Abstract
Combination of active and thermally stable amino acid-functionalized ionic liquids (AAILs) with high surface area and porosity of mesocellular silica foams (MCF) to form a robust CO2 sorbent is investigated in this study. These sorbent composites (MCF-x) are synthesized by immobilizing three AAILs (Gly, Lys, and Arg) into MCF by a simple wet-impregnation method. The prepared AAILs and MCF-x sorbents are characterized by N2 adsorption/desorption, small-angle X-ray scattering (SAXS), elemental analysis (EA), and Fourier-transformed infrared (FTIR) spectroscopies. Their corresponding CO2 sorption–desorption performance at 348 K under ambient pressure using dry 15 % CO2 is also studied. The obtained results show that the AAILs have low CO2 sorption capacities and rates because of their high viscosities. The MCF-x sorbents, however, exhibit remarkable enhancement of sorption capacities and fast kinetics. Among these sorbents, MCF-Lys possesses the superior sorption capacity of 1.38 mmolCO2/gsorbent, the higher tolerance to water moisture and much better long-term durability which may be a promising sorbent for CO2 capture applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Recently, global warming caused by the increased raise of CO2 concentration in the atmosphere because of the extensive use of fossil fuels has been a severe environmental problem (Raskar et al. 2013; Bhatta et al. 2015; Granados-Correa et al. 2015). Therefore, it is urgent to develop technologies to efficiently and economically capture CO2 from large stationary sources, for instance, coal-fired power plants, cement, steel factories, and so on. Generally, amine absorption technologies, which have been mostly used in the post-combustion process, are limited by the high capital, operation costs, and high-energy consumption during regeneration of the sorbents.
Alternatively, it has been reported that nanoporous solids such as microporous zeolites, porous carbon materials, porous coordination polymers, and organic nanostructures can be used in sequestration of CO2 via physisorption (Hyun et al. 1999; Zhu et al. 2012; Wang et al. 2014; Shi et al. 2015). However, these materials show low-CO2 sorption capacity, poor selectivity, poor tolerance to water, and high-temperature regeneration which are unable to be utilized for practical industrial applications.
During the recent years, amine-functionalized ordered mesoporous silicas (OMSs), which can be carried out in two routes, chemical grafting and physical impregnation, are widely studied for applications as CO2 sorbents. In terms of the former method, primary, secondary, and tertiary amine-containing compounds have been modified onto the OMSs which were able to capture CO2 with enhanced durability and resistance to moisture as compared with other supports such as activated carbon, metal-organic frameworks (MOFs), silica gel, and pristine silica (Houshmand et al. 2012; Khan et al. 2014; Kishor and Ghoshal 2015; Yao et al. 2015; Arellano et al. 2016). In terms of latter method, many kinds of amine-containing compounds such as diamines, polyamines, polyaniline (PANI), tetraethylenepentamine (TEPA), and polyethylenimine (PEI), etc. were immobilized into mesoporous supports (MCM-41, MCM-48, KIT-6, and SBA-15) which can exhibit high-CO2 sorption capacities that is ca. 2–30 times higher than that of pristine supports and amine compounds (Sanz et al. 2012; Lin and Bai 2013; Linneen et al. 2013; Sanz-Perez et al. 2013; Ma et al. 2014; Cogswell et al. 2015; Ullah et al. 2015; Wang et al. 2015). However, this preparation route can suffer from the worse durability of long-term operation.
Ionic liquids (ILs), which consist of organic molten salts, possess many unique properties, such as negligible vapor pressures, good thermal stability, tunable solubility for both organic and inorganic molecules, and much synthetic flexibility (Bara et al. 2010; Gurkan et al. 2010). The first report on the solubility of CO2 in imidazolium-based ILs was proposed by Blanchard et al. (1999) in 1999. However, the physical absorption of CO2 between imidazolium rings was too low to be sufficient for practical applications. Accordingly, many works (Bates et al. 2002; Fukumoto et al. 2005; Gutowski and Maginn 2008; Zhang et al. 2009; Goodrich et al. 2011a; Wang et al. 2011) were intensively focused on the synthesis of amine-functionalized ILs to improve CO2 absorption capacity. Even though the CO2 absorption in amine-functionalized ILs was significantly enhanced, the use of ILs is still hindered due to their highly viscous properties. Thus, it may be a promising strategy to incorporate the viscous amine-functionalized ILs into high surface area of porous support in order to increase the CO2 mass transfer and maintain the thermal stability of solid sorbents as well.
