Abstract
Fe3O4 decorated multi-walled carbon nanotube (Fe3O4/MWCNT) nanocomposites were synthesized by co-precipitation process and used as heterogeneous Fenton catalyst for degradation of phenol and p-nitrophenol (p-NP). The Fe3O4 nanoparticles with size less than 20 nm were well-dispersedly coated on the surface of MWCNTs at relatively low loading. Some aggregations appear at high Fe3O4 content in composite. The Fe3O4/MWCNT with about 25 wt.% of Fe3O4 is the most cost-effective catalyst compared with others, whose phenol conversion and COD removal rates could, respectively, reach to 99.20 and 58.09%. And a high H2O2 utilization efficiency was achieved (about 132.41%) for this catalyst. For the p-NP degradation, the optimal reaction condition was that: 2.0 mg/L of catalyst dosage, 3 mmol/L of initial H2O2 concentration, 3 of pH value, and 40 °C of reaction temperature. At this condition, the removal rates of p-NP and COD in 120 min achieved 97.16 and 67.71%, respectively. And the Fe3O4/MWCNT nanocomposite also exhibits an acceptable stability and reusability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Phenolic compounds, especially phenol and p-nitrophenol (p-NP), usually appear in manufactures of pharmaceutical, coal gasification, and dyes and produce large amounts of industrial wastewater (Li et al. 2014; Lu et al. 2015; Wan et al. 2016). Every year, a large quantity of phenolic compounds was leaked into the environment. It is well known that phenolic compounds have high toxicity, potential carcinogenicity, and non-biodegradation (Nie et al. 2015; Ahmed et al. 2011; Boruah et al. 2016). The American Environmental Protection Agency has stipulated that the total phenol effluent is less than 0.01 mg/L. Thus, efficient technologies for treatment of phenolic-contained wastewater are urgently needed. Nowadays, many technologies have been applied, such as adsorption (Li et al. 2016; Liu et al. 2016; Farrokhi et al. 2014), homogeneous/heterogeneous Fenton oxidation, photocatalytic oxidation, microwave degradation, catalytic ozonation, and electrochemical degradation (Pugazhenthiran et al. 2011; Xu and Wang 2012; Munoz et al. 2015; Garciasegura et al. 2012; Wang et al. 2012; Gu et al. 2011). Fenton and Fenton-like oxidation technologies have been successfully used for degradation of phenolic effluents, because of the strong oxidation capacity of hydroxyl radical (.OH). As we know, homogeneous Fenton reaction has some disadvantages, such as narrow working pH range, large quantity of iron sludge, and low H2O2 utilization efficiency (Pugazhenthiran et al. 2011). For these reasons, heterogeneous Fenton-like oxidation has become a promising technology for wastewater treatment. And an efficient heterogeneous, catalyst is the key. Up till now, various Fenton-like catalysts have been exploited and could be categorized into natural minerals and artificial novel composites. Goethite, magnetite, and pyrite volcanic rocks all belong to the first kind. On the other hand, many catalysts have been synthesized as heterogeneous Fenton catalysts. And Fe3O4-based catalyst could be looked as the most important one (Xu et al. 2015). Fe3O4 nanoparticles (NPs) easily aggregate in aqueous solution. So supporting Fe3O4 NPs is a fantastic way to resolve this problem. Recently, many materials were used to support Fe3O4 NPs, such as chitosan hollow fibers (Dorraji et al. 2015), graphene (Ahmed et al. 2011; Zubir et al. 2014), multi-walled carbon nanotubes (MWCNTs) (Xu et al. 2015; Deng et al. 2012), coke (Li et al. 2014; Lu et al. 2016), and active carbon (Zhang et al. 2014).
Carbon nanotube (CNT) as a kind of carbonaceous material has been applied into various fields, such as adsorbent, lithium ion battery, capacitor, catalyst, photo-electrode, and sensor, due to its unique morphology and structure (Mallakpour and Khadem 2016). And CNT was also used for inorganic hybrid carbon-based catalytic material for pollutant treatment. The Fe3O4/MWCNT nanocomposite as a Fenton-like catalyst to degrade 17-α-methyltestosterone has a high removal efficiency and could be used in wide working pH range (3.5 ~ 8.0) (Hu et al. 2011). The Fe3O4/MWCNT nanocomposite could also have a high removal rate of bisphenol A (Cleveland et al. 2014).
