Abstract
Invasive earthworms perturb natural forest ecosystems that initially developed without them, mainly by consuming the forest floor (an organic rich surficial soil horizon) and by mixing the upper parts of the soil. The fate of mercury (Hg) formerly contained in the forest floor is largely unknown. We used two mass balance approaches (simple mass balance and geochemical mass balance) to quantitatively assess the impact of exotic earthworms on Hg cycling in forest soils and the fate of Hg formerly contained in the forest floor. Two sets of soils, collected in 2009 and 2014 from an earthworm invasion transect in north central Minnesota, were analyzed in this study. We observed a substantial loss of Hg from the soil system following earthworm invasion. Mass balance calculations showed that 35 to 65 % of the forest floor Hg was retained in the underlying mineral soil with the remainder unaccounted. The most likely explanation for the loss of forest floor Hg from the system is microbially induced volatilization of Hg by soil microbes and possibly by earthworm gut microbes during consumption of the forest floor. Our calculations suggest that exotic earthworms can substantially perturb mercury cycling in the topsoil. Forest floor Hg may accumulate within worm biomass; however, this process can account for only a small fraction (1 to 3 %) of the lost Hg. Additionally, we calculated the mass balance of organic carbon (C) due to Hg’s high affinity for organic matter and found that a portion of the organic carbon also appears to be lost from the soil system.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Mercury (Hg) is a global pollutant that is highly volatile (Hg0), reactive (Hg2+), and toxic. Mercury contamination of the environment is widespread due to its long residence time in the atmosphere (Slemr et al. 1985, Mason et al. 1994), contaminating even the most remote and pristine areas. Atmospheric deposition of Hg, in the form of wet and dry deposition (St. Louis et al. 2001), is the main source of Hg to most landscapes. Mercury deposition pathways in forested ecosystems not only include open air precipitation but also include throughfall, stemflow, and litterfall, which in combination can increase total Hg deposition two- to threefold above deposition in open, unforested areas (Kolka et al. 2001). In addition, gaseous Hg0 is also taken up directly by mineral soil horizons, but the overall quantity is relatively minor compared to other mass fluxes (Obrist et al. 2014). Mercury deposited in surficial soil horizons is predominantly in ionic forms (Hg2+, Hg2 2+) and is strongly bound to various functional groups, but especially by reduced sulfur groups, on organic matter (Andersson 1979; Schuster 1991; Skyllberg et al. 2003; Gabriel and Williamson 2004).
The forest floor is a surficial organic (OFootnote 1) horizon formed from partially decomposed leaf and plant litter (Binkley and Fisher 2012). Forest floor horizons typically range from 2 to >20 cm in thickness and, unlike most other O horizons, do not require saturated conditions to form, but can develop in well-drained, unsaturated uplands. The forest floor has a very low mineral content due to a lack of mixing between it and underlying mineral soil horizons. The forest floor generally overlies a thin A horizon over an E horizon or, where the A horizon is absent, directly overlies the E horizon. Forest floor horizons are usually absent where earthworms, ants, or other soil mixing organisms are abundant (Alban and Berry 1994) because these organisms partially consume the forest floor and mix it with the underlying mineral soil material, forming surficial A horizons. Soil organic matter does not readily leach into the lower horizons unless the pH is very low (<4.5), as in Spodosols, or high (>8.5), as in highly alkaline soils.
Due to Hg’s close association with organic matter, its concentrations in forest soils are generally highest in the forest floor (100–250 ng Hg g−1), lower in the A horizon (30–100 ng Hg g−1), and much lower (typically 7–30 ng Hg g−1) in subsurface horizons (Nater and Grigal 1992). The Hg load (as expressed on a mass per unit area per depth), however, is often much higher in the A and subsurface horizons than in the forest floor due to the lower bulk density of the forest floor (0.1–0.4 g cm−3) compared to mineral soil (0.8–1.5 g cm−3) horizons.
