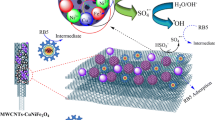
Abstract
A magnetic composite of CoFe2O4 and carbon nanotube (CNT) was prepared using the solvothermal approach and then employed for the activation of peroxydisulfate (PDS) to degrade reactive black 5 (RB5) and other organic pollutants. Characterization results of the composite catalyst revealed the successful loading of spherical CoFe2O4 particles on CNTs, possessing abundant porosity as well as magnetic separation capability. Under the degradation conditions of 0.2 g/L CoFe2O4-CNT dosage and 4 mM PDS dosage, the removal efficiencies of 10 mg/L RB5 and other pollutants were in the range of 94.5 to ~ 100%. The effects of pH, co-existing ions/humic acid, and water matrices as well as the reusability of the catalyst were also investigated in detail. Furthermore, the degradation mechanism and pathway were proposed based on quenching experiments, LC–MS analysis, and density functional theory (DFT) calculations, and the toxicity of the degradation products was evaluated in the quantitative structure–activity relationship approach.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The presence of various organic pollutants in the water environmental poses significant threats to human health and the survival of various organisms, causing severe harm to the ecological environment. Currently, several technologies have been adopted for treating organic pollutants in water, including adsorption, membrane separation, and the advanced oxidation process (Collivignarelli et al. 2019; Shi et al. 2022a). Among these, the advanced oxidation process has gained widespread use in wastewater treatment due to its high removal efficiency towards various organic pollutants. Recently, persulfate-based advanced oxidation is receiving increasing attention. In this approach, the SO4·− radical is generated through the activation of peroxymonosulfate (PMS) or peroxydisulfate (PDS) (Kohantorabi et al. 2021). The redox potential of SO4·− in the range of 2.5–3.1 V (Zhang et al. 2021) is close to that of the ·OH radical produced in other advanced oxidation processes such as Fenton, photocatalytic and ozone oxidation. Notably, SO4·− is more stable than ·OH and can function effectively across a wide pH range (Giannakis et al. 2021). Consequently, persulfate-based advanced oxidation has been extensively investigated to degrade a variety of organic pollutants in water including dyes, antibiotics, endocrine disruptors, and others (Sarkar et al. 2022).
In comparison to PMS, PDS in the form of potassium or sodium salts is considered to be more stable and cost-effective (Li et al. 2021). However, PDS exhibits slow self-decomposition rates to produce active radicals. Consequently, activation methods are typically employed to enhance its reactivity towards the oxidation of organic pollutants. Thermal (Ren et al. 2021), ultraviolet (Tian et al. 2022), ultrasound (Monteagudo et al. 2018), and electrochemical (Araújo et al. 2022) activation approaches have been studied, where the introduction of external energy in different forms boosted the activation of PDS and the generation of active species. Another approach relies on the employment of catalysts, including homogenous and heterogeneous ones. Although homogenous catalysts, usually transition metal ions, such as Fe2+, Co2+, Ag+, etc., are easy to handle and highly efficient, the remaining metal ions in treated water may pose additional risks. Considering this, heterogeneous catalysts are more welcome from an environmental viewpoint.
Carbon materials as a large group of heterogeneous catalysts have been regarded as promising candidates for persulfate activation. Different types of carbon materials have been investigated, and carbon nanotubes (CNTs) stand out due to their large surface areas and strong stabilities (Apul and Karanfil 2015). Although CNTs showed outstanding performance in the activation of persulfate, the separation of them after the degradation of organic pollutants remains a challenging issue. In our previous work, magnetic Fe3O4 particles were combined with CNTs by a facile one-pot solvothermal method to form a magnetic Fe3O4-CNT composite, which was then employed for PDS activation towards the degradation of several organic pollutants (Shi et al. 2022b). However, Fe3O4 only benefited magnetic separation and was found to show limited catalytic effect in that reaction system (Shi et al. 2022b), which was probably attributed to the relatively low reactivity of iron species. As an alternative, Co3O4 also possesses magnetic property and has been proved to be an efficient catalyst for persulfate activation (Peng et al. 2022; Qin et al. 2022). However, compared to iron species, cobalt species are more toxic. When using single Co3O4 as the catalyst, the leached high concentration of cobalt ions may form secondary pollution. In our another previous work (Wang et al. 2022b), Bi2O3 was introduced in combination with Co3O4 to reduce the leaching of cobalt. Nevertheless, Bi2O3 shows no magnetic property and the Co3O4-Bi2O3 catalyst is not suitable for magnetic separation. In comparison with Co3O4-Bi2O3, the spinel catalyst of CoFe2O4 may be more applicable, which is also magnetic and has a better resistance to cobalt leaching thanks to its strong Co–Fe interactions (Wang et al. 2020).
Based on these previous works, we further prepared a CoFe2O4-CNT composite using the same one-pot solvothermal method by replacing the single iron salt precursor with mixed precursors of both iron and cobalt salts. The CoFe2O4-CNT/PDS system was then investigated for PDS activation towards the degradation of reactive black 5 (RB5) and other organic pollutants. The effects of various operational and environmental factors have been studied, as well as the reaction mechanism, the degradation pathway, and toxicity evaluation of degradation products.