In this study, we prepared the Fe3O4/MWCNT nanocomposites by co-precipitation process and used them as heterogeneous Fenton catalyst for degradation of phenol and p-NP. And we investigated the effect of Fe3O4 loading, reaction conditions (catalyst dosage, H2O2 concentration, initial pH, and working temperature) on the degradation of phenol and p-NP and corresponding COD removal efficiency. And stability and reusability of Fe3O4/MWCNT were also investigated. Furthermore, the effect of H2O2 decomposition and Fe leaching on Fenton oxidation was also discussed.
2 Experimental
2.1 Chemicals and Materials
Phenol, p-NP, ferric chloride, ferrous chloride, and hydrogen peroxide (H2O2) were all bought from Tianjin FUCHENG Chemical Reagents Factory (China). All chemicals were of analytical grade. MWCNTs were purchased from Shenzhen SUSN Sinotech New Materials Co.Ltd.
2.2 Synthesis of Fe3O4/MWCNT Nanocomposites
MWCNTs were firstly treated by acid oxidation. MWCNTs (1.0 g) were added into a round bottom flask containing 300 mL of mixture acid of concentrated sulfuric acid, concentrated nitric acid and deionized water (3: 1: 1, v/v/v), and sonicated for 30 min; then, the mixture was transferred to a water bath at 100 °C and stirred for 4 h. After that, the mixture was filtered and washed with deionized water and ethanol several times, and dried at 80 °C under vacuum for 12 h.
The Fe3O4/MWCNT nanocomposites were prepared by the in-situ co-precipitation process. Mixture (0.5 g of modified MWCNTs and 300 mL of deionized water) in a round bottom flask was sonicated for 30 min, and further transferred to a water bath (90 °C). Next, ferric chloride (0.5 mg) and ferrous chloride (0.25 mg) were added into the flask (deaerated with N2) under stirring in inert atmosphere for 10 min. Then, ammonium hydroxide was slowly dropped until the pH value reached to 8.0. After that, the reactant was further agitated at 90 °C for 4 h. Finally, the product was separated with an external magnet and washed with deionized water and ethanol for several times followed by drying overnight at 80 °C under vacuum. The nanocomposites with different Fe3O4 weight loadings were synthesized as above method, named Fe3O4/MWCNT-1 (14.29 wt.%), Fe3O4/MWCNT-2 (25 wt.%), and Fe3O4/MWCNT-3 (40 wt.%), respectively. And the Fe3O4 nanoparticles were synthesized as above process just without MWCNTs. The Fe3O4 content in nanocomposite was confirmed according to the Chinese national standard GB/T 1429–2009 (Carbon materials-Determination of the ash content).
2.3 Characterization and Analytical Methods
The crystal structure and microstructure of the samples were characterized by X-ray powder diffractometer (XRD, Rigaku D/max-2400, Japan) with a Cu Kα radiation source and transmission electron microscope (TEM, Tecnai-G2), respectively. The surface chemical state of the sample was identified by Fourier transform infrared spectrometer (FT-IR, Nicolet 6700, America).
The phenol and p-NP concentrations were measured by UV-Vis spectroscopy (YU-1810, Beijing Purikinje General Co. China) at wavelength of 510 and 400 nm, respectively (Yu et al. 2016; Wang et al. 2014). The chemical oxygen demand (COD) was analyzed by dichromate method (Wan et al. 2016). The concentrations of total dissolved Fe and Fe2+ were tested by 1, 10-phenatroline spectrophotometric method (Wan et al. 2016). And H2O2 concentration in aqueous solution was quantified by UV-Vis spectroscopy with titanium oxalate at wavelength of 395 nm (Kan and Huling 2009).
