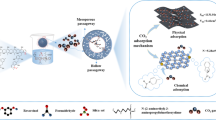
Abstract
The objective of this research was to develop a new CO2 sorbent, three-dimensional nitrogen-doped mesoporous carbon (KIT-6-CN). KIT-6-CN was synthesized by nano-replication using carbon tetrachloride and ethylenediamine as precursors, and KIT-6 with cubic Ia3d symmetry as a hard template. The new CO2 sorbent has a relatively high BET surface area of 587 m2/g, a high pore volume of 0.91 cm3/g, and the mesostructures with pore diameters centered at 1.7 and 6.3 nm. The transmission electron microscopy of the KIT-6-CN shows that after removal of silica by NaOH, KIT-6-CN possesses three-dimensional mesoporous structure. CO2 adsorption–desorption isotherms indicate that this nitrogen-doped material has relatively high CO2 capture capacities of 2.11 mmol/g at 25 °C and 3.09 mmol/g at 0 °C, which are superior to those of the pure carbon material (KIT-6-C) with analogous mesostructures. Cyclic CO2 sorption–desorption tests demonstrated the stability of the sorbent. Thus, the new sorbent can potentially be a good candidate for CO2 capture.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the recent decades, carbon dioxide is gradually recognized as the most important anthropogenic greenhouse gas (GHG) leading to global warming [1–8], so the removal of CO2 from industrial flue gas has become an important issue [2, 9]. Carbon materials were considered as potential candidates for CO2 capture because the weak interaction between CO2 and carbon requires lower-energy requirements with respect to other sorbents, such as zeolites.
In recent years, research people paid much attention to the ordered mesoporous carbon materials [10–14], because of their advantages of separation, adsorption, catalysis of large molecules, and energy storage. There are many ways to synthesize ordered mesoporous carbon materials [13–17]. For example, the soft-template method as a facile route has been applied to fabricate mesoporous carbon very recently. In another way, Vinu et al. [18] successfully reported the preparation of mesoporous carbon nitride (C3N4) with an uniform pore-size distribution using SBA-15 as a template. In another study, Deng et al. synthesized mesoporous carbon nitride using SBA-15 as hard template for CO2 capture [19]. It is popular and viable to synthesize porous carbons adopting the ordered mesoporous silicas as the hard templates, since the pore structure and the textural parameters of mesoporous silica materials can be easily controlled by many methods. It is well known that three-dimensional (3D) pore channels are more advantageous for mass diffusion than 2D pores. Unfortunately, the synthesis of 3D nitrogen-doped mesoporous carbon has rarely been reported.
In this work, 3D-ordered nitrogen-doped mesoporous carbon was synthesized using 3D mesoporous silica KIT-6 as the hard template and ethylenediamine (EDA) as the nitrogen source. Compared with undoped mesoporous carbon, the nitrogen-doped mesoporous carbon shows better CO2 adsorption performance.
Experimental
Chemicals
All chemicals were used as received from the chemical vendors without further purification. Triblock copolymer poly (ethylene oxide)-b-poly (propylene oxide)-b-poly (ethylene oxide) (Pluronic P123, EO20-PO70-EO20, Mw = 5800 g/mol) and EDA were purchased from Sigma-Aldrich. Butanol, carbon tetrachloride (CTC), tetraethyl orthosilicate (TEOS) and sucrose were obtained from Quzhou Reagent. Pure N2 and 10 % CO2/N2 gases were obtained from Jinhua Gas. Distilled water was used in all experiments.
Synthesis
Preparation of the KIT-6 silica template
Mesoporous silica KIT-6 was synthesized following the procedures available in the literature by Ryong Ryoo [20]. In a typical synthesis batch, a homogeneous solution was obtained by dissolving 6.0 g of P123 in 217 g of distilled water and 11.8 g of concentrated HCl solution (35 wt%). To this solution, 6 g of butanol was added under stirring at 35 °C for an hour, followed by slow addition of 12.9 g of TEOS. The mixture was kept under magnetic stirring for 24 h at 35 °C, and subsequently heated to 100 °C for 24 h under static conditions in a teflon-lined autoclave. The solid product was filtered and washed to neutral with extensive deionized water, and then the sample was dried at room temperature overnight. The final material was obtained by calcinations at 550 °C (heating rate 5 °C/min) for 5 h to remove the P123 template in air.