Experimental
Material and characterization
Multi-walled CNTs with outer diameters of 4–6 nm, lengths of 10–20 µm, and purity over 98% were purchased from Chengdu Organic Chemical Co., Ltd. CoFe2O4-CNT was fabricated in a one-pot solvothermal method similar to the one reported in our previous work when preparing Fe3O4-CNT (Shi et al. 2022b), except that a mixture of FeCl3‧6H2O and Co(NO3)2‧6H2O (molar ratio of Fe/Co = 2:1) was added instead of single FeCl3‧6H2O. A series of methods have been conducted to characterize the composite CoFe2O4-CNT catalyst, including scanning electron microscope (SEM), X-ray diffraction (XRD), nitrogen sorption, X-ray photoelectron spectroscopy (XPS), vibrating sample magnetometer (VSM), and zeta potential analysis. Inductively coupled plasma-mass spectrometry (ICP-MS), open circuit potential (OCP) test, and liquid chromatography-mass spectrometer (LC–MS) analysis were also employed when investigating the degradation mechanism and pathway. More details for the reagents and characterization methods have been provided in Text S1 in Supplementary Materials.
Catalytic oxidation experiments
For the catalytic degradation experiments, appropriate amounts of the CoFe2O4-CNT catalyst and PDS were introduced into a 250-mL three-necked flask containing 100 mL of pollutant solution prepared in advance. The flask was immersed in a water bath connected to a mechanical stirring apparatus set at 500 r/min. The concentration of the pollutants was determined using a UV–visible spectrophotometer (TU-1901, Beijing Puxi, China). More details for the catalytic oxidation experiments have been provided in Text S2 in Supplementary Materials.
Theoretical calculation and toxicity evaluation
The density functional theory (DFT) calculations were conducted with Gaussian 16 program (Frisch et al. 2016) and the Multiwfn software (Lu and Chen 2012). The results were then visualized with the Virtual Molecular Dynamic (VMD) software (Humphrey et al. 1996). The Ecological Structure Activity Relationships (ECOSAR, v2.2) software was used for toxicity evaluation. More details have been provided in Text S3 in Supplementary Materials.
Results and discussion
Characterization of the catalyst
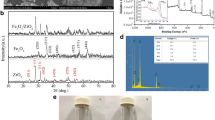
SEM analysis was first used to characterize the morphology of the CoFe2O4-CNT composite catalyst. As shown in Fig. 1 a and b, aggregates of CoFe2O4 particles possessed a spherical morphology, which were dispersed with the CNT branches. XRD was then used to characterize the crystal structure of the catalyst, which revealed distinct diffraction peaks at various 2θ values (Fig. 1c). Most of the peaks were in accordance with the standard pattern of CoFe2O4 (JCPDS 22–1086) except for the broad band at around 26°, which was ascribed to the (002) crystal plane of CNT (Yang et al. 2023). This again verified the co-existence of CoFe2O4 and CNT in the composite catalyst, in consistent with the SEM results. To further investigate the chemical structure of the composite, XPS analysis was performed. As illustrated in Fig. 1d, the peaks of C 1 s, O 1 s, Fe 2p, and Co 2p could be clearly observed as expected. Furthermore, deconvolution of the Fe 2p and Co 2p peaks was conducted and the results were summarized in Figure S1 and Table S1-S2. In the Fe 2p spectrum (Figure S1a), the two binding energies at around 726.0 and 713.5 eV were attributed to Fe(III) in tetrahedral sites (A-sites) in the spinel-type CoFe2O4, while the two at 724.1 and 710.9 eV were assigned to Fe(III) in octahedral sites (B-sites) (Wang et al. 2012; Zou et al. 2021). Similarly, for the Co 2p spectrum (Figure S1b), the two binding energies at 797.5 and 782.3 eV, and the other two at 796.3 and 780.6 eV, indicated the presence of Co(II) in tetrahedral (A-sites) and octahedral sites (B-sites), respectively (Wang et al. 2012; Zou et al. 2021).
Adsorption of the reactants is important for the catalytic oxidation process with the CoFe2O4-CNT/PDS system. On the one hand, activation of PDS molecules took place on the surface of the CoFe2O4-CNT catalyst after they were adsorbed. On the other hand, the produced reactive oxidative species (ROSs) usually possessed short lifetimes, and it is more favorable to oxidize the adsorbed pollutants because they were closer in space. Generally, a large specific surface area and abundant porosity indicated that there was more space on the catalyst surface for the adsorption of the reactants. Thus, nitrogen sorption test was used to characterize porosity of the catalyst. According to the nitrogen sorption isotherm (Fig. 1e), the composite possessed a large specific area (SBET) of 204 m2/g and abundant porosity with a pore volume (Vt) of 0.716 cm2/g, which were favorable for the adsorption of the reactants and the subsequent catalytic oxidation reaction. In addition, the catalyst showed a saturation magnetization of 48.0 emu/g as revealed by VSM analysis (Fig. 1f). When other conditions are identical, a larger saturation magnetization means that it is easier to separate the catalyst magnetically. The large saturation magnetization of the CoFe2O4-CNT catalyst indicated that it could be facially magnetically separated, and this was further confirmed in Fig. 1f inset, which showed that the catalyst could be facially separated with an external magnet.