2.4 Heterogeneous Fenton-Like Reaction
The degradation of phenol or p-NP was conducted in a conical flask in dark which contains 100 mL of phenol or p-NP solution and a certain mass of catalyst at different temperatures and pH values. The initial pH of the reaction solution was adjusted by H2SO4 or NaOH solution. Then, H2O2 was added to start the degradation process. And 5 mL of sample was taken out each time in different time for further analysis. Moreover, the reusability of Fe3O4/MWCNT was evaluated by collecting with an external magnet and simply washing with deionized water and ethanol for several times. And after dried under a vacuum, the catalyst was used for next cyclic reaction under similar conditions.
3 Results and Discussion
3.1 Characterization of Fe3O4/MWCNT Composites
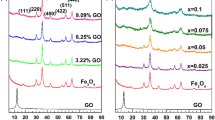
Figure 1 shows the X-ray diffraction patterns of the samples. It is obvious that the diffraction peaks at 2θ of about 26.2o is assigned to MWCNTs (Wan et al. 2016). The diffraction peaks of the Fe3O4 material are at 2θ of 18.3o, 30.2o, 35.6o, 43.1o, 53.6o, 57.0o, and 62.6o, respectively. In the patterns of the Fe3O4/MWCNT composites, the diffraction peaks of Fe3O4 and MWCNTs appear that indicates the loading of Fe3O4 on MWCNTs successfully (Cleveland et al. 2014). With increase of the Fe3O4 content in the composites, the peak intensity rate of Fe3O4 to MWCNTs increases gradually. The actual contents of Fe3O4 in the composites were measured by the ash determination method for carbon materials. For Fe3O4/MWCNT-1, Fe3O4/MWCNT-2, and Fe3O4/MWCNT-3, the content are, respectively, 14.96, 25.16, and 41.11 wt.%. Those measured values are very close to the theoretical values (14.29, 25, and 40 wt.%). The little difference may originate from the MWCNT losing during nanocomposite preparation process. In the later, the theoretical contents were used.
The FT-IR spectrum of the MWCNTs was shown in Fig. 2. The peaks at 3430, 1709, 1637, and 1568 cm−1, respectively, correspond to the stretch vibrations of O-H, C = O, ─COOH and R-C = O, while that at 1381 and 1173 cm−1 could be ascribed to the bending vibration of O-H and stretching vibration of C-O. It indicates that the acid-treated MWCNTs have many oxygen-containing functional groups.
The TEM images of the pure Fe3O4 NPs, MWCNTs, and Fe3O4/MWCNT were shown in Fig. 3. Compared with the Fe3O4 NPs supported on MWCNTs, the pure Fe3O4 NPs have many big aggregates and relatively wide size distribution. It is clear that the Fe3O4 NPs were successfully loaded on the surface of MWCNTs. And more Fe3O4 NPs were supported on the MWCNTs with increasing the Fe3O4 content in the composite. The loaded Fe3O4 NPs have a relatively uniform size that is less than 20 nm. At low loading, the Fe3O4 NPs are well dispersed on the surface of the MWCNTs. When the loading reaches to 40 wt.%, some aggregations appear that may affect the nanocomposite’s catalytic ability.
3.2 Effect of Catalysts on Degradation of Phenol
The effect of different catalysts on the degradation of phenol was investigated firstly under the same condition (100 mg/L of phenol, 1 g/L of catalyst dosage, 10 mmol/L of H2O2, 3.0 of pH, and 25 °C of reaction temperature). Figure 4a shows that the phenol removal rate is as low as 1.05% for only H2O2 without catalyst. The result indicates H2O2 cannot oxide phenol individually. The phenol removal rate for just Fe3O4/MWCNT without H2O2 is also no more than 4%, which could be contributed to the phenol absorption of Fe3O4/MWCNT. When the Fe3O4 and Fe3O4/MWCNT were used as heterogeneous catalyst, the phenol removal rates could reach to 77.77 and 89.25% in 30 min, respectively. With increase of the reaction time to 4 h, the corresponding removal rates arrive at 85.01 and 99.20%, respectively. The reason is that the Fe3O4/MWCNT or Fe3O4 could effectively catalyze H2O2 to decompose phenol. That using the Fe3O4/MWCNT nanocomposite could achieve a higher phenol removal rate may be contributed to the best dispersion of Fe3O4 on the surface of MWCNTs (Wan et al. 2016; Xu and Wang 2012; Xu and Wang 2015). Furthermore, the H2O2 decomposition rate was evaluated as shown in Fig. 4b. H2O2 was nearly not consumed without catalyst. When the Fe3O4 and Fe3O4/MWCNT were added, the H2O2 decomposition rates are as high as about 69.70 and 70.76%, respectively.