Synthesis of nitrogen-doped mesoporous carbon material
The nitrogen-doped mesoporous carbon material was synthesized according to the route reported by Li [21] and Deng [19]. The difference is that 3D-ordered mesoporous silica KIT-6 rather was used as a hard template in this study with the aim to synthesize mesoporous carbon with 3D structure. In the typical procedure, 0.5 g of the silica KIT-6 template was added to a mixed solution containing 1.1 mL EDA and 1.3 g of CTC at room temperature. Then, the above solution was heated at 90 °C for 6 h with stirring under reflux to induce polymerization of the precursor. The resulting dark-brown mixture was pre-pyrolyzed at 400 °C for 2 h in a helium atmosphere. The pyrolyzed sample was refilled with 0.75 mL EDA and 0.8 mL of CTC at 90 °C. After pyrolysis at 600 °C for 5 h in helium, the final mesoporous carbon product was obtained by using 2 mol/L NaOH aqueous solution to remove the silica template from the carbon–silica composite.
Synthesis of mesoporous carbon materials
For comparison, the mesoporous carbon materials were synthesized mainly according to the procedures available in the literature [10]. 1.25 g sucrose as the carbon source was dissolved in mixture solution containing 5 mL distilled water and 80 uL H2SO4, and then 1 g KIT-6 was added into the formed solution. The mixed solution was heated to 100 °C for 6 h in drying oven, after which period, the temperature was raised 160 °C for 6 h. The impregnation and drying procedures were repeated twice using 66 % sucrose. After carbonization, the sample was heated to 900 °C for 7 h under helium flow. The final mesoporous carbon product was obtained using 2 mol/L of NaOH aqueous solution to remove the silica template.
Characterizations of KIT-6, KIT-6-NC, and KIT-6-C
The X-ray diffraction patterns was recorded on a Philips PW3040/60 automatic powder diffractometer using CuKα radiation (λ = 0.1542 nm) at 40 kV and 40 mA. The scans were recorded in the 2θ values between 0° and 5° with a step width of 0.014°.
The transmission electron microscopy (TEM) images were recorded on a JEOL-2100F TEM working at 200 kV. The samples were diluted in ethanol and sonicated for 10 min. The ethanol slurry was then dripped onto a Cu grid covered with a thin film of carbon.
Surface areas of the carbon materials were calculated using the multipoint Brunauer–Emmett–Teller (BET) equation from nitrogen adsorption–desorption isotherms measured at liquid nitrogen temperature (Quantachrome Autosorb-1). The pore-size distribution curves were obtained from the desorption branches using the non-local density functional theory (NLDFT) method, and the total pore volume values were estimated from the adsorbed amount at a relative pressure P/P0 of 0.99.
X-ray photoelectron spectra (XPS) were recorded on an ESCALAB 250Xi spectrometer with an Al anode Ka radiation (1486.6 eV). Carbonaceous C1 s line (284.6 eV) was used as a reference to calibrate the binding energies.
The CO2 adsorption–desorption isotherms were measured using the Quantachrome Autosorb-1 equipment at 0 and 25 °C. Prior to each adsorption experiment, the sample was degassed for 5 h at 200 °C to remove the guest molecules from the pores.
Results and discussion
Structural characterization
Figure 1 shows the XRD patterns of KIT-6, mesoporous carbon (KIT-6-C), and nitrogen-doped mesoporous carbon (KIT-6-CN) in the small angle region. It can be seen that the template has three obvious diffraction peaks, indicating that KIT-6 exhibits ordered mesoporous structure. The intensity of diffraction peaks for KIT-6-C and KIT-6-CN became weaker. For KIT-6-CN, the diffraction peaks shifted to slightly higher 2θ values, indicating that KIT-6-CN has smaller pore sizes than KIT-6. In comparison, KIT-6-C synthesized using sucrose as carbon source has larger pore sizes than those of KIT-6.
