, the higher the buffering capacity, which further resulted in a lower rate of pH reduction, ORP increase, and sulfate production in the bioleaching process. Under the experimental conditions, the rate and degree of PM bioleaching were significantly affected by solid contents, and a solid content higher than 60 g/L led to an unsatisfied solubilization of HMs, which did not meet the requirements for agricultural application. When lowering the solid content to 40 g/L, the solubilization of Cu, Zn, and Mn achieved 82, 88, and 72%, respectively, and the content of heavy metals that remained in PM at this solid content was relatively low and safe for agriculture application. The rates of metal solubilization were well-described by a solid content-related kinetic equation. The results of regression analysis showed that Cu solubilization was primarily controlled by ORP, whereas the pH seemed to be the sole factor responsible for the solubilization of Zn and Mn. In addition, PM nutrient solubilization was also greatly affected by the solid content, and the solubilization rates of TN, TP, TK, and organic matter were 15.7, 53, 58, and 24.4%, respectively, at a solid content of 40 g/L.
References
APAH. (1995). Standard methods for the examination of water and wastewater (19th ed.). Washington, DC: American Public Health Association.
Bayat, B., & Sari, B. (2010). Comparative evaluation of microbial and chemical leaching processes for heavy metal removal from dewatered metal plating sludge. Journal of Hazardous Materials, 174, 763–769.
Brierley, J. A., & Brierley, C. L. (2001). Present and future commercial applications of biohydrometallurgy. Hydrometallurgy, 9, 81–89.
Buelna, G., Dubé, R., & Turgeon, N. (2008). Pig manure treatment by organic bed biofiltration. Desalination, 231, 297–304.
Chen, P., Yan, L., Leng, F., Nan, W., Yue, X., Zheng, Y., Feng, N., & Li, H. (2011). Bioleaching of realgar by Acidithiobacillus ferrooxidans using ferrous iron and elemental sulfur as the sole and mixed energy sources. Bioresource Technology, 102(3), 3260–3267.
Chen, S. Y., & Lin, J. G. (2000). Influence of solid content on bioleaching of heavy metals from contaminated sediment by Thiobacillus spp. Journal of Chemical Technology and Biotechnology, 75, 649–656.
Chen, S. Y., & Lin, J. G. (2004a). Bioleaching of heavy metals from contaminated sediment by indigenous sulfur-oxidizing bacteria in an air-lift bioreactor: effects of sulfur concentration. Water Research, 38, 3205–3214.
Chen, S. Y., & Lin, J. G. (2004b). Bioleaching of heavy metals from livestock sludge by indigenous sulfur-oxidizing bacteria: effects of sludge solids concentration. Chemosphere, 54, 283–289.
Chen, S. Y., & Pan, S. H. (2010). Simultaneous metal leaching and sludge digestion by thermophilic microorganisms: effect of solids content. Journal of Hazardous Materials, 179, 340–347.
Chen, Y. X., Hua, Y. M., Zhang, S. H., & Tian, G. M. (2005). Transformation of heavy metal forms during sewage sludge bioleaching. Journal of Hazardous Materials, 123, 196–202.
Chen, Z., Hu, M., Cui, B., Liu, S., Guo, D., & Xiao, B. (2015). The effect of bioleaching on sewage sludge pyrolysis. Waste Management, 48, 383.
Ehrlich, H. L. (2001). Past, present and future of biohydrometallurgy. Hydrometallurgy, 59, 127–134.
Gascó, G., Blanco, C. G., Guerrero, F., & Lázaro, A. M. M. (2005). The influence of organic matter on sewage sludge pyrolysis. Journal of Analytical and Applied Pyrolysis, 74, 413–420.
Huang, W., Cai, W., Huang, H., Lei, Z., Zhang, Z., Tay, J. H., & Lee, D. J. (2015). Identification of inorganic and organic species of phosphorus and its bio-availability in nitrifying aerobic granular sludge. Water Research, 68, 423–431.
Li, L., Xu, Z., Wu, J., & Tian, G. (2010). Bioaccumulation of heavy metals in the earthworm Eisenia fetida in relation to bioavailable metal concentrations in pig manure. Bioresource Technology, 101, 3430–3436.
Liu, Y. G., Zhou, M., Zeng, G. M., Wang, X., Li, X., Fan, T., & Xu, W. H. (2008). Bioleaching of heavy metals from mine tailings by indigenous sulfur-oxidizing bacteria: effects of substrate concentration. Bioresource Technology, 99, 4124–4129.
Lu, X. M., Lu, P. Z., Chen, J. J., Zhang, H., & Fu, J. (2015). Effect of passivator on Cu form transformation in pig manure aerobic composting and application in soil. Environmental Science and Pollution Research, 22, 14727–14737.
Maccari, A. P., Baretta, D., Paiano, D., Leston, S., Freitas, A., Ramos, F., Sousa, J. P., & Klauberg-Filho, O. (2016). Ecotoxicological effects of pig manure on Folsomia candida in subtropical Brazilian soils. Journal of Hazardous Materials, 314, 113–120.
Mccarthy, G., Lawlor, P. G., Gutierrez, M., & Gardiner, G. E. (2013). Assessing the biosafety risks of pig manure for use as a feedstock for composting. Science of the Total Environment, 463-464, 712–719.
Medeiros, J. J. G., Cid, B. P., & Gómez, E. F. (2005). Analytical phosphorus fractionation in sewage sludge and sediment samples. Analytical and Bioanalytical Chemistry, 381, 873–878.