3.3 Effect of Fe3O4 Content on Phenol Degradation
The effect of Fe3O4 loading amount in the nanocomposite on phenol degradation was further evaluated (100 mg/L of phenol, 1 g/L of catalyst dosage, 10 mmol/L of H2O2, 3.0 of pH, and 25 °C of temperature) as displayed in Fig. 5a. The phenol removal rate increases obviously with the increase of the Fe3O4 content in Fe3O4/MWCNT and reaches the maximum (99.20%) when at about 25 wt.% of the Fe3O4 content. It is possible that more Fe3O4 NPs can provide more active sites for the .OH generation. On the contrary, the phenol removal rate slightly decreases to 98.07% for Fe3O4/MWCNT with 40 wt.% of Fe3O4 content. And the phenol removal rate for the Fe3O4 NPs is only about 85.01%. It is possible the agglomeration of Fe3O4 NPs. The H2O2 utilization rate was tested as shown in Fig. 5b. It shows that the H2O2 decomposition rate increases with the increase of Fe3O4 content. More Fe3O4 NPs in nanocomposite can provide more active sites for H2O2 decomposition to produce .OH. By comparison, the Fe3O4/MWCNT with 25 wt.% of Fe3O4 content is cost-effective and used for the following research. The loaded Fe3O4 NPs in different nanocomposites have the same crystal structure and similar size. So, the Fe3O4 NPs could affect the nanocomposite’s catalytic ability in two main ways (loading content and dispersion state). The real amount of the active catalytic sites in the nanocomposite is the key factor affecting the catalytic ability. At high loading, the aggregations could increase and even become big as shown in Fig. 3. It is possible that the amount of the really active catalytic sites in the nanocomposite did not have obvious increase.
3.4 Iron Leaching and H2O2 Decomposition
The iron leaching, H2O2 decomposition, phenol conversion rate, and COD removal rate were investigated as shown in Fig. 6 (100 mg/L of phenol, 1 g/L of catalyst dosage, 10 mmol/L of H2O2, 3.0 of pH, and 25 °C of temperature). In the first 120 min, phenol was removed completely. And the corresponding total dissolved iron and Fe2+ are 2.5 and 2.27 mg/L, respectively. The dissolved Fe2+ has a downtrend in 180 to 240 min. The dissolved iron and Fe2+ are also as low as 5.25 and 2.39 mg/L in 240 min, respectively. It is possible that the ferrous ions were oxidized to ferric ions by remaining H2O2 and .OH (Wan et al. 2016; Cleveland et al. 2014). The Fe3O4 NP as catalyst for 2, 4-dichloropheonl degradation has higher leached total Fe ion (about 9.8 mg/L in 180 min) (Xu and Wang 2012), which may have a serious impact on its stability and reusability. The H2O2 consumption speed was quick in the first 120 min and then decreased. It indicates the great impact of ferrous ions on H2O2 decomposition. The phenol conversion and COD removal rates increase rapidly in 30 min and then slowly reach to 99.20 and 58.09% in 240 min, respectively.
In addition, the H2O2 utilization efficiency E was used to evaluate the nanocomposite’s catalytic ability from another perspective and calculated according to the following equation (Gao et al. 2015):
where △COD is the removed COD (mg/L); 0.47 is the conversion factor; △[H2O2] is the H2O2 decomposed amount (mg/L). Here, the H2O2 consumption and removed COD are about 240.6 and 149.7 mg/L, respectively. Thus, the calculated H2O2 utilization efficiency is about 132.41% that is a very high value (Wan et al. 2016; Xu and Wang 2012). The E value exceeds 100%. It is possible the two main reasons: (1) the Fe3O4/MWCNT nanocomposite could absorb the organic pollutants; (2) the iron-containing matter formed in the reaction could also absorb the organic pollutants. Both reasons lead the increase of the removed COD. So the calculated E value reaches about 132.41%.