Forest floor organic horizons were very common in forested landscapes in the Great Lakes States region and other temperate forests due to the lack of earthworms following the last glaciation (Alban and Berry 1994; Reynolds 1994). Over the last century, however, large areas of temperate region forests have become invaded by exotic earthworms (Bohlen 2004a, Hale et al. 2005a, Eisenhauer et al. 2007). Human activities, such as agriculture, logging, and fishing, are the most common vectors for exotic earthworm invasion of forests (Alban and Berry 1994, Gates 1982).
Earthworms ingest soil organic matter, digest a portion of it, and excrete the remainder, either below the surface or in the form of earthworm casts at the surface. Thus, they mix and homogenize the soil, mixing partially decomposed organic matter with mineral soil materials. This process leads to an increase in the thickness of the A horizon or the development of one where none previously existed (Hale et al. 2005b). This bioturbation perturbs existing elemental cycles and can potentially change the elemental composition of the soil (Hale et al. 2005b). The effects of earthworm consumption of the forest floor on the fate and biogeochemical cycling of Hg contained therein are largely unknown.
The objectives of this study are to assess the impacts of exotic earthworms on the geochemical cycling of Hg in surface soil and determine the fate of Hg originally stored in the forest floor pool. We hypothesize that, following earthworm invasion, nearly all of the Hg originally contained in the forest floor will be transferred to the underlying A horizon. Our study was conducted on an earthworm invasion transect that was established in 1997 by Hale et al. (2005a, b) and has been studied extensively since then (Hale et al. 2005a, b, 2006; Resner et al. 2011, 2014; Lyttle et al. 2011, 2014).
We utilized two mass balance approaches to quantitatively assess the degree to which soil Hg cycling is perturbed by invasive earthworms. The first approach is based on simple calculations of Hg pools in invaded and uninvaded areas and the second approach utilizes geochemical normalization. The geochemical normalization method has been used reliably to assess major elemental mass changes in the soil for geochemical processes such as weathering and erosion (Brimhall and Dietrich 1987; Merritts et al. 1992; Riebe et al. 2003; Oh and Richter 2005). Elemental mass losses and gains can be detected more reliably when the element of interest is normalized to a biogeochemically immobile element such as Zr, Ti, or Nb. The method has also been used to study the geochemistry of numerous metals such as Pb, Cu, Zn, Ni, Co, and Cr in forest soils (Jersak et al. 1997) highlighting the possible benefit of using this method to study Hg. Resner et al. (2014) recently used this method to assess the effects of bioturbation on a number of biogeochemical cycles. To our knowledge, however, this method has not been used to study the impacts of bioturbation on Hg cycling even though organic matter is considered an important sorbent of metals in surficial soils (Gustafsson et al. 2003). Bioturbators can, therefore, impact metal cycling in surface soils through the manipulation of organic matter.
2 Methods
2.1 Field Site
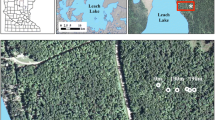
The field site is located in a productive upland hardwood forest dominated by sugar maple (Acer saccharum Marsh.) in north central Minnesota near Leech Lake (Fig. 1). The transect is located on the east side of Sucker Bay Road which was constructed in the 1960s and is the most likely source for exotic earthworm invasion in this forest. The most invaded areas are located closest to the road with the leading edge of the invasion advancing about 5 m per year (Resner 2013). This earthworm invasion transect has been extensively studied to determine the effects of a relatively recent earthworm invasion on elemental biogeochemical cycles (Hale et al. 2005a, b, 2006; Resner et al. 2011, 2014; Lyttle et al. 2011, 2014). This previous body of work enabled us to better understand how earthworms affect the cycles of major elements (K, Ca, Mg, Na, P, C, and Fe) in the soil (Resner 2013) while also streamlining our sampling efforts.