Catalytic oxidation performance of organic pollutants
Using the CoFe2O4-CNT/PDS system, the catalytic oxidation performances of RB5 and other organic pollutants were studied. The effects of several operation factors have been investigated, including dosage of CoFe2O4-CNT, dosage of PDS, initial concentration of RB5, initial pH, and reaction temperature (Fig. 2). The corresponding kinetic fitting results and kobs values are shown in Figure S2 in Supplementary Materials. As illustrated in Fig. 2a, RB5 removal was 20.4% in the presence of PDS only, which was attributed to the direct oxidation of RB5 by PDS. With the addition of 0.1 g/L CoFe2O4-CNT, RB5 removal was significantly increased to 80.4%, indicating the high catalytic efficiency of the catalyst. An increased dosage of 0.2 g/L provided more active sites, leading to a further increase in RB5 removal to 96.8%. However, further increasing the dosage to 0.3 g/L did not result in obvious improvement in RB5 removal. From a practical viewpoint, the dosage of 0.2 g/L was selected to decrease the catalyst cost. At this catalyst dosage, the effect of PDS dosage was further investigated (Fig. 2b). In the absence of PDS, RB5 removal was found to be 44.4%. This high removal by adsorption was attributed to the abundant porosity of the catalyst, which was verified above in Fig. 1e. Note that the sum of RB5 removals with catalyst only (44.4%) and with PDS only (20.4%) was 64.8%, which was much lower than the removal obtained in the presence of both (96.8%). This clearly proved the high catalytic efficiency of the CoFe2O4-CNT/PDS system. With an increase in the PDS dosage, RB5 removal was enhanced first and reached a maximum at 4 mM. Nevertheless, at a higher dosage of 5 mM, excessive PDS resulted in decreased RB5 removal, which was probably due to self-quenching reactions occurred between PDS and the produced active species (Maifadi et al. 2022). Therefore, the dosage of PDS was selected as 4 mM. The effect of initial RB5 concentration was investigated in the range of 5–40 mg/L. As shown in Fig. 2c, as the initial RB5 concentration increases, its removal gradually decreases. This is because when the amounts of catalyst and oxidant remain constant, the catalytic system can provide limited active sites and reactive species. At a higher initial RB5 concentration, there will be more RB5 and more oxidation intermediates in the system, leading to more fierce competition and resulting in a decrease in the removal efficiency. However, even at a RB5 concentration of 40 mg/L, a high removal of 81.6% could be achieved, indicating the high catalytic performance of the CoFe2O4-CNT/PDS system. Although decreasing the initial concentration led to enhanced removal efficiency, dilution led to the consumption of more oxidant and catalyst at a fixed mass of pollutant. Therefore, to make a compromise, the initial concentration was selected as 10 mg/L to achieve a moderate removal efficiency.
Effects of CoFe2O4-CNT dosage (a), PDS dosage (b), initial concentration of RB5 (c), initial pH (d), temperature (e), and linear fitting in the Arrhenius model (f) Experimental conditions unless otherwise stated: CoFe2O4-CNT dosage = 0.2 g/L, PDS dosage = 4 mM, initial RB5 concentration = 10 mg/L, no pH adjustment, temperature = 25 ℃
The influence of initial pH was depicted in Fig. 2d. The removal of RB5 was above 90% in the initial pH range of 3–9, but it declined to 82.1% at a higher pH value of 11. The highest removal efficiency of 97.7% was obtained at pH 3. From a practical viewpoint, additional acid is required to tailor the solution pH, leading to increased cost. At the spontaneous pH of the RB5 solution (around 6.7), the removal efficiency was 96.8%, which was only slightly lower than the value at pH 3. Therefore, the initial pH was not tailored for subsequent experiments. The decreased removal efficiency under alkaline conditions may be attributed to several reasons. As revealed by zeta potential measurements (Figure S3), the surface of the CoFe2O4-CNT catalyst surface carries negative charges under alkaline conditions. On the one hand, this will lead to enhanced electrostatic repulsion between the anionic RB5 and the catalyst surface, inhibiting its adsorption and degradation. On the other hand, this will also hinder the adsorption and subsequently activation of the PDS oxidant, which is also a negatively charged species in the form of S2O82−. In addition, the large amount of OH− under alkaline conditions may react with surface metal species to form iron hydroxide complexes, suppressing the catalytic performance of the catalyst (Xu et al. 2019).
Finally, the impact of reaction temperature on the removal of RB5 was studied. As revealed in Fig. 2e, RB5 removal was improved from 96.8 to ~ 100% from 25 to 45 ℃, and kobs increased sharply from 0.0142 to 0.0304/min. Based on the Arrhenius equation, the activation energy (Ea) was calculated to be 29.9 kJ/mol (Fig. 2f), which was a relatively low value and again indicated the high catalytic performance of the reaction system (Ma et al. 2020). Additional heat is required to maintain a higher temperature, leading to increased cost. Therefore, the temperature was selected to be 25 ℃, which was the ambient room temperature.