3.5 p-NP Heterogeneous Fenton Oxidation
The Fenton-like catalyst Fe3O4/MWCNT nanocomposite was further used for p-NP catalytic degradation. Figure 7 shows the effect of catalyst dosage on the p-NP removal. In our researched range, the p-NP removal rates all exceed 96% at any dosage. But at 2.0 mg/L of the catalyst dosage, the p-NP degradation velocity is quickest and the removal rate reaches to 96.0% in 60 min.
Figure 8 shows the effect of H2O2 concentration on the removal of p-NP and corresponding COD. With the increase of the H2O2 concentration, the removal rates of p-NP and COD increase and approach the maximal values at 3 mmol/L of the concentration. The above said values are 97.52 and 60.60%, respectively. And the H2O2 decomposition rate constant also reaches the maximal value (0.8673 h−1). At low H2O2 concentration, enough.OH could not be generated to degrade p-NP and intermediate products. On the other hand, excess H2O2 will lead to low H2O2 utilization efficiency (Fig. 9).
The reaction temperature has great effect on the p-NP removal and COD degradation. The p-NP removal rate increases with increasing the reaction temperature in range of 20 to 40 °C. The p-NP removal rate constants at 20, 25, 30, and 40 °C are 0.01556, 0.02848, 0.05797, and 0.0833 min−1, respectively. It is possible that the reaction of ferric ion with H2O2 (Eq. 3) needs higher activation energy than that of ferrous ion with .OH (Eq. 2) (Cleveland et al. 2014). Higher temperature can provide high energy and prompt the generation of Fe2+ ion.
3.6 Stability and Reusability of Fe3O4/MWCNT
The stability and reusability of the Fe3O4/MWCNT nanocomposite were measured at the optimal reaction condition (100 mg/L of phenol, 2.0 g/L of catalyst dosage, 3 mmol/L of H2O2, 3.0 of pH, and 40 °C of temperature). After each cycle, the catalyst was easily separated with an external magnet as shown in Fig. 10a. From Fig. 10b, it can be seen that the p-NP degradation efficiency of the reused catalyst had a little decrease. The p-NP removal rates in 1 to 4 time cycles of the catalyst are 97.16, 93.92, 93.19, and 88.84%, respectively. And the corresponding COD removal rates are 67.71, 56.09, 45.68, and 39.29%, respectively. It is possible that the total iron on surface of MWCNTs was reduced with the increase of the reuse cycles. And the accumulation of pollutants and intermediates on the surface of particles may also have a negative effect.
Furthermore, the Fe3O4/MWCNT nanocomposite (25 wt.%) after 4-time use were characterized by XRD (Fig. 11). It is obvious that the diffraction pattern of the Fe3O4 NPs in the nanocomposite has no obvious change. This results show that the Fe3O4 NPs have no crystal structure change after use. But, the peak intensity ratio of MWCNTs and Fe3O4 NPs decreased after use. That suggests the amount decrease of MWCNTs in the nanocomposite. It is possible that the Fe3O4 NPs were consumed or fell off from the MWCNTs. So the MWCNTs were lost in the magnetic recovery process. Tightly fixing Fe3O4 NPs on MWCNTs is in favor of lift span of the nanocomposite as Fenton catalyst.
4 Conclusion
In this paper, the Fe3O4/MWCNT nanocomposites were prepared by a co-precipitation method, and further used as Fenton-like catalyst for the degradation of phenol and p-NP. The Fe3O4/MWCNT nanocomposite has excellent catalytic performance for the Fenton-like oxidation of phenol and p-NP. The Fe3O4/MWCNT nanocomposite with 25 wt.% of Fe3O4 loading has highest catalytic efficiency among those nanocomposites. At an appropriate reaction condition, the phenol conversion and COD removal rates are 99.20 and 58.09%, respectively. At the optimal reaction condition (catalyst dosage, 2.0 mg/L; initial H2O2 concentration, 3 mmol/L; pH value, 3; reaction temperature, 40 °C) for the p-NP oxidation using this Fe3O4/MWCNT nanocomposite, the removal rates of p-NP and COD, respectively, achieve 97.16 and 67.71% in 120 min.