In this work, the physical wet-impregnation method was used to immobilize amino acid-functionalized ionic liquids (AAILs, (3-aminopropyl)tributylphosphonium amino acid salts)) into mesocellular silica foams (MCF) which may enhance the durability and maintain the high-sorption capacity of solid sorbents. Three different AAILs were synthesized by using a three-step process. A fixed amount of AAILs was dissolved in water and then immobilized into MCF by treating with a simple impregnation method. The fabricated MCF-x sorbents (x denotes the different kind of amino acids, i.e., glycine (Gly), l-lysine (Lys), and l-arginine (Arg). These MCF-x sorbents were found to have enhanced CO2 sorption capacities, rates, and excellent durability of long-term operation, revealing potential opportunities for practical applications in post-combustion CO2 capture.
2 Experimental
2.1 Sample Preparation
The pure MCF samples were prepared based on the methods reported earlier (Schmidt-Winkel et al. 1999). Typically, 2 g of Pluronic 123 was dissolved in 75 mL of aqueous 1.6 N HCl at room temperature, followed by addition of 12 mg of NH4F (ACROS) and 1.5 g of trimethylbenzene (TMB; 98 %, ACROS). Then, 4.3 g of TEOS was added to the mixture after stirring for 1 h at 313 K. The resultant solution was stirred at 313 K for 20 h and then aged at 373 K for 24 h. The solid products of as-synthesized MCF were filtrated with deionized (DI) water, dried at room temperature overnight and followed by removal of organic template by calcination at 823 K.
(3-Aminopropyl)tributylphosphonium l-α-diaminocaproic acid salts were synthesized by a three-step route. Firstly, (3-aminopropyl)tributylphosphonium bromide hydrobromide was prepared by mixing tri-n-butylphosphine and 3-bromopropylamine hydrobromide in acetonitrile at 353 K for 24 h. After acetonitrile was evaporated at 353 K, the solid was dried under vacuum at 353 K for 24 h. The resultant solid was washed with hexane under reflux until the upper hexane phase had no tributylphosphine. The (3-aminopropyl)tributylphosphonium bromide hydrobromide was obtained by drying at 353 K for 24 h. Secondly, the (3-aminopropyl)tributylphosphonium bromide hydrobromide was dissolved in deionized water and passed through a OH-type anion exchange resin column until Cl- species were not be detected. The (3-aminopropyl)tributylphosphonium hydroxide solution can be obtained by rotation evaporation under reduced pressure at 313 K. Thirdly, the synthesis of amine-functionalized ionic liquids (glycine (Gly), l-lysine (Lys) and l-arginine (Arg)) was carried out by neutralizing the (3-aminopropyl)tributylphosphonium hydroxide solution with calculated amino acid (Gly, Lys, and Arg). Then the solvents were removed by rotating evaporation, followed by drying at 353 K under vacuum for 24 h.
The synthesized AAILs-incorporating MCF sorbents were prepared by a facile physical impregnation method. In a typical run, calcined MCF (0.5 g) were first dried at 398 K for 6 h in air, then, deposited the mixture solution with the calculated amounts of AAILs. The products (denoted as MCF-x, x = Gly, Lys, and Arg) were obtained by drying at 353 K overnight.
2.2 Sample Characterizations
Fourier transform infrared (FTIR) spectra were obtained on a spectrometer (Bruker Tensor 27) with 4 cm−1 resolution using KBr pellets at room temperature. To obtain bulk compositions of samples, elemental analyses (EA) were performed by using a CHN elemental analyzer (Heraeus varioIII). Small-angle X-ray scattering (SAXS) of all samples was measured on a Nanostar U system (Bruker, AXS Gmbh). Nitrogen adsorption isotherms were conducted at 77 K using a Micromeritics ASAP 2020 system and the corresponding specific surface areas were calculated from nitrogen adsorption data in the relative pressure (P/P0) range from 0.05 to 0.2 via Brunauer–Emmett–Teller (BET) equation. The pore volumes were derived from the adsorption branch using Barrett–Joyner–Halenda (BJH) model.