In summary, for the selection of specific operation conditions, both the goal of a higher removal efficiency and the economy of the treatment process are considered. The selected conditions were as follows: CoFe2O4-CNT dosage = 0.2 g/L, PDS dosage = 4 mM, initial RB5 concentration = 10 mg/L, no pH adjustment, temperature = 25 ℃.
Inorganic ions and natural organic matter widely presented in real water matrices may impact the degradation performance. Considering this, their effects were investigated furthermore. When Cl− and H2PO4− were added, the removal efficiencies of RB5 decreased from 96.8 to 95.7% and 94.9% (Fig. 3a), and the corresponding kobs dropped from 0.0142 to 0.0129 and 0.0125/min, respectively (Figure S4a). The added Cl− and H2PO4− could react with the active species such as ·OH and ·SO4·− radicals generated in the catalytic system, forming weaker radicals and thus slightly inhibiting the degradation process (Fu et al. 2019; Shi et al. 2020). Compared to Cl− and H2PO4−, the addition of HCO3− and HPO42− could not only react with active radicals to form less reactive ones but also lead to an increase in the initial pH of the solution. As a result, they exerted more significant suppression effects, resulting in decreased RB5 removal to 94.5% and 91.3% (Fig. 3a) as well as decreased kobs to 0.0118 and 0.0099/min (Figure S4a). The impact of HA was limited compared to these inorganic ions, leading to a similar removal efficiency of 96.4% and a similar kobs of 0.0141/min.
Effects of anions and HA (a), water matrices (b), and pollutant type (c) on the degradation performance and reusability of the CoFe2O4-CNT catalyst (d). Experimental conditions: CoFe2O4-CNT dosage = 0.2 g/L, PDS dosage = 4 mM, initial pollutant concentration = 10 mg/L, no pH adjustment, temperature = 25 ℃
When investigating the performance of the CoFe2O4-CNT/PDS system above, deionized water was used and RB5 was employed as the target pollutant. For practical application of the catalyst, different water matrices and various pollutants may be faced with. To address this issue, the performance of the catalytic system was further evaluated in several water matrices including tap water, lake water and sea water. In these different matrices, RB5 removal efficiencies all remained above 95% (Fig. 3b) although the kobs values decreased to some extent (Figure S4c), which could be ascribed to the inorganic ions and organic matters in them as well as higher pH values of them (Table S3). This indicated the potential of this reaction system for utilization in real water matrices. After that, several other organic pollutants were tested as the target, including another two dye pollutants (Congo red (CR) and methyl orange (MO)) and an antibiotic pollutant (tetracycline, TC). As depicted in Fig. 3c, these organic pollutants with different structures could be efficiently removed, achieving high removal efficiencies of ~ 100%, 99.3%, and 94.5%, respectively. These results showed that the CoFe2O4-CNT/PDS system is applicable to various water matrices and various organic pollutants, suggesting its wide practical applicability.
Finally, reusability of the CoFe2O4-CNT catalyst was evaluated. The RB5 removal efficiencies were 96.8%, 93.8%, 90.2% and 80.7% in four consecutive catalytic runs (Fig. 3d). Metal leaching may be a possible reason for catalytic deactivation. According to ICP results, the amounts of leached Fe and Co were 0.38 and 1.30 mg/L, respectively. Based on this result, an additional degradation experiment was conducted by adding a homogenous mixture of Fe2+ and Co2+ instead of the heterogenous CoFe2O4-CNT catalyst. The result showed that compared to the case of single PDS (20.4%), the Fe2+/Co2+/PDS system only slightly promoted RB5 removal (29.7%) (Figure S5). In addition, the XRD pattern of used CoFe2O4-CNT was similar to that of the fresh one (Fig. 1c), indicating the structural stability of the catalyst. Therefore, the main reason for the deactivation of CoFe2O4-CNT was ascribed to the accumulation of residue degradation products on the catalyst surface, which occupied the active sites and inhibited the adsorption and subsequently oxidation of organic pollutants (Wang et al. 2022a, b).
Reaction mechanism
In PDS-based catalytic oxidation processes, several ROSs may be involved, including ·OH, SO4·−, O2·−, 1O2, and high-valent metal-oxo species. To investigate their contributions in the CoFe2O4-CNT/PDS system, several quenchers were added, including MeOH, PMSO, FFA, and BQ. MeOH can efficiently quench both ·OH and SO4·− (Wang et al. 2023), while PMSO is a quencher for high-valent metal-oxo species (Chi et al. 2021; Feng et al. 2021). FFA and BQ are known as quenchers for 1O2 (Guo et al. 2021) and O2·− (Yang et al. 2021), respectively. As illustrated in Fig. 4a and Figure S6a, all the four quenchers led to declined RB5 removal and kobs value, indicating the contributions of all these reactive species in the catalytic oxidation process. To gain further insights into the contributions of these species, the four quenchers were simultaneously introduced into the system. This led to a significant decrease in RB5 removal, reducing it to 42.1% (Fig. 4b). This value approached the removal efficiency of 44.4% when only the catalyst was present, and the obtained kobs values were also close to each other in these two cases (Figure S6b). This indicated the near-complete inhibition of the catalytic reaction, ruling out the involvement of other reaction mechanisms.