The soil on site is identified as the Warba series which is classified as a fine-loamy, mixed, superactive, frigid Haplic Glossudalf (National Cooperative Soil Survey 2006, USDA). Warba soils formed in loamy calcareous glacial till on moraines and typically have a thin (<0.5 m) loess cap. The Warba soil is extensively mapped throughout north central Minnesota and is moderately well- to well-drained. For a more detailed description of the site and soil refer to Resner et al. (2014). The climate is temperate and humid continental with 66 cm mean annual precipitation. Mean temperatures range from −15 °C in January to 20 °C in July with snow cover from late November to early April (Hale et al. 2005a). Earthworm ecological groups and species were identified by both Hale et al. (2005a) and Resner (2013) and include epigeic, epi-endogeic, endogeic, and anecic species.
2.2 Sampling and Sample Processing
Two sets of soil samples were analyzed in this study. The first set was collected by Resner (2013) in 2009 as part of her master’s thesis research at the University of Minnesota. Briefly, Resner (2013) collected soil samples from 6 soil pits located at 0, 50, 100, 150, 160, and 190 m along the transect with the 0 m pit located closest to the road (Fig. 1). Soils were sampled in 2–5 cm increments to a depth of 25 cm and 10 cm increments thereafter to a depth of 1 m or greater. We analyzed a subsample of each soil sample for total Hg content after the soil had been oven-dried, ground, and homogenized. For this study, we were only interested in mineral soil to a depth of 24 cm for the purpose of comparison to our second set of soil.
We collected a second set of samples in summer of 2014 with 15 samples collected in the invaded area near the start of the transect and 12 samples in an uninvaded area approximately 100 m beyond the 190 m transect distance Resner (2013) collected (Fig. 1). Where present, the forest floor was sampled by cutting a 10 cm diameter circle (area = 78.54 cm2) into the forest floor with a sharp knife and then carefully separating the forest floor from the underlying mineral horizons. Once the forest floor sample had been removed, we collected mineral soil samples in the center of the same location with a 1.8-cm-diameter push probe to a depth of 24 cm from the top of the mineral soil horizon. All samples extended well into the E horizon but did not reach the glacial till underlying the loess cap, which occurs at a depth of 40–50 cm (Resner 2013). The mineral soil sample was then separated into A and E horizons based on visual morphology of the distinct horizon boundary. All samples were stored in zipper-type polyethylene bags in a cooler for transport to the University of Minnesota.
Mineral soil samples were dried at 105 °C for 48 h, ground with a mortar and pestle, passed through a 2-mm brass sieve to remove coarse fragments, and stored in small plastic zipper bags until analysis. Forest floor samples were dried at 95 °C for 48 h and ground in a food blender to homogenize the samples. Bulk density was determined for all samples by computing the volume of the sample from core diameter and length of each sample.
There is an important distinction between the two sets of soil concerning their sampling design. Soil pits sampled in 2009 were sampled along an invasion transect with varying degrees of earthworm invasion. The 2014 samples were collected in only two areas—an invaded area approximately 10 to 20 m prior to (i.e., the −10 or −20 location) the 0 m pit from 2009 sampling and an uninvaded area 100 m beyond the least invaded pit from 2009 (Fig. 1). For the purpose of comparing the 2009 soil to the 2014 soil, the pits from 2009 were split into the two groups—the least invaded group and the most invaded group. Least invaded soils include pits 190, 160, 150, and 100 as all of these pits still had some forest floor present above the mineral soil. Pits located at 50 and 0 m were grouped together to represent the most invaded soils and had no forest floor present.