2.3 CO2 Sorption–Desorption Tests
The CO2 sorption and desorption properties of various sorbents were investigated by a thermogravimetric analyzer (TGA, PerkinElmer Pyris 6). Firstly, ca. 10 mg of sorbent loaded in a ceramic cell was ramped to 373 K by flowing N2 with a flow rate of 50 mL min−1, followed by maintaining at 373 K for at least 30 min to remove the moisture in the sorbents. Secondly, the sample temperature was lowered to 348 K under N2 flow and then introduced 15 % dry CO2/85 % N2 into the TGA cell at a flow rate of 50 mL min−1. After 40 min of sorption process, the gas was switched to pure N2 flow with a flow rate of 50 mL min−1 to carry out desorption process at the same temperature for 110 min. The sensitivity and accuracy of TGA microbalance are 10 μg and 0.1 %, respectively. To evaluate the effect of moisture in the flue gas on the CO2 sorption performance, a modified TGA system with a water saturator (Liu et al. 2011, 2012) was used. For sorbents durability tests, the cyclic sorption/desorption measurements were repeated for ten times while monitoring the sorption/desorption capacities over multiple cycles.
3 Results and Discussion
As shown in Fig. 1(a), the FTIR spectrum of the pure MCF indicates two distinctive peaks at 1,079 and 968 cm−1 attributed to Si–O–Si and Si–OH stretching, respectively. In terms of AAILs (Gly, Lys, and Arg), at least three weak peaks can be observed at ca. 1,590 cm−1 (NH2 scissoring vibration), 2,931 and 2,818 cm−1 (CH stretching vibration). The nitrogen contents of AAILs can be found in Table 1. After incorporating them onto the MCF, the features of FTIR spectra mentioned above are also observed, indicating the existence of AAILs in the MCF. Furthermore, nitrogen contents measured by EA were observed to range from 7.6 to 11.9 wt% in the MCF-x samples (Table 2). The small-angle X-ray scattering patterns of the pristine MCF and MCF-x can be seen in Fig. 1(b). The MCF has one predominant (100) peak and two weak (110) and (200) diffraction features at 2θ of ca. 0.34°, 0.58°, and 0.85°, suggesting the possible formation of the monodisperse spheres (cells) of MCFs that the uniformly sized spherical cells are interconnected by windows of uniform size (Schmidt-Winkel et al. 1999). Nevertheless, upon impregnating Gly, Lys, and Arg into the pristine substrate, the diffraction peak intensities are notably decreased probably because of the incorporation of AAIL compounds, which result in less ordering of mesostructure. Additionally, the N2 adsorption/desorption curves of the pristine MCF samples (see Fig. 1(c)) also revealed typical features of mesoporosity, i.e., type-IV isotherms with evident hysteresis loops. However, the diminishing of hysteresis loops can be observed upon incorporating a fixed amount of AAILs in the MCF-x samples. This may be due to the blockage of mesoporous channels. As summarized in Table 2, it is worthy to note that total pore volume (V tot) and BET surface area (S BET) are remarkable decreased for MCF-x samples as compared to the pristine MCF samples, indicating a large amount of pore filling of MCF by AAILs. Combined the data from FTIR, SAXS, and N2 adsorption–desorption isotherms, the successful synthesis of MCF-supported amine-functionalized ILs is confirmed.