It should be noted that the reaction mechanism in this CoFe2O4-CNT/PDS system was different from the Fe3O4-CNT/PDS system in our previous work, where the non-radical surface-mediated electron-transfer mechanism played an indispensable role (Shi et al. 2022b). In the current work, in-situ OCP test was conducted as well. Nevertheless, although the potential of the CoFe2O4-CNT catalyst was increased with the addition of PDS, the subsequent addition of RB5 did not result in a potential drop (Figure S7). This phenomenon was inconsistent with the result in the Fe3O4-CNT/PDS system where an apparent drop was observed (Shi et al. 2022b). Thus, the contribution of non-radical surface-mediated electron-transfer mechanism was ruled out.
XPS measurements further revealed the divergent reaction mechanisms. As discussed in the “Characterization of the catalyst” section, Fe and Co elements in the catalyst existed in form of Fe(III) and Co(II). After the catalytic run, the Fe 2p spectrum of the used catalyst was similar with that of the fresh one, showing the same number of deconvoluted peaks at close binding energies (Figure S8a and Table S1). In contrast, two additional peaks appeared at 792.7 and 780.0 eV in the Co 2p spectrum of the used catalyst, which could be ascribed to Co(III) species (Figure S8b and Table S1) (Zou et al. 2021). Based on this result, Fe(III) species did not take part in the catalytic process but Co(II) did, which activated PDS and was partly transformed into Co(III). Although Co(III) can also activate PDS to form reactive species, the transformation from Co(III) to Co(II) is much slower than the transformation from Co(II) to Co(III) (Liu et al. 2021b). As a result, an increase in the Co(III) fraction was observed for the used catalyst. Again, this was inconsistent with the Fe3O4-CNT/PDS system, where it was found that Fe3O4 only played a role in magnetic separation and made little contribution to the activation of PDS (Shi et al. 2022b).
Degradation pathway and toxicity evaluation
To scrutinize the oxidation products generated in the degradation process of RB5, LC–MS analysis has been conducted on the treated RB5 solution. To assist the analysis, DFT calculations have been performed to obtain the orbital-weighted Fukui functions (fOW− and fOW0) of RB5 (Fig. 5). The condensed Fukui indexes were summarized in Table S4. According to previous reports (Deng et al. 2021), fOW− and fOW0 represent the tendency of different sites in the molecule towards radical attack and electrophilic attack. The larger the values, the more susceptible the sites. Based on Fig. 5 and Table S4, O12-14, O16-18, N19-20, and N37-38 sites exhibited higher susceptibility in the oxidative degradation process.
Combining the DFT calculation results above and the LC–MS results (Figure S9), the plausible degradation pathway of RB5 in the CoFe2O4-CNT/PDS system has been proposed (Fig. 6). The attack on O12-14 and O16-18 sites of compound A (the target pollutant of RB5) results in the formation of compound B. Subsequently, the N19 and N20 sites of compound B undergo cleavage, giving rise to the generation of compound D and compound E. The N20 site in compound D is attacked, leading to the production of compound G, which is subsequently subjected to desulfurization to yield compound J. Meanwhile, the N37 and N38 sites in compound E are susceptible to attack, culminating in the formation of compound H, which is further protonated to yield compound K (Hisaindee et al. 2013). Additionally, the N19 and N38 sites of compound A are subject to attack, leading to the formation of compound C and compound D. Subsequently, the N37 site in compound C is targeted for attack, resulting in the creation of compound F. Compound F further engages in an attack on the O12-14 and O16-18 sites, ultimately producing compound I (Liu et al. 2021a).
Moreover, predictions regarding the potential toxicological effects of these oxidation products have been conducted. As shown in Fig. 7, RB5 (designated as compound A) and its oxidation products (designated as compounds B-K) exhibited varying degrees of acute and chronic toxicity towards three indicator species. Compound G exhibited higher acute and chronic toxicity values for the three indicator species, indicating a decrease in both acute and chronic toxicity compared to RB5. Compound J has higher toxicity values for daphnia and fish (Fig. 7c, d, f), indicating that its toxicity to daphnia and chronic toxicity to fish are reduced compared to RB5. For other products, the EC50 and ChV values are relatively lower, indicating increased toxicity compared to RB5. In addition, the orders of acute and chronic toxicity of these products for the same species are different. For example, for daphnia, compound D ranks eighth in terms of acute toxicity among all products (Fig. 7c) and is considered non-toxic (LC50 > 10 mg/L), but its chronic toxicity ranks fifth (Fig. 7d) and is considered toxic (ChV > 10 mg/L) (Zhu et al. 2021). Therefore, when conducting toxicity prediction, both acute and chronic toxicity should be considered to draw a comprehensive conclusion. For different species, the toxicity trends of most products are similar except for compound C and D. These two compounds are relatively safe for green algae and fish, but have significant chronic toxicity for daphnia (Fig. 7d). In general, toxicity prediction indicated that some oxidation products with stronger toxicity than RB5 are obtained after treated with the CoFe2O4-CNT/PDS system. Therefore, from a practical perspective, further remediation technologies such as adsorption and membrane separation may be required to remove these residual toxic products.