2.3 Laboratory Analysis
For mineral soil samples, approximately 1 g of sample was digested in 10 ml of concentrated nitric acid (HNO3) at 70 °C overnight and subsequently diluted by a factor of 5 with Barnstead E-Pure 18 milli-Ohm distilled and deionized (DDI) water prior to analysis. For forest floor samples, 0.5–1.0 g of sample was digested in 20 ml of concentrated HNO3 at 70 °C overnight and subsequently diluted by a factor of 10 with DDI water. All mineral soil and forest floor samples were analyzed for total mercury (THg) by the double gold amalgamation method (Bloom and Crecelius 1983) using a Brooks Rand Model III cold vapor atomic fluorescence spectroscopy (CVAFS) analyzer via US EPA method 1631. Bubbler blanks and standard curve were completed at the start of each analytical run. A matrix standard curve with eight Hg concentrations (1.6, 0.8, 0.4, 0.2, 0, 0.2, 0.4, and 0.8 ng mL−1) was performed; R 2 higher than 0.995 had to be achieved before sample analysis could proceed. Quality assurance and quality control (QA/QC) measures included analytical blanks (n = 21), digestion blanks (n = 12), analytical standards (n = 22), sample duplicates (n = 29), digestion duplicates (n = 15), matrix spikes (n = 6) and use of National Institute of Standards and Testing (NIST) reference material 2976, freeze-dried mussel tissue (n = 7). QA/QC mean recovery percentages were excellent ranging from 101.3 to 107.5 % with % relative standard deviations of 2.8–5.8 % (Supplementary Information, Table 1).
For samples collected in 2014, 0.5 g of mineral soil sample and 0.3 g of forest floor sample were also analyzed for soil organic carbon by a VarioMax CN Elemental Analyzer. Recoveries of sample duplicates were well within acceptable values (97–116 %). Loss on ignition (LOI) analysis was performed on 2009 samples to determine organic matter content (%OM) (Resner 2013). We determined %C rather than %LOI for the 2014 soil samples and therefore used the conventional Van Bemmelen factor of 1.724, which assumes that 58 % of organic matter is organic carbon (Sutherland 1998), to calculate %LOI. Zirconium (Zr) concentrations were determined for 2009 samples with a lithium borate fusion and subsequent inductively coupled plasma-mass spectrometry (ICP-MS) analysis at Australian Laboratory Services (ALS) Chemex (Resner 2013).
2.4 Simple Mass Balance Calculations
Mercury and organic carbon pools (mass of Hg or C in a known volume of soil) were calculated to assess the mass of Hg and C transferred from the forest floor into the mineral soil following the invasion of soil mixing earthworms. Pools were determined in a 1 m2 area of soil and forest floor multiplied by the depth of each horizon. The total pool of each mineral soil profile was calculated to a depth of 24 cm which is the total mass of Hg or C in 240,000 cm3 or 0.24 m3 of mineral soil. Forest floor pools were calculated based on the concentration and mass of the forest floor samples apportioned to a 1 m2 area times the depth of the forest floor.
2.5 Geochemical Mass Balance Calculations
We used zirconium (Zr) as an immobile index element in our geochemical mass balance calculations because it is well conserved throughout the O, A, and E horizons, similar to Resner et al. (2014). We focused on the calculations of absolute mass losses or gains of Hg and C, our elements of interest (δ j ) (Eq. 1):
Where C is the concentration [ng g−1] of the element of interest (j) and index element (i) in the A horizon (w) relative to the underlying loess parent material (E horizon) (p), ρ w is the soil bulk density (g/cm3), and Δh is the thickness (cm) of the A horizon. The loess of the E horizon was used as parent material in our geochemical mass balance calculations because the A horizon was shown to be a mixture of organic matter and loess (Resner et al. 2014). Earthworm mixing was not observed to extend beyond the upper few centimeters of the E horizon.
Geochemical reference elements are commonly used to determine mass losses (related to intense chemical weathering) or gains (usually illuviation) in soils or soil horizons (Riebe et al. 2003; Oh and Richter 2005). The soils at this site, however, are all the same age and have not been subjected to significant weathering or erosional losses. Some of the surficial horizons have, however, experienced additions in the form of organic matter. Consequently, the Zr concentration in the mineral fraction is relatively uniform across the transect, but because of the dilution with organic matter, the Zr concentration in the soil is a function of the Zr concentration in the mineral fraction within the soil, that is, it is inversely proportional to the OM fraction in the soil as shown by the strong correlation (R 2 = 0.80, data now shown) between Zr and %OM observed in the 2009 data (Resner 2013). Since Zr concentrations are well conserved in the soil (Resner et al. 2014) and the soils have undergone negligible alteration in the intervening 5 years, the Zr concentrations in the 2014 soil samples can be estimated from regression relationships between %LOI and Zr from the 2009 soil data (Resner 2013). The two end member soils (pit 190 and pit 0) were used to graph the relationship of %LOI to Zr and the resulting equations were used to calculate Zr values for 2014 soil (uninvaded soil: y = −457.7x + 377.31, R 2 = 0.99, P < 0.01 and invaded soil: y = −964.32x + 377.25, R 2 = 0.94, P < 0.01).