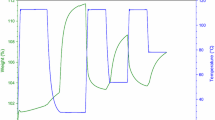
CO2 sorption/desorption cycles of various AAILs and MCF-x at 348 K were obtained by using TGA under atmospheric pressure and their corresponding curves are shown in Fig. 2. Due to the switch of gas flow stream in the CO2 sorption–desorption system, a sudden increase of CO2 sorption at ca. 40 min was observed. The CO2 sorption capacities of various sorbents are also listed in Table 1 and Table 2. Theoretically, about 1–2 mol CO2 can be sorbed per mol of Gly, Lys, and Arg which contain two to four amino groups in one molecule based on the 1:2 stoichiometry. However, the AAILs (Gly, Lys, and Arg) can only sorb ca. 0.09–0.34 mmolCO2/gsorbent (ca. 0.028–0.116 mol CO2/mol AAILs), which was far below the theoretical value. This finding may be ascribed to the relatively high viscosities of AAILs which hinder the mass transfer of CO2. In this work, the decrease of CO2 sorption capacities, amine efficiencies (CO2/N), and rates in the AAILs (Gly > Lys > Arg; see Table 1) may be due to an increase in the viscosities of the AAILs such that Gly < Lys < Arg (54.2 < 81.7 < 124.8 mPas at 348 K; Zhang et al. 2009). In addition, formation of hydrogen bonds in the AAILs via amino groups among themselves and hence may cause ineffectiveness for CO2 sorption (Zhang et al. 2009). Interestingly, it is noteworthy that the CO2 desorption capacity of Lys was ca. 47 % which may be due to occurrence of the solidification (Ren et al. 2012) after the sorption of CO2 by Lys. To circumvent these problems, the amine-functionalized ILs were immobilized into MCF which has hierarchical structure and high BET surface area (677 m2 g−1). It can be seen that MCF-x sorbents exhibited remarkably enhanced sorption capacities, higher amine efficiencies (CO2/N) and sorption rates (Table 2) compared to pristine AAILs. In particular, because of the high primary amino groups (three amino groups in one molecule), the sorption capacities of MCF-Lys and MCF-Arg were increased by 700 % that of Lys and Arg, respectively. The obtained results show that the MCF-x sorbents can overcome the high viscosities of AAILs and possess significantly enhanced CO2 sorption capacities (0.58–1.38 mmolCO2/gsorbent) and rates (reach the equilibrium plateau within 15 min). This could be due to the enhancement of mass transfer area in the AAILs upon incorporation. As can be seen in Table 2, there are no obvious correlations between the CO2 sorption capacities of the synthesized samples and their corresponding physicochemical properties (BET surface areas, total pore volumes and nitrogen contents). Nonetheless, the CO2 sorption capacities may be related to the ratios of primary amino groups (Ko et al. 2011) and the viscosities of ILs in the MCF-x sorbents. Compared with the sorption capacity of MCF-Lys, sorption capacity of MCF-Arg was found to decrease remarkably, which could be due to the possible formation of hydrogen bonds among the Arg molecules (Goodrich et al. 2011b), thus rendering them ineffective for CO2 sorption. In terms of desorption capacity, almost 100 % of CO2 can be desorbed within ca. 80 min in the MCF-Gly and MCF-Arg. However, the lowest desorption rate of MCF-Lys was observed, which may be due to the above-mentioned solidification phenomenon of Lys during CO2 capture. To further study the CO2 sorption mechanism, IR spectroscopic studies of MCF-Lys sorbents before and after CO2 sorption were conducted. As can been seen in Fig. 3, the sorbents possess a band at ca. 1,600 cm−1 which can be assigned to the COO- group in the AAILs prior to CO2 sorption. After sorption of CO2, a new shoulder feature can be found at ca. 1650 cm−1, which is attributed to the formation of carbamate because of CO2 chemical absorption (Ren et al. 2012) on MCF-Lys sorbents. The possible CO2 adsorption mechanism can be illustrated as below. Generally, aqueous amine can absorb CO2 in a 1:2 (CO2 to amino) stoichiometry (Rochelle 2009). However, in terms of amino-functionalized ILs, both mechanisms of 1:2 (Bates et al. 2002) and 1:1 (Goodrich et al. 2011b) stoichiometry have been reported. In this study, the MCF-x sorbents may follow 1:2 stoichiometry which the amino groups would react with CO2 to form carbamic acid, followed by further reacting with another amino groups to form carbamate and ammonium ion, as illustrated in the following equations.
For practical applications in CO2 capture, these solid sorbents were further evaluated in terms of moisture in flue gas and cyclic sorption–desorption performance during long-term operation. As shown in Fig. 4, the effects of water vapor contents in the flue gas on CO2 sorption of the MCF-x sorbents were examined. It was found that while the water vapor content increased from 0 to 2.5 %, the CO2 sorption capacities of MCF-Gly and MCF-Arg were decreased by 33 %. This observation may be associated with the hydrogen bond interaction between AAILs and water, which leads to the sorption competition between water and CO2 (Ludwig and Kragl 2007; Ren et al. 2012). However, CO2 sorption capacities were almost intact as the water vapor content further increased from 2.5 to 10.6 %. Among these sorbents, the MCF-Lys has the superior tolerance to the water vapor since only 8 % loss of sorption capacity was observed in the presence of 10.6 % water moisture.