The nitrogen adsorption–desorption isotherms of the KIT-6, KIT-6-C, and KIT-6-CN, are given in Fig. 2. It is clear that the isotherms of the silica KIT-6 show typical type IV curves with a steep H 1 hysteresis loop within the relative pressure (P/P 0) range of ca. 0.63–0.82. Its BET specific surface area and pore volume are as large with values of 566 m2/g and 0.77 cm3/g, respectively. These results are consistent with those reported by Ryoo et al. [20, 22], indicating that the mesoporous silica template was successfully synthesized. From the nitrogen isotherms of KIT-6-C and KIT-6-CN shown in Fig. 2, it can be deduced that the two isotherms also exhibit type IV curves and H1 hysteresis. KIT-6-CN shows a different hysteresis loop compared to those of KIT-6 and KIT-6-C, which may be due to two possible reasons. First, the processors for KIT-6-C and KIT-6-CN are different. For KIT-6-C, sucrose was used as processor, while EDA were used as processors for KIT-6-CN. Sucrose and EDA may show different properties during the carbonization process. Second, the preparation methods for KIT-6-C and KIT-6-CN are different. For KIT-6-C, the sucrose solution and KIT-6 template were treated in oven at 100 °C for 6 h. While EDA and KIT-6 were heated at 90 °C for 6 h with stirring under reflux to induce polymerization of the precursor. These two reasons may lead to different pore structures. The surface area and total pore volume of KIT-C are 740 m2/g and 0.88 cm3/g, and the corresponding values for KIT-6-CN are 587 m2/g and 0.91 cm3/g, respectively. The pore-size distribution is deduced using NLDFT method from adsorption isotherms, which allows for an accurate pore-size analysis over the complete range of micro- and mesopores. As shown in Fig. 2b, both KIT-6-C and KIT-6-CN possess pore-sizes of both microporous and mesoporous ranges. The micropores may result from the removal of the silica walls [21], and the mesopores may be due to the corresponding ordered structure of KIT-6. The micropores of both samples are centered around 1.7 nm, while their mesoporous sizes are 8.7 and 6.3 nm, respectively.
The structure and morphology of KIT-6-C and KIT-6-CN are also characterized by TEM. As shown in Fig. 3, both samples exhibit well-ordered mesopores with 3D porous network, although intensities of XRD diffraction peaks of carbon materials are weaker than that of the KIT-6 template as shown in Fig. 1. Thus, it can be concluded that both the nitrogen-doped and undoped mesoporous carbons with 3D porous network have been successfully synthesized using the hard template route.
Table 1 also gives the results of elemental analysis of KIT-6-CN. The contents of carbon, nitrogen, hydrogen, and silicon of KIT-6-CN are 76.5, 15.9, 4.2, and 1.6 wt%, respectively, which indicate that nitrogen has been effectively introduced into the mesoporous carbon. It should be noted that in previous studies, Vinu [18] and Li [21] et al. claimed that carbon nitrides (C3N4) were synthesized using EDA and CTC as carbon and nitrogen sources. However, our results indicate that the carbon content of the obtained product is much higher than that of C3N4, while the nitrogen content is much lower than that of C3N4. Furthermore, the synthesized materials are black in color, while it is known that C3N4 is yellow in color. Hence, the synthesized materials should be named as nitrogen-doped mesoporous carbon (KIT-6-CN) rather than C3N4 [23].
To investigate the chemical state of the doped nitrogen atoms, the KIT-6-C and KIT-6-CN samples were characterized by X-ray photoelectron spectroscopy (XPS). For KIT-6-C, no nitrogen signal was observed (the spectrum is not shown here), since the carbon precursor sucrose does not contain nitrogen content. For KIT-6-CN, C, N, and O signals were observed as shown in Fig. 4a. The O signal may be from the trace SiO2 and the contaminant on sample surface. Figure 4b shows the XPS spectrum in N1 s region which can be deconvoluted into three peaks centered at 398.1, 399.8, and 400.4 eV. These three peaks are corresponding to different types of nitrogen species, i.e., pyridinic-type nitrogen, pyrrolic-type nitrogen, and quaternary nitrogen, respectively. The strong peaks at 398.1 and 399.8 eV indicate that most nitrogen atoms are pyridinic-type nitrogen and pyrrolic-type nitrogen species. It has been reported that pyrrolic-type nitrogen species favor the CO2 capture performance of carbon [9, 24]. Therefore, KIT-6-CN is expected to possess better CO2 capture performance than the KIT-6-C.
The FTIR spectra of KIT-6-C and KIT-6-CN are shown in Fig. 5. For KIT-6-C, the band at 1560 cm−1 is related to C–C stretching of aromatic ring structures, and the band at 3430 cm−1 is attributed to O–H stretching vibration of absorbed water molecules. For KIT-6-CN, a strong band at 1618 cm−1 can be ascribed to N–H deformation vibration, suggesting that the KIT-6-CN is mainly composed of pyridinic-type and pyrrolic-type nitrogen species.