In addition to calculating absolute mass changes of Hg in the A horizons of 2009 soil, fractional mass changes (τ) of Hg were also calculated for these soil samples (Eq. 2). The 2009 soil samples were collected in small increments (2–5 cm increments) whereas the 2014 soil samples were simply separated by horizon. Mercury concentrations of smaller increments of soil, therefore, allowed us to use the following equation to calculate fractional mass changes:
2.6 Hg:C Ratios in the 2014 Samples
The ratio of Hg to C in the 2014 soil cores is also of interest due to Hg’s affinity for organic matter. The Hg:C ratio in the earthworm invaded A horizons are derived from the Hg:C ratio initially present in the horizons and the Hg:C ratios in the forest floor. Assuming that the properties of the A horizon prior to invasion were the same as the same properties in the uninvaded cores, we can subtract the Hg and C concentrations from the A horizons in the invaded core to calculate the Hg:C ratios of the added organic matter (Table 1). We only included the average depth (10.60 cm) of the A horizon in the invaded site in our calculations because there was no significant difference (p = 0.48) in the Hg:C ratios of the invaded and uninvaded E.
3 Results and Discussion
3.1 Simple Mass Balance Approach: Mercury Pools
In general, the forest floor has smaller Hg pools due to its low bulk density and volume than mineral soil. The 2009 forest floor Hg pool is 2.30 ± 0.92 mg Hg m−2 while the Hg pools of the least and most invaded mineral soil are 3.36 ± 0.71 and 4.86 ± 0.34 mg Hg m−2, respectively (Table 2). The 2014 forest floor Hg pool is 2.85 ± 0.77 mg Hg m−2 with Hg pools of uninvaded and invaded mineral soil of 4.42 ± 0.88 and 5.47 ± 0.98, respectively (Table 2). The forest floor or organic soil pools found in this study correspond well with pools reported by Friedli et al. (2007) for organic soil of an upland boreal forest in Canada—2.92 ± 0.87 mg m−2 for an old forest stand (130 years) and 1.01 ± 0.28 mg m−2 for a younger stand (39 years). In another recent study, Tipping et al. (2011) calculated Hg pools for the top 15 cm of British soils and found that the majority of rural soils had Hg pools in the range of 5–20 mg m−2. The pools reported in this study fall on the lower end of that spectrum since our site is not located near any point sources of Hg.
For the 2009 soil, the total Hg pool in the least invaded area is 5.66 ± 1.63 mg Hg m−2 (forest floor + least invaded mineral soil) while the total Hg pool in the most invaded area (mineral soil only) is 4.86 ± 0.34 mg Hg m−2 (Table 2). Based on these calculations, 0.80 mg Hg m−2 (35 %) is missing or unaccounted as we move from the least earthworm invaded area to the most heavily invaded area. Similarly, for the 2014 soil samples, the total Hg pool in the uninvaded area (forest floor + uninvaded mineral soil) is larger (7.27 ± 1.65 mg m−2) than in the invaded area (5.47 ± 0.98 mg m−2) leaving 1.80 mg Hg m−2 (63 %) unaccounted.
These data suggest that, following earthworm invasion and consumption of the forest floor, a portion (1.05–1.50 mg m−2) of the Hg originally present in the forest floor is transported to and retained in the underlying mineral soil. This leaves a substantial fraction (0.8–1.8 mg m−2) of the Hg that was originally present in the forest floor now missing from the mass balance calculations. The regression relationship of forest floor (R 2 = 0.66) and mineral soil (R 2 = 0.43) pools along the 2009 sampling transect also demonstrates this general trend (Fig. 2). As the Hg pools in the forest floor decrease and eventually reach zero in the 0 and 50 m pit, the mineral soil Hg pools increase moving from the least invaded pit at 190 m to the most invaded pit at 0 m (Fig. 2). This seems to indicate a translocation of a fraction of the forest floor bound Hg into the underlying mineral soil by the action of soil mixing earthworms.