References
Ahmed, S., Rasul, M. G., Martens, W. N., Brown, R., & Hashib, M. A. (2011). Advances in heterogeneous photocatalytic degradation of phenols and dyes in wastewater: a review. Water, Air, & Soil Pollution, 215(1), 3–29.
Boruah, P. K., Sharma, B., Karbhal, I., Shelke, M. V., & Das, M. R. (2016). Ammonia-modified graphene sheets decorated with magnetic Fe3O4 nanoparticles for the photocatalytic and photo-Fenton degradation of phenolic compounds under sunlight irradiation. Journal of Hazardous Materials, 325, 90–100.
Cleveland, V., Bingham, J. P., & Kan, E. (2014). Heterogeneous Fenton degradation of bisphenol a by carbon nanotube-supported Fe3O4. Separation and Purification Technology, 133, 388–395.
Deng, J., Wen, X., & Wang, Q. (2012). Solvothermal in situ synthesis of Fe3O4-multi-walled carbon nanotubes with enhanced heterogeneous Fenton-like activity. Materials Research Bulletin, 47(11), 3369–3376.
Dorraji, M. S. S., Mirmohseni, A., Carraro, M., Gross, S., Simone, S., Tasselli, F., et al. (2015). Fenton-like catalytic activity of wet-spun chitosan hollow fibers loaded with Fe3O4 nanoparticles: batch and continuous flow investigations. Journal of Molecular Catalysis A: Chemical, 398, 353–357.
Farrokhi, M., Hosseini, S. C., Yang, J. K., & Shirzad-Siboni, M. (2014). Application of ZnO–Fe3O4, nanocomposite on the removal of azo dye from aqueous solutions: kinetics and equilibrium studies. Water, Air, & Soil Pollution, 225(9), 1–12.
Gao, Y., Wang, Y., & Zhang, H. (2015). Removal of rhodamine B with Fe-supported bentonite as heterogeneous photo-Fenton catalyst under visible irradiation. Applied Catalysis B: Environmental, 178, 29–36.
Garciasegura, S., Garrido, J. A., Rodríguez, R. M., Cabot, P. L., Centellas, F., Arias, C., et al. (2012). Mineralization of flumequine in acidic medium by electro-Fenton and photoelectro-Fenton processes. Water Research, 46(46), 2067–2076.
Gu, L., Song, F. Y., & Zhu, N. W. (2011). An innovative electrochemical degradation of 1-diazo-2-naphthol-4-sulfonic acid in the presence of Bi2Fe4O9. Applied Catalysis B: Environmental, 110(2), 186–194.
Hu, X., Liu, B., Deng, Y., Chen, H., Luo, S., Sun, C., et al. (2011). Adsorption and heterogeneous Fenton degradation of 17α-methyltestosterone on nano Fe3O4/MWCNT in aqueous solution. Applied Catalysis B: Environmental, 107(3), 274–283.
Kan, E., & Huling, S. G. (2009). Effects of temperature and acidic pre-treatment on Fenton-driven oxidation of MTBE-spent granular activated carbon. Environmental Science & Technology, 43(5), 1493–1499.
Li, Y., Zhu, G., Ng, W. J., & Tan, S. K. (2014). A review on removing pharmaceutical contaminants from wastewater by constructed wetlands: design, performance and mechanism. Science of the Total Environment, 468-469(1), 908–932.
Li, X., Liu, Y., Di, D., Wang, G., & Yi, L. (2016). A formaldehyde carbonyl groups-modified self-crosslinked polystyrene resin: synthesis, adsorption and separation properties. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 500, 1–9.
Liu, F., Wu, Z., Wang, D., Yu, J., Jiang, X., & Chen, X. (2016). Magnetic porous silica–graphene oxide hybrid composite as a potential adsorbent for aqueous removal of p-nitrophenol. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 490, 207–214.
Lu, M., Yao, Y., Gao, L., Mo, D., Lin, F., & Lu, S. (2015). Continuous treatment of phenol over an Fe2O3 /γ-Al2O3 catalyst in a fixed-bed reactor. Water, Air, & Soil Pollution, 226(4), 1–13.