CO2 adsorption test
The CO2 capture performances of the obtained carbon materials were also tested. The CO2 adsorption–desorption isotherms of KIT-6-C and the KIT-6-CN at 25 and 0 °C are shown in Fig. 6. Apparently, the CO2 uptake of KIT-6-CN is higher than that of KIT-6-C almost in all pressure ranges at 25 °C. It can reach 2.11 mmol/g at ~120 kPa, while KIT-6-C can only adsorb 1.62 mmol/g CO2 at the same pressure. When the temperature was decreased to 0 °C, the CO2 capture performances of both samples were greatly improved. For example, the CO2 uptake of the KIT-6-CN at 0 °C can reach 3.09 mmol/g which is 1.46 times of that at 25 °C. This indicates that the adsorption of CO2 on carbon is mainly attributed to physical adsorption, and that lower temperature can improve the CO2 capture performance. As is known to all, the interaction forces between carbon dioxide and sorbent surface are the weak van der Waals forces during the physisorption. When the temperature becomes higher, the thermal motion of the CO2 molecules is accelerated; therefore, it is detrimental to the adsorption. While it is also well known that gas uptake by physisorption is related to the porous texture of sorbents, as shown in Table 2, however, KIT-6-CN shows a similar porous texture as that of KIT-6-C. Then the superior CO2 capture capacities of KIT-6-CN may be due to the nitrogen-doped species, the existence of which has been proven by XPS and FTIR analyses (Figs. 4, 5). It has been known that the nitrogen-doped atoms can change the chemical state of carbon materials and then improve the CO2 capture performance of carbon materials [9].
Recently, Xu et al. reported some nitrogen-doped porous carbons synthesized using metal-organic frameworks (MOFs) [25, 26] as precursors. These carbons with high surface areas show ultrahigh CO2 capture capacities which can reach 5.7 mmol/g at 277 K, which can be attributed to the dominant presence of micropores. For KIT-6-C and KIT-6-CN, the mesopores are dominant as shown in Fig. 2. This explains why they show lower CO2 capture capacities.
The reversible nature of CO2 capture performance on KIT-6-C and KIT-6-CN at 0 °C was also tested for five cycles. As shown in Fig. 7, the adsorption capacities for the five cycles did not show noticeable changes, indicating that both KIT-6-C and KIT-6-CN have good recyclability. Sethia et al. [27] also demonstrated that nitrogen-doped carbons show remarkable stability for CO2 capture. This agrees well with our results. The good recyclability will improve the feasibility of these carbon materials for applications in CO2 capture.
Conclusions
In summary, mesoporous nitrogen-doped carbon material (KIT-6-CN) has been successfully synthesized using Ia3d silica KIT-6 as a hard template, and CTC and EDA as precursors via a pyrolyzing process. KIT-6-CN has a relatively high BET surface area of 587 m2/g and a pore volume of 0.91 cm3/g. The CO2 adsorption–desorption isotherms show that the adsorption capacities of KIT-6-CN at 25 and 0 °C are 2.11 and 3.09 mmol/g, respectively, which are superior to those of the pure carbon materials with analogous mesostructures. This can be attributed to the doped alkaline nitrogen group into the carbon framework.
References
Kamarudin KSN, Alias N (2013) Adsorption performance of MCM-41 impregnated with amine for CO2 removal. Fuel Process Technol 106:332–337
Song C (2006) Global challenges and strategies for control, conversion and utilization of CO2 for sustainable development involving energy, catalysis, adsorption and chemical processing. Catal Today 115:2–32
Yu KMK, Curcic I, Gabriel J, Tsang SCE (2008) Recent advances in CO2 capture and utilization. Chemsuschem 1:893–899
Cui S, Cheng WW, Shen XD, Fan MH, Russell A, Wu ZW, Yi XB (2011) Mesoporous amine-modified SiO2 aerogel: a potential CO2 sorbent. Energy Environ Sci 4:2070–2074
Yao M, Dong Y, Feng X, Hu X, Jia A, Xie G, Hu GS, Lu J, Luo M, Fan M (2014) The effect of post-processing conditions on aminosilane functionalization of mesocellular silica foam for post-combustion CO2 capture. Fuel 123:66–72