3.2 Geochemical Mass Balance Mercury Calculations
3.2.1 2009 Soil
A similar trend is apparent with the geochemical mass balance calculations with the mean δ of the combined forest floor and mineral soil in the least invaded area of 1.45 ± 0.79 mg Hg m−2, which is larger than that of the most invaded mineral soil (0.70 ± 0.63 mg m−2) (Table 2). Based on these calculations, 0.64 mg m−2 (46 %) of the Hg initially present in the forest floor was presumably transported into the underlying mineral soil while 0.75 mg Hg m−2 (54 %) remains unaccounted.
Fractional mass change (τ) calculations show an enrichment of Hg in the A horizon along the entire mineral soil transect (Fig. 3). In the least invaded group (pits 190, 160, 150, and 100), Hg is enriched from the top of the mineral soil horizons to about 10 cm. The depth of Hg enrichment also increases as earthworm invasion progresses. The more invaded pits (50 and 0) have higher enrichment values which are also present to a greater depth—about 15 to 17 cm. The enrichment values of the top mineral soil increase from 3.7 in the least invaded soil (pit 190) to 6.8 in the most invaded soil (pit 0). In the least invaded pits, Hg enrichment values are generally less than 1.0 for all depths below the uppermost sampling increment, indicating that there is little translocation of the Hg present in the forest floor into the underlying horizons. As earthworms invade and consume the forest floor, they appear to incorporate the organic matter plus its associated Hg into the A horizon, thereby increasing the enrichment values in the invaded areas.
Direct atmospheric deposition of Hg to the bare soil surface also occurs, however, in forested ecosystems this is a smaller fraction of the total Hg deposited to the soil with the larger portion deposited through throughfall and litterfall (Grigal et al. 2000). Freshly deposited atmospheric Hg also has a tendency to preferentially re-volatilize, a mechanism named prompt recycling (Selin et al. 2008), and is therefore not the largest contributor to the retained soil Hg pool in forests. The Hg deposited with litterfall is mostly in its ionic form (Hg2+) and tightly bound to organic matter constituents (Skyllberg et al. 2003). Consequently, it is not available for immediate volatilization unless subjected to organic matter decomposition and microbially induced volatilization or a disturbance such as bioturbation.
3.2.2 2014 Soil
Absolute mass change (δ) Hg calculations for the 2014 soil follow the same trend as discussed for 2009 soil. Means of δ show that 1.57 ± 0.52 mg Hg m−2 is present in the combined forest floor and uninvaded mineral soil while 0.65 ± 0.35 mg Hg m−2 is present in the invaded mineral soil (Table 2). Consequently, 0.49 mg Hg m−2 (35 %) was transported from the forest floor into the mineral soil, while 0.92 mg Hg m−2 (65 %) is missing. Fractional mass changes (τ) were not calculated for the 2014 samples since the soil was not collected in small enough depth increments to make these calculations meaningful.
3.3 Simple Mass Balance vs. Geochemical Mass Balance
Based on the simple mass balance calculations, 35 and 63 % of the Hg initially present in the forest floor of the least invaded and uninvaded soils is missing in the 2009 and 2014 soils, respectively (Table 2). The geochemical mass balance calculations indicate that 54 and 65 % of the Hg initially present in the forest floor is missing in the 2009 and 2014 soils, respectively.
The two mass balance approaches agree well for the 2014 soils, with both methods reporting over 60 % of the forest floor Hg missing after earthworm consumption of forest floor and mixing of upper mineral soil horizons. There is less agreement between the two methods for the 2009 soil where 35 % of the forest floor Hg is missing in the simple mass balance calculations and 54 % of the forest floor Hg is missing in the geochemical mass balance calculations. Some of these disparities may be explained by differences in sampling design between the two sets of samples—the 2009 soil was sampled along a transect that had varying degrees of invasion while 2014 soil was sampled only at the extremes, most invaded and uninvaded areas. Although there are some disagreements among the calculations and sample sets, all soil/mass balance combinations show that a substantial portion of Hg from the forest floor is missing following invasion by exotic earthworms. While other forest disturbances such as fires have been shown to deplete Hg in the soil (Woodruff and Cannon 2010; Kolka et al. 2014), to our knowledge no other studies have used a mass balance approach to assess the fate of forest floor Hg from a disturbance caused by earthworms.
3.4 Mercury and Organic Carbon Relationship
The results of the C mass balance calculations agree, for the most part, with the Hg results (Table 2). Simple C pool calculations for the 2009 soil point to 32 % C loss after accounting for the C that presumably moved from the forest floor into the mineral soil in the most invaded area. Absolute mass change (δ) calculations for the 2009 soil samples show that 57 % of C is missing. Similarly, for the 2014 soil, we observe that 34 % of C is missing based on the simple mass balance and 67 % is missing based on the geochemical mass balance. The percentages of lost C for both sets of soils using the geochemical mass balance agree well with the Hg percentages (i.e., 2009 soil: 54 % Hg loss with 57 % C loss and 2014 soil: 65 % Hg loss with 67 % C loss), while there is slightly less agreement based on the simple mass balance but only for the 2014 soil samples, which is most likely due to delta calculations only being done on A horizon material while pools were calculated to a depth of 24 cm (i.e., 2009 soil: 35 % Hg loss with 32 % C loss and 2014 soil: 63 % Hg loss with 34 % C loss).
Other studies have also reported a loss of C from the soil system following earthworm invasion (Alban and Berry 1994, Burtelow et al. 1998, Bohlen et al. 2004a, b). Alban and Berry (1994) reported a decline in total soil C to a depth of 50 cm during their 14-year study while Bohlen et al. (2004a, b) calculated a 25–28 % loss of C from the soil profile to a depth of 12 cm. While we sampled to a greater depth than Bohlen et al. (2004a, b), the percentage C losses agree closely with the percentage losses reported here based on the simple mass balance—32–34 %.
Next, we assessed the Hg:C ratios (Table 1). The Hg:C ratio of the added material (292.76 ng g−1) in the invaded area is considerably lower than that of the forest floor from the uninvaded area (711.21 ng g−1), suggesting that Hg was preferentially lost compared to C during the incorporation process. Our results also indicate that the ratio of Hg:C in the uninvaded soils is higher than that of the invaded soil (Table 1), suggesting that recently added organic carbon present in the A horizons of the invaded soils is less decomposed than the organic carbon present in the A horizons of the uninvaded soils. This implies that exotic earthworms are mixing organic carbon from the forest floor into the mineral soil but are not decomposing it fully in the heavily invaded areas.
3.5 Loss of Mercury from Forest Floor
Based on our findings, a substantial portion of the forest floor Hg is missing from the mass balance calculations following invasion by exotic earthworms. While 35–65 % of the forest floor Hg was transferred into the upper depths of the A horizon, the remainder is unaccounted. There are at least four potential explanations for the fate of the missing Hg: (1) accumulation in earthworm biomass and their movement out of the depths analyzed in this study, (2) microbial reduction of organically bound Hg2+ to Hg0 and subsequent volatilization to the atmosphere, (3) movement of Hg to greater depths in the soil than were analyzed in this study possibly associated with elevated dissolved organic carbon (DOC) transport, and (4) erosion of soils following loss of forest floor and removal of Hg-rich surficial sediments.