Based on the findings above, more research is needed in future works before using the CoFe2O4-CNT/PDS system for practical water treatment. On the one hand, although the CoFe2O4-CNT/PDS system showed high performance for the removal of several single organic pollutant, different organic pollutants may co-exist for real water treatment. According to the results in Fig. 3c, different removal efficiencies were obtained for different pollutants although their concentrations were the same. Therefore, the selectivity and the overall removal efficiency for mixed pollutants should be investigated. On the other hand, based on the toxicity evaluation results in Fig. 7, some of the oxidation products showed decreased toxicity compared to the parent RB5 while some others showed increased toxicity. Since these products were in different concentrations in the treated water, the overall toxicity of the treated water remained unknown. In future works, it is suggested to conduct some toxicity evaluation experiments using some indicator species to provide a better understanding of the change in toxicity after being treated with the CoFe2O4-CNT/PDS system.
Conclusion
The composite of CoFe2O4-CNT was synthesized in a facile solvothermal approach, serving as a catalyst in activation of PDS towards the degradation of RB5 and other organic pollutants. SEM, XRD, and XPS measurements verified the successfully preparation of the composite catalyst. In addition, nitrogen-sorption and VSM results showed that the catalyst possessed a large surface area of 204 m2/g and a saturation magnetization of 48.0 emu/g. When employed in combination with PDS for pollutant degradation, the effects of various operational conditions have been investigated. Under the experimental conditions of 0.2 g/L CoFe2O4-CNT, 4 mM PDS, initial pollutant concentration of 10 mg/L and temperature of 25 ℃, the removal efficiencies of several organic pollutants including RB5, CR, MO, and TC were in the range of 94.5 to ~ 100%. The effects of pH, co-existing inorganic salts and HA as well as water matrix were also studied, verifying the potential application of the CoFe2O4-CNT/PDS system for treatment of real water matrices. Based on quenching experiments, OCP and XPS analysis, the reaction mechanism in CoFe2O4-CNT/PDS was found to be different from the Fe3O4-CNT/PDS system in our previous work. The additional Co(II) species played an indispensable role in the catalytic oxidation process, which helped to active PDS towards the generation of various reactive oxidation species. Finally, the plausible degradation pathway of RB5 was proposed based on DFT calculations and LC–MS analysis, and the toxicity of those degradation products were predicted by ECOSAR using indicator species of green algae, daphnia, and fish.
Data availability
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
References
Apul OG, Karanfil T (2015) Adsorption of synthetic organic contaminants by carbon nanotubes: A critical review. Water Res 68:34–55. https://doi.org/10.1016/j.watres.2014.09.032
Araújo KCF, dos Santos EV, Nidheesh PV, Martínez-Huitle CA (2022) Fundamentals and advances on the mechanisms of electrochemical generation of persulfate and sulfate radicals in aqueous medium. Curr Opin Chem Eng 38:100870. https://doi.org/10.1016/j.coche.2022.100870
Chi H, Wan J, Zhou X, Sun J, Yan B (2021) Fe@C activated peroxymonosulfate system for effectively degrading emerging contaminants: analysis of the formation and activation mechanism of Fe coordinately unsaturated metal sites. J Hazard Mater 419:126535. https://doi.org/10.1016/j.jhazmat.2021.126535
Collivignarelli MC, Abba A, Carnevale Miino M, Damiani S (2019) Treatments for color removal from wastewater: state of the art. J Environ Manage 236:727–745. https://doi.org/10.1016/j.jenvman.2018.11.094
Deng J, Ye C, Cai A, Huai L, Zhou S, Dong F, Li X, Ma X (2021) S-doping α-Fe2O3 induced efficient electron-hole separation for enhanced persulfate activation toward carbamazepine oxidation: experimental and DFT study. Chem Eng J 420:129863. https://doi.org/10.1016/j.cej.2021.129863
Feng Y, Li Y, Yang B, Yang Z, Fan Y, Shih K, Li H, Wu D, Zhang L (2021) Mechanistic insight into the generation of high-valent iron-oxo species via peroxymonosulfate activation: an experimental and density functional theory study. Chem Eng J 420:130477. https://doi.org/10.1016/j.cej.2021.130477
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR et al (2016) Gaussian 16 Rev. C.01, Wallingford, CT