The CO2 sorption–desorption cycles of MCF-x are displayed in Fig. 5. It can be seen that ca. 26 % loss in CO2 sorption capacities, which may result from the weight loss of the incorporated AAILs, were found for MCF-Gly and MCF-Arg after the tenth sorption–desorption cycle (i.e., total operation period of ca. 25 h) at 348 K under dry 15 % CO2 concentration. Previously, ILs have been reported to have some volatility even though they have ultralow vapor pressure (Jiang et al. 2013; Wang et al. 2013; Zhao et al. 2013). The AAILs attached to the porous silica surface were formed via weak physical forces and may leach out to the gas stream owing to the concentration difference. However, CO2 sorption capacity of MCF-Lys showed a surpassingly stable (2 % loss) in the tenth adsorption–desorption cycle, which can be probably attributed to the lower volatility and solidification posting the CO2 sorption. The results show that the MCF-Lys sorbents possess the higher CO2 sorption capacity, better resistance to water moisture, and excellent long-term stability among all the MCF-x sorbents.
4 Conclusions
Three different amine-functionalized ILs were prepared and incorporated into the mesocellular silica foam (MCF-x) as sorbents for post-combustion CO2 capture. The synthesized ILs and sorbents were thoroughly characterized by various spectroscopies and their CO2 sorption–desorption performance were also investigated. The MCF-x possesses the well-dispersed amine-functional groups of ILs on porous support which can enhance the CO2 sorption capacities and rates. In addition, the good thermal stability of ILs can assist the durability of sorbents. Among them, MCF-Lys has the highest CO2 sorption capacity of 1.38 mmolCO2/gsorbent and best long-term durability which may be a potential candidate for post-combustion CO2 capture.
References
Arellano, I. H., Madani, S. H., Huang, J. H., & Pendleton, P. (2016). Carbon dioxide adsorption by zinc-functionalized ionic liquid impregnated into bio-templated mesoporous silica beads. Chemical Engineering Journal, 283, 692–702.
Bara, J. E., Camper, D. E., Gin, D. L., & Noble, R. D. (2010). Room-temperature ionic liquids and composite materials: platform technologies for CO2 capture. Accounts of Chemical Research, 43, 152–159.
Bates, E. D., Mayton, R. D., Ntai, I., & Davis, J. H. (2002). CO2 capture by a task-specific ionic liquid. Journal of the American Chemical Society, 124, 926–927.
Bhatta, L. K. G., Subramanyam, S., Chengala, M. D., Olivera, S., & Venkatesh, K. (2015). Progress in hydrotalcite like compounds and metal-based oxides for CO2 capture: a review. Journal of Cleaner Production, 103, 171–196.
Blanchard, L. A., Hancu, D., Beckman, E. J., & Brennecke, J. F. (1999). Green processing using ionic liquids and CO2. Nature, 399, 28–29.
Cogswell, C. F., Jiang, H., Ramberger, J., Accetta, D., Willey, R. J., & Choi, S. (2015). Effect of pore structure on CO2 adsorption characteristics of aminopolymer impregnated MCM-36. Langmuir, 31, 4534–4541.
Fukumoto, K., Yoshizawa, M., & Ohno, H. (2005). Room temperature ionic liquids from 20 natural amino acids. Journal of the American Chemical Society, 127, 2398–2399.
Goodrich, B. F., de la Fuente, J. C., Gurkan, B. E., Lopez, Z. K., Price, E. A., Huang, Y., & Brennecke, J. F. (2011a). Effect of water and temperature on absorption of CO2 by amine-functionalized anion-tethered ionic liquids. Journal of Physical Chemistry B, 115, 9140–9150.
Goodrich, B. F., de la Fuente, J. C., Gurkan, B. E., Zadigian, D. J., Price, E. A., Huang, Y., & Brennecke, J. F. (2011b). Experimental measurements of amine-functionalized anion-tethered ionic liquids with carbon dioxide. Industrial and Engineering Chemistry Research, 50, 111–118.