Quan, W., Zhen, W., Awasthi, M. K., Jiang, Y., Li, R., Ren, X., Zhao, J., Feng, S., Wang, M., & Zhang, Z. (2016). Evaluation of medical stone amendment for the reduction of nitrogen loss and bioavailability of heavy metals during pig manure composting. Bioresource Technology, 220, 297.
Qureshi, A., Lo, K. V., Liao, P. H., & Mavinic, D. S. (2008). Real-time treatment of dairy manure: implications of oxidation reduction potential regimes to nutrient management strategies. Bioresource Technology, 99, 1169–1176.
Sreekrishnan, T. R., & Tyagi, R. D. (1996). A comparative study of the cost of leaching out heavy metals from sewage sludges. Process Biochemistry, 31, 31–41.
Sreekrishnan, T. R., Tyagi, R. D., Blais, J. F., & Campbell, P. G. C. (1993). Kinetics of heavy metal bioleaching from sewage sludge—I. Effects of process parameters. Water Research, 27, 1641–1651.
Tessier, A., Campbell, P. G. C., & Bisson, M. (1979). Sequential extraction procedure for the speciation of particulate trace metals. Analytical Chemistry, 51, 844–851.
Tigini, V., Franchino, M., Bona, F., & Varese, G. C. (2016). Is digestate safe? A study on its ecotoxicity and environmental risk on a pig manure. Science of the Total Environment, 551-552, 127–132.
Tsai, L. J., Yu, K. C., Chen, S. F., Kung, P. Y., Chang, C. Y., & Lin, C. H. (2003). Partitioning variation of heavy metals in contaminated river sediment via bioleaching: effect of sulfur added to total solids ratio. Water Research, 37, 4623–4630.
Verdonck, O. (1998). Compost specifications. Acta Hortic, (469), 169–177.
Wang, L., Chen, G., Owens, G., & Zhang, J. (2016). Enhanced antibiotic removal by the addition of bamboo charcoal during pig manure composting. RSC Advances, 6, 27575–27583.
Wong, J. W. C., Xiang, L., & Chan, L. C. (2002). pH requirement for the bioleaching of heavy metals from anaerobically digested wastewater sludge. Water Air & Soil Pollut, 138, 25–35(11).
Xu, Y., Yu, W., Ma, Q., & Zhou, H. (2013). Accumulation of copper and zinc in soil and plant within ten-year application of different pig manure rates. Plant, Soil and Environment, 59, 492–499.
Yang, H. Y., Liu, W., Chen, G. B., Liu, Y. Y., Tong, L. L., Jin, Z. N., & Liu, Z. L. (2015). Function of microorganism and reaction pathway for carrollite dissolution during bioleaching. Transactions of the Nonferrous Metals Society of China, 25, 2718–2724.
Yang, Z., Zhang, Z., Chai, L., Wang, Y., Liu, Y., & Xiao, R. (2016). Bioleaching remediation of heavy metal-contaminated soils using Burkholderia sp. Z-90. Journal of Hazardous Materials, 301, 145–152.
Zeng, J., Gou, M., Tang, Y. Q., Li, G. Y., Sun, Z. Y., & Kida, K. (2016). Effective bioleaching of chromium in tannery sludge with an enriched sulfur-oxidizing bacterial community. Bioresource Technology, 218, 859–866.
Zhang, P., Yi, Z., Zhang, G., Zou, S., Zeng, G., & Zhen, W. (2008). Sewage sludge bioleaching by indigenous sulfur-oxidizing bacteria: effects of ratio of substrate dosage to solid content. Bioresource Technology, 100, 1394–1398.
Zheng, G., & Zhou, L. (2011). Supplementation of inorganic phosphate enhancing the removal efficiency of tannery sludge-borne Cr through bioleaching. Water Research, 45, 5295–5301.
Zhou, H. B., Zeng, W. M., Yang, Z. F., Xie, Y. J., & Qiu, G. Z. (2009). Bioleaching of chalcopyrite concentrate by a moderately thermophilic culture in a stirred tank reactor. Bioresource Technology, 100, 515–520.
Zhou, J., Zhou, L., Liu, F., Zheng, C., & Deng, W. (2012). Transformation of heavy metals and the formation of secondary iron minerals during pig manure bioleaching by the co-inoculation acidophilic Thiobacillus. Environmental Technology, 33, 2553–2560.
Zhou, S. G., Zhou, L. X., & Fang, D. (2008). Enhancing metal removal by coaddition of Fe2+ and S0 as substrates of Acidithiobacillus ferrooxidans for sewage sludge bioleaching. Practice Periodical of Hazardous, Toxic, Waste Management, 12, 159–164.
Acknowledgements
This work was supported by the Tianjin Rural Work Committee of China (grant number 201601190) and the Agro-Environment Protection Institute (AEPI) of the Ministry of Agriculture (MOA) of China (grant number 2016YFD0801002-01). In addition, Xiaocheng Wei wants to thank, in particular, the inimitable care and support from Qian Wang over the past ten years. Will you marry me?
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wei, X., Huang, W., Liu, D. et al. Bioleaching of Heavy Metals from Pig Manure Employing Indigenous Sulfur-Oxidizing Bacteria: Effects of Solid Content. Water Air Soil Pollut 230, 39 (2019). https://doi.org/10.1007/s11270-019-4087-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-019-4087-z