Fu H, Zhao P, Xu S, Cheng G, Li Z, Li Y, Li K, Ma S (2019) Fabrication of Fe3O4 and graphitized porous biochar composites for activating peroxymonosulfate to degrade p-hydroxybenzoic acid: insights on the mechanism. Chem Eng J 375:121980. https://doi.org/10.1016/j.cej.2019.121980
Giannakis S, Lin K-YA, Ghanbari F (2021) A review of the recent advances on the treatment of industrial wastewaters by sulfate radical-based advanced oxidation processes (SR-AOPs). Chem Eng J 406:127083. https://doi.org/10.1016/j.cej.2020.127083
Guo Y, Yan L, Li X, Yan T, Song W, Hou T, Tong C, Mu J, Xu M (2021) Goethite/biochar-activated peroxymonosulfate enhances tetracycline degradation: Inherent roles of radical and non-radical processes. Sci Total Environ 783:147102. https://doi.org/10.1016/j.scitotenv.2021.147102
Hisaindee S, Meetani MA, Rauf MA (2013) Application of LC-MS to the analysis of advanced oxidation process (AOP) degradation of dye products and reaction mechanisms. TrAC-Trend Anal Chem 49:31–44. https://doi.org/10.1016/j.trac.2013.03.011
Humphrey W, Dalke A, Schulten K (1996) VMD: visual molecular dynamics. J Mol Graph 14:33–38. https://doi.org/10.1016/0263-7855(96)00018-5
Kohantorabi M, Moussavi G, Giannakis S (2021) A review of the innovations in metal- and carbon-based catalysts explored for heterogeneous peroxymonosulfate (PMS) activation, with focus on radical vs. non-radical degradation pathways of organic contaminants. Chem Eng J. 411:127957. https://doi.org/10.1016/j.cej.2020.127957
Li H, Yang Z, Lu S, Su L, Wang C, Huang J, Zhou J, Tang J, Huang M (2021) Nano-porous bimetallic CuCo-MOF-74 with coordinatively unsaturated metal sites for peroxymonosulfate activation to eliminate organic pollutants: Performance and mechanism. Chemosphere 273:129643. https://doi.org/10.1016/j.chemosphere.2021.129643
Liu F, Wang X, Liu Z, Miao F, Xu Y, Zhang H (2021a) Peroxymonosulfate enhanced photocatalytic degradation of Reactive Black 5 by ZnO-GAC: key influencing factors, stability and response surface approach. Sep Purif Technol 279:119754. https://doi.org/10.1016/j.seppur.2021.119754
Liu J, Li Z, Wang M, Jin C, Kang J, Tang Y, Li S (2021b) Eu2O3/Co3O4 nanosheets for levofloxacin removal via peroxymonosulfate activation: performance, mechanism and degradation pathway. Sep Purif Technol 274:118666. https://doi.org/10.1016/j.seppur.2021.118666
Lu T, Chen F (2012) Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem 33:580–592. https://doi.org/10.1002/jcc.22885
Ma Q, Nengzi L-c, Zhang X, Zhao Z, Cheng X (2020) Enhanced activation of persulfate by AC@CoFe2O4 nanocomposites for effective removal of lomefloxacin. Sep Purif Technol 233:115978. https://doi.org/10.1016/j.seppur.2019.115978
Maifadi S, Mhlanga SD, Nxumalo EN, Motsa MM, Kuvarega AT (2022) Treatment of salon wastewater by peroxydisulfate based advanced oxidation process (PDS-AOP) under solar light: Synergy through integrated technologies. J Water Process Eng 49:103062. https://doi.org/10.1016/j.jwpe.2022.103062
Monteagudo JM, El-taliawy H, Durán A, Caro G, Bester K (2018) Sono-activated persulfate oxidation of diclofenac: degradation, kinetics, pathway and contribution of the different radicals involved. J Hazard Mater 357:457–465. https://doi.org/10.1016/j.jhazmat.2018.06.031
Peng J, Chang Y, Wang Z, Liu J, Wang S, Zhang Y, Shao S, Liu D, Zhang Y, Shi J, Liu H, Yan G, Cao Z, Gao S (2022) Amlodipine removal via peroxymonosulfate activated by carbon nanotubes/cobalt oxide (CNTs/Co3O4) in water. Environ Sci Pollut Res 29:11091–11100. https://doi.org/10.1007/s11356-021-16399-5
Qin Q, Yan L, Liu Z, Liu Y, Gu J, Xu Y (2022) Efficient activation of peroxymonosulfate by nanotubular Co3O4 for degradation of Acid Orange 7: performance and mechanism. Environ Sci Pollut Res 29:50135–50146. https://doi.org/10.1007/s11356-022-19434-1
Ren W, Huang X, Wang L, Liu X, Zhou Z, Wang Y, Lin C, He M, Ouyang W (2021) Degradation of simazine by heat-activated peroxydisulfate process: a coherent study on kinetics, radicals and models. Chem Eng J 426:131876. https://doi.org/10.1016/j.cej.2021.131876
Sarkar P, Roy D, Bera B, De S, Neogi S (2022) Enhanced photodegradation of reactive dyes in textile effluent with CoFe2O4/g-CN heterostructure-mediated peroxymonosulphate activation. Environ Sci Pollut Res 29:50566–50583. https://doi.org/10.1007/s11356-022-18944-2
Shi Y, Zhu J, Yuan G, Liu G, Wang Q, Sun W, Zhao B, Wang L, Zhang H (2020) Activation of persulfate by EDTA-2K-derived nitrogen-doped porous carbons for organic contaminant removal: radical and non-radical pathways. Chem Eng J 386:124009. https://doi.org/10.1016/j.cej.2019.124009