Granados-Correa, F., Bonifacio-Martinez, J., Hernandez-Mendoza, H., & Bulbulian, S. (2015). CO2 Capture on metallic oxide powders prepared through chemical combustion and calcination methods. Water, Air, and Soil Pollution, 226, 281.
Gurkan, B. E., de la Fuente, J. C., Mindrup, E. M., Ficke, L. E., Goodrich, B. F., Price, E. A., Schneider, W. F., & Brennecke, J. F. (2010). Equimolar CO2 absorption by anion-functionalized ionic liquids. Journal of the American Chemical Society, 132, 2116–2117.
Gutowski, K. E., & Maginn, E. J. (2008). Amine-functionalized task-specific ionic liquids: a mechanistic explanation for the dramatic increase in viscosity upon complexation with CO2 from molecular simulation. Journal of the American Chemical Society, 130, 14690–14704.
Houshmand, A., Daud, W. M. A. W., Lee, M. G., & Shafeeyan, M. S. (2012). Carbon dioxide capture with amine-grafted activated carbon. Water, Air, and Soil Pollution, 223, 827–835.
Hyun, S. H., Song, J. K., Kwak, B. I., Kim, J. H., & Hong, S. A. (1999). Synthesis of ZSM-5 zeolite composite membranes for CO2 separation. Journal of Materials Science, 34, 3095–3103.
Jiang, B., Wang, X., Gray, M. L., Duan, Y., Luebke, D., & Li, B. (2013). Development of amino acid and amino acid-complex based solid sorbents for CO2 capture. Applied Energy, 109, 112–118.
Khan, N. A., Hasan, Z., & Jhung, S. H. (2014). Ionic liquids supported on metal-organic frameworks: remarkable adsorbents for adsorptive desulfurization. Chemical Engineering Journal, 20, 376–380.
Kishor, R., & Ghoshal, A. K. (2015). APTES grafted ordered mesoporous silica KIT-6 for CO2 adsorption. Chemical Engineering Journal, 262, 882–890.
Ko, Y. G., Shin, S. S., & Choi, U. S. (2011). Primary, secondary, and tertiary amines for CO2 capture: designing for mesoporous CO2 adsorbents. Journal of Colloid and Interface Science, 361, 594–602.
Lin, L. Y., & Bai, H. L. (2013). Facile and surfactant-free route to mesoporous silica-based adsorbents from TFT-LCD industrial waste powder for CO2 capture. Microporous and Mesoporous Materials, 170, 266–273.
Linneen, N., Pfeffer, R., & Lin, Y. S. (2013). CO2 capture using particulate silica aerogel immobilized with tetraethylenepentamine. Microporous and Mesoporous Materials, 176, 123–131.
Liu, S. H., Lin, Y. C., Chien, Y. C., & Hyu, H. R. (2011). Adsorption of CO2 from flue gas streams by a highly efficient and stable aminosilica adsorbent. Journal of the Air and Waste Management Association, 61, 226–233.
Liu, S. H., Hsiao, W. C., & Sie, W. H. (2012). Tetraethylenepentamine-modified mesoporous adsorbents for CO2 capture: effects of preparation methods. Adsorption, 18, 431–437.
Ludwig, R., & Kragl, U. (2007). Do we understand the volatility of ionic liquids? Angewandte Chemie International Edition, 46, 6582–6584.
Ma, J. J., Liu, Q. M., Chen, D. D., Zhou, Y., & Wen, S. (2014). Carbon dioxide adsorption using amine-functionalized mesocellular siliceous foams. Journal of Materials Science, 49, 7585–7596.
Raskar, R., Rane, V., & Gaikwad, A. (2013). The applications of lithium zirconium silicate at high temperature for the carbon dioxide sorption and conversion to syn-gas. Water, Air, and Soil Pollution, 224, 1569.
Ren, J., Wu, L. B., & Li, B. G. (2012). Preparation and CO2 sorption/desorption of N-(3-aminopropyl) aminoethyl tributylphosphonium amino acid salt ionic liquids supported into porous silica particles. Industrial and Engineering Chemistry Research, 51, 7901–7909.