Lu, L., Li, W., WANG, G., Zhang, Z., Wan, D., Lü, L., et al. (2016). Synthesis and characterization of biomimetic Fe3O4/coke magnetic nanoparticles composite material. Journal of Wuhan University of Technology-Materials Science Edition, 31(2), 254–259.
Mallakpour, S., & Khadem, E. (2016). Carbon nanotube-metal oxide nanocomposites: fabrication, properties and applications. Chemical Engineering Journal, 302, 344–367.
Munoz, M., Pedro, Z. M. D., Casas, J. A., & Rodriguez, J. J. (2015). Preparation of magnetite-based catalysts and their application in heterogeneous Fenton oxidation—a review. Applied Catalysis B: Environmental, 176-177, 249–265.
Nie, M., Yan, C., Li, M., Wang, X., Bi, W., & Dong, W. (2015). Degradation of chloramphenicol by persulfate activated by Fe2+ and zerovalent iron. Chemical Engineering Journal, 279, 507–515.
Pugazhenthiran, N., Sathishkumar, P., Murugesan, S., & Anandan, S. (2011). Effective degradation of acid orange 10 by catalytic ozonation in the presence of Au-Bi2O3 nanoparticles. Chemical Engineering Journal, 168(3), 1227–1233.
Wan, D., Li, W., Wang, G., Lu, L., & Wei, X. (2016). Degradation of p-nitrophenol using magnetic Fe0/Fe3O4/coke composite as a heterogeneous Fenton-like catalyst. Science of the Total Environment, 574, 1326–1334.
Wang, K., Wang, C., Xu, Q., & Yang, L. (2012). Study on the degradation of acidic red B by microwave/catalyst/H2O2. Industrial Water Treatment, 32(5), 35–38.
Wang, N., Zheng, T., Jiang, J., Lung, W. S., Miao, X., & Wang, P. (2014). Pilot-scale treatment of p -nitrophenol wastewater by microwave-enhanced Fenton oxidation process: effects of system parameters and kinetics study. Chemical Engineering Journal, 239(3), 351–359.
Xu, L., & Wang, J. (2012). Fenton-like degradation of 2, 4-dichlorophenol using Fe3O4 magnetic nanoparticles. Applied Catalysis B: Environmental, s 123-124(30), 117–126.
Xu, L., & Wang, J. (2015). Degradation of 2, 4, 6-trichlorophenol using magnetic nanoscaled Fe3O4/CeO2 composite as a heterogeneous Fenton-like catalyst. Separation and Purification Technology, 149, 255–264.
Xu, H. Y., Shi, T. N., Zhao, H., Jin, L. G., Wang, F. C., Wang, C. Y., et al. (2015). Heterogeneous Fenton-like discoloration of methyl orange using Fe3O4/MWCNT as catalyst: process optimization by response surface methodology. Frontiers of Materials Science, 10(1), 45–55.
Yu, L., Chen, J., Liang, Z., Xu, W., Chen, L., & Ye, D. (2016). Degradation of phenol using Fe3O4 -GO nanocomposite as a heterogeneous photo-Fenton catalyst. Separation and Purification Technology, 171, 80–87.
Zhang, J., Wang, G., Zhang, L., Fu, X., & Liu, Y. (2014). Catalytic oxidative desulfurization of benzothiophene with hydrogen peroxide catalyzed by Fenton-like catalysts. Reaction Kinetics, Mechanisms and Catalysis, 113(2), 347–360.
Zubir, N. A., Yacou, C., Motuzas, J., Zhang, X., & Costa, J. C. D. D. (2014). Structural and functional investigation of graphene oxide-Fe3O4 nanocomposites for the heterogeneous Fenton-like reaction. Scientific Reports, 4(4594), 1–8.
Acknowledgements
The authors are grateful for the financial support provided by the Fundamental Research Funds for the Central Universities of China (JD1406), the National Natural Science Foundation of China (Grant No. 21174011 and U1462102).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Tian, X., Liu, Y., Chi, W. et al. Catalytic Degradation of Phenol and p-Nitrophenol Using Fe3O4/MWCNT Nanocomposites as Heterogeneous Fenton-Like Catalyst. Water Air Soil Pollut 228, 297 (2017). https://doi.org/10.1007/s11270-017-3485-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-017-3485-3