Most organisms take up and accumulate heavy metals and earthworms are no exception. Numerous researchers (Talmage and Walton 1993; Hinton and Veiga 2002; Zagury et al. 2006; Ernst and Frey 2007; Ernst et al. 2008; Rieder et al. 2011; Richardson et al. 2015) have shown that the rate of Hg uptake and accumulation by earthworms varies by ecological group. Rieder et al. (2011) showed that endogeic species in forest soils accumulate three times more Hg than epigeic species and two times more than anecic species. These differences in Hg bioaccumulation are largely attributed to the different burrowing and feeding behaviors of the different ecological groups. Using the highest earthworm tissue Hg concentrations (1.74 and 0.88 μg g−1) found by two recent studies (Rieder et al. 2011 and Richardson et al. 2015, respectively) and the highest worm biomass (13 g m−2 dry weight) found at our site (Resner 2013), we can estimate the maximum quantity of Hg we would expect to bioaccumulate in the earthworms. These simple calculations yield 0.023 and 0.012 mg Hg m−2 that can be accounted for through bioaccumulation, a small fraction (1–3 %) of the Hg missing from the system.
Mercury can also leave the soil system through microbial detoxification and subsequent volatilization of Hg0 during organic matter decomposition. Mercury resistance genes, known as the mer operon (Osborn et al. 1997), are widespread in soil microbes and allow organisms to effectively detoxify themselves of Hg. The most important gene involved in Hg resistance is mercuric reductase (merA) which catalyzes the reduction of Hg2+ into volatile Hg0 (Barkay et al. 2010). Elemental Hg can then readily pass through cell walls and into the soil atmosphere, where it may leave the soil system by volatilization.
The volatilization of Hg through the reduction of ionic Hg may potentially also occur in the earthworm gut. The gut of Lumbricus terrestris L., an anecic species, has been shown to contain bacterial strains related to the Firmicutes, Actinobacteria, and Proteobacteria phyla (Rieder et al. 2013) all of which are known to contain mercury resistance genes (Robinson and Tuovinen 1984; Osborn et al. 1997; Oregaard and Sorensen 2007; Barkay et al. 2003). Consequently, it is possible that a portion of the Hg taken up by earthworms is lost via merA detoxification while in the earthworm gut and subsequent volatilization. This hypothesis is supported by the large change in Hg:C ratio observed between forest floor and the A horizon in recently invaded sites, described above. However, mer genes tend to occur mainly in aerobic environments (Barkay et al. 2010) and the gut of earthworms is anaerobic, highlighting the need to further study this hypothesis. Direct measurement of Hg gas fluxes in invaded and uninvaded soils would clearly help answer this question. Although it is certainly not conclusive, the decrease in the Hg:C ratio in the underlying A and/or E horizons following loss of the forest floor strongly suggests that there is a preferential loss of Hg during the worm invasion process. While microbes in the soil might be responsible for this decrease, one has to question why the Hg:C ratio is higher in the underlying A horizons in the uninvaded soils where these processes have occurred for many tens or hundreds of years but occur rapidly during the relatively short span of the invasion process.
Abiotic pathways of Hg2+ reduction and volatilization also exist but occur mainly in very dry soils (Selin et al. 2008), which is not the case for this study site.
Mercury could also be lost from the invaded soils if it is leached to depths beyond those sampled in this study. This potential pathway is unlikely in fine to medium-fine textured soils, where Hg concentrations show little or no enrichment below the A horizon, indicating that Hg leaching is limited or non-existent (Nater and Grigal 1992). Additionally, 2009 soils, which were collected to a depth of 1 m or greater by Resner (2013), show lower concentrations of organic carbon in the horizons directly below the E horizon than in the E horizon itself, indicating that Hg translocation associated with DOC transport is negligible.
Another potential pathway for Hg loss is through the removal of surficial sediments with high Hg concentrations through erosional processes. The loss of the forest floor typically leaves bare mineral soils exposed which can significantly increase the erosion potential of the soil surface. However, the soils in this area are well-drained and there is little slope throughout the transect. Additionally, during our 2014 sampling in the invaded area, we sampled from a variety of micro depressions and micro highs. If significant erosional processes were occurring, they should have left accumulations of Hg-enriched surficial sediments in the micro depressions and some of the sites should have shown higher Hg pools than were present in the uninvaded areas. However