Rochelle, G. T. (2009). Amine scrubbing for CO2 capture. Science, 325, 1652–1654.
Sanz, R., Calleja, G., Arencibia, A., & Sanz-Perez, E. S. (2012). Amino functionalized mesostructured SBA-15 silica for CO2 capture: exploring the relation between the adsorption capacity and the distribution of amino groups by TEM. Microporous and Mesoporous Materials, 158, 309–317.
Sanz-Perez, E. S., Olivares-Marin, M., Arencibia, A., Sanz, R., Calleja, G., & Maroto-Valer, M. M. (2013). CO2 adsorption performance of amino-functionalized SBA-15 under post-combustion conditions. International Journal of Greenhouse Gas Control, 17, 366–375.
Schmidt-Winkel, P., Lukens, W. W., Zhao, D. Y., Yang, P. D., Chmelka, B. F., & Stucky, G. D. (1999). Mesocellular siliceous foams with uniformly sized cells and windows. Journal of the American Chemical Society, 121, 254–255.
Shi, Q., Sun, H. X., Yang, R. X., Zhu, Z. Q., Liang, W. D., Tan, D. Z., Yang, B. P., Li, A., & Deng, W. Q. (2015). Synthesis of conjugated microporous polymers for gas storage and selective adsorption. Journal of Materials Science, 50, 6388–6394.
Ullah, R., Atilhan, M., Aparicio, S., Canlier, A., & Yavuz, C. T. (2015). Insights of CO2 adsorption performance of amine impregnated mesoporous silica (SBA-15) at wide range pressure and temperature conditions. International Journal of Greenhouse Gas Control, 43, 22–32.
Wang, C. M., Luo, X. Y., Luo, H. M., Jiang, D. E., Li, H. R., & Dai, S. (2011). Tuning the basicity of ionic liquids for equimolar CO2 capture. Angewandte Chemie International Edition, 50, 4918–4922.
Wang, X. F., Akhmedov, N. G., Duan, Y. H., Luebke, D., Hopkinson, D., & Li, B. Y. (2013). Amino acid-functionalized ionic liquid solid sorbents for post-combustion carbon capture. ACS Applied Materials & Interfaces, 5, 8670–8677.
Wang, J. Y., Huang, L., Yang, R. Y., Zhang, Z., Wu, J. W., Gao, Y. S., Wang, Q., O’Hare, D., & Zhong, Z. Y. (2014). Recent advances in solid sorbents for CO2 capture and new development trends. Energy and Environmental Science, 7, 3478–3518.
Wang, J. T., Wang, M., Li, W. C., Qiao, W. M., Long, D. H., & Ling, L. C. (2015). Application of polyethylenimine-impregnated solid adsorbents for direct capture of low-concentration CO2. AIChE Journal, 64, 972–980.
Yao, M. L., Wang, L., Hu, X., Hu, G. S., Luo, M. F., & Fan, M. H. (2015). Synthesis of nitrogen-doped carbon with three-dimensional mesostructures for CO2 capture. Journal of Materials Science, 50, 1221–1227.
Zhang, Y. Q., Zhang, S. J., Lu, X. M., Zhou, Q., Fan, W., & Zhang, X. P. (2009). Dual amino-functionalised phosphonium ionic liquids for CO2 capture. Chemistry-A European Journal, 15, 3003–3011.
Zhao, W. Y., Zhang, Z., Li, Z. S., & Cai, N. S. (2013). Investigation of thermal stability and continuous CO2 capture from flue gases with supported amine sorbent. Industrial and Engineering Chemistry Research, 52, 2084–2093.
Zhu, X., Fu, Y., Hu, G., Shen, Y., Dai, W., & Hu, X. (2012). CO2 capture with activated carbons prepared by petroleum coke and KOH at low pressure. Water, Air, and Soil Pollution, 224, 1387–1392.
Acknowledgments
Financial support of this work from the Ministry of Science and Technology of Taiwan (Grant No.: NSC 101-2628-E-151-003-MY3) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, SH., Sie, WH. CO2 Capture on Mesocellular Silica Foam Supported Amino Acid-Functionalized Ionic Liquids. Water Air Soil Pollut 227, 263 (2016). https://doi.org/10.1007/s11270-016-2925-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-016-2925-9