Shi Y, Chang Q, Zhang T, Song G, Sun Y, Ding G (2022a) A review on selective dye adsorption by different mechanisms. J Environ Chem Eng 10:108639. https://doi.org/10.1016/j.jece.2022.108639
Shi Y, Zhang Y, Song G, Tong L, Sun Y, Ding G (2022b) Efficient degradation of organic pollutants using peroxydisulfate activated by magnetic carbon nanotube. Water Sci Technol 86:2611–2626. https://doi.org/10.2166/wst.2022.371
Tian D, Zhou H, Zhang H, Zhou P, You J, Gang Y, Pan Z, Liu Y, Lai B (2022) Heterogeneous photocatalyst-driven persulfate activation process under visible light irradiation: from basic catalyst design principles to novel enhancement strategies. Chem Eng J 428:131166. https://doi.org/10.1016/j.cej.2021.131166
Wang WP, Yang H, Xian T, Jiang JL (2012) XPS and magnetic properties of CoFe2O4 nanoparticles synthesized by a polyacrylamide gel route. Mater Trans 53:1586–1589. https://doi.org/10.2320/matertrans.M2012151
Wang Y, Zhang Y, Wang J (2020) Nano spinel CoFe2O4 deposited diatomite catalytic separation membrane for efficiently cleaning wastewater. J Membrane Sci 615:118559. https://doi.org/10.1016/j.memsci.2020.118559
Wang B, Li S, Wang H, Yao S (2022a) Insight into the performance and mechanism of magnetic Ni0.5Cu0.5Fe2O4 in activating peroxydisulfate for ciprofloxacin degradation. Water Sci Technol 85:1235–1249. https://doi.org/10.2166/wst.2022.043
Wang L, Wang L, Shi Y, Zhu J, Zhao B, Zhang Z, Ding G, Zhang H (2022b) Fabrication of Co3O4-Bi2O3-Ti catalytic membrane for efficient degradation of organic pollutants in water by peroxymonosulfate activation. J Colloid Interf Sci 607:451–461. https://doi.org/10.1016/j.jcis.2021.08.086
Wang J, Lv H, Tong X, Ren W, Shen Y, Lu L, Zhang Y (2023) Modulation of radical and nonradical pathways via modified carbon nanotubes toward efficient oxidation of binary pollutants in water. J Hazard Mater 459:132334. https://doi.org/10.1016/j.jhazmat.2023.132334
Xu M, Li J, Yan Y, Zhao X, Yan J, Zhang Y, Lai B, Chen X, Song L (2019) Catalytic degradation of sulfamethoxazole through peroxymonosulfate activated with expanded graphite loaded CoFe2O4 particles. Chem Eng J 369:403–413. https://doi.org/10.1016/j.cej.2019.03.075
Yang B, Luo Q, Li Q, Meng Y, Lingli L, Liu Y (2021) Selective oxidation and direct decolorization of cationic dyes by persulfate without activation. Water Sci Technol 83:2744–2752. https://doi.org/10.2166/wst.2021.177
Yang F, Hu P, Yang FF, Chen B, Yin F, Hao K, Sun R, Gao L, Sun Z, Wang KJS (2023) CNTs bridged basal-plane-active 2H-MoS2 nanosheets for efficient robust electrocatalysis. Small 19:2301468. https://doi.org/10.1002/smll.202301468
Zhang H, Wang X, Li Y, Zuo K, Lyu C (2021) A novel MnOOH coated nylon membrane for efficient removal of 2,4-dichlorophenol through peroxymonosulfate activation. J Hazard Mater 414:125526. https://doi.org/10.1016/j.jhazmat.2021.125526
Zhu L, Shi Z, Deng L (2021) Enhanced heterogeneous degradation of sulfamethoxazole via peroxymonosulfate activation with novel magnetic MnFe2O4/GCNS nanocomposite. Colloid Surface A 621:126531. https://doi.org/10.1016/j.colsurfa.2021.126531
Zou L, Xiao X, Chu C, Chen B (2021) Facile synthesis of porous CoFe2O4/graphene aerogel for catalyzing efficient removal of organic pollutants. Sci Total Environ 775:143398. https://doi.org/10.1016/j.scitotenv.2020.143398
Funding
This work is financially supported by the Joint Research Fund Liaoning-Shenyang National Laboratory for Materials Science (20180510004), the National Natural Science Foundation of China (51479016, 51908409), and the Fundamental Research Funds for the Central Universities (3132023162).
Author information
Authors and Affiliations
Contributions
Yawei Shi: writing—original draft, writing—review and editing, conceptualization. Yi Zhang: methodology, data curation, investigation. Guobin Song: formal analysis, data curation. Ya Sun: resources. Guanghui Ding: resources, supervision.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Guilherme Luiz Dotto
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shi, Y., Zhang, Y., Song, G. et al. Efficient removal of organic pollutants by activation of peroxydisulfate with the magnetic CoFe2O4/carbon nanotube composite. Environ Sci Pollut Res 31, 6835–6846 (2024). https://doi.org/10.1007/s11356-023-31567-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-31567-5