Yao M, Dong Y, Hu X, Feng X, Jia A, Xie G, Hu GS, Lu J, Luo M, Fan M (2013) Tetraethylenepentamine-modified silica nanotubes for low-temperature CO2 capture. Energy Fuels 27:7673–7680
Ma L, Bai R, Hu GS, Chen R, Hu X, Dai W, Dacosta HFM, Fan M (2013) Capturing CO2 with amine-impregnated titanium oxides. Energy Fuels 27:5433–5439
Feng XX, Hu GS, Hu X, Xie GQ, Xie YL, Lu JQ, Luo MF (2013) Tetraethylenepentamine-modified siliceous mesocellular foam (MCF) for CO2 capture. Ind Eng Chem Res 52:4221–4228
Liu L, Deng QF, Hou XX, Yuan ZY (2012) User-friendly synthesis of nitrogen-containing polymer and microporous carbon spheres for efficient CO2 capture. J Mater Chem 22:15540–15548
An HB, Yu MJ, Kim JM, Jin M, Jeon JK, Park SH, Kim SS, Park YK (2012) Indoor formaldehyde removal over CMK-3. Nanoscale Res Lett 7:7–12
Xia Y, Mokaya R (2004) Synthesis of ordered mesoporous carbon and nitrogen-doped carbon materials with graphitic pore walls via a simple chemical vapor deposition method. Adv Mater 16:1553–1558
Fang B, Kim JH, Kim M-S, Yu J-S (2013) Hierarchical nanostructured carbons with meso macroporosity: design, characterization, and applications. Acc Chem Res 46:1397–1406
Xia Y, Yang Z, Mokaya R (2010) Templated nanoscale porous carbons. Nanoscale 2:639–659
Sun J-K, Xu Q (2014) Functional materials derived from open framework templates/precursors: synthesis and applications. Energy Environ Sci 7:2071–2100
Zelenak V, Badanicova M, Halamova D, Cejka J, Zukal A, Murafa N, Goerigk G (2008) Amine-modified ordered mesoporous silica: effect of pore size on carbon dioxide capture. Chem Eng J 144:336–342
Zelenak V, Halamova D, Gaberova L, Bloch E, Llewellyn P (2008) Amine-modified SBA-12 mesoporous silica for carbon dioxide capture: effect of amine basicity on sorption properties. Microporous Mesoporous Mater 116:358–364
Zhao C, Chen X, Zhao C (2009) CO2 absorption using dry potassium-based sorbents with different supports. Energy Fuels 23:4683–4687
Vinu A (2008) Two-dimensional hexagonally-ordered mesoporous carbon nitrides with tunable pore diameter, surface area and nitrogen content. Adv Funct Mater 18:816–827
Deng Q-F, Liu L, Lin X-Z, Du G, Liu Y, Yuan Z-Y (2012) Synthesis and CO2 capture properties of mesoporous carbon nitride materials. Chem Eng J 203:63–70
Kleitz F, Choi SH, Ryoo R (2003) Cubic Ia3d large mesoporous silica: synthesis and replication to platinum nanowires, carbon nanorods and carbon nanotubes. Chem Commun 7:2136–2137
Li Q, Yang JP, Feng D, Wu ZX, Wu QL, Park SS, Ha CS, Zhao DY (2010) Facile synthesis of porous carbon nitride spheres with hierarchical three-dimensional mesostructures for CO2 capture. Nano Res 3:632–642
Choi DH, Ryoo R (2010) Template synthesis of ordered mesoporous organic polymeric materials using hydrophobic silylated KIT-6 mesoporous silica. J Mater Chem 20:5544–5550
Thrower PA (2004) What’s in a name? Carbon 42:2367–2368
Liu L, Deng QF, Ma TY, Lin XZ, Hou XX, Liu YP, Yuan ZY (2011) Ordered mesoporous carbons: citric acid-catalyzed synthesis, nitrogen doping and CO2 capture. J Mater Chem 21:16001–16009
Aijaz A, Fujiwara N, Xu Q (2014) From metal organic framework to nitrogen-decorated nanoporous carbons: high CO2 uptake and efficient catalytic oxygen reduction. J Am Chem Soc 136:6790–6793
Aijaz A, Akita T, Yang H, Xu Q (2014) From ionic-liquid@metal-organic framework composites to heteroatom-decorated large-surface area carbons: superior CO2 and H2 uptake. Chem Commun 50:6498–6501
Sethia G, Sayari A (2014) Nitrogen-doped carbons: remarkably stable materials for CO2 capture. Energy & Fuels 28:2727–2731
Acknowledgement
We gratefully acknowledge the financial support to this work from the Science and Technology Department of Zhejiang Province (2013C31049), and the National Natural Science Foundation of China (21203167, 21106136), as well as from Wyoming Clean Coal Program.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yao, M., Wang, L., Hu, X. et al. Synthesis of nitrogen-doped carbon with three-dimensional mesostructures for CO2 capture. J Mater Sci 50, 1221–1227 (2015). https://doi.org/10.1007/s10853-014-8678-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-014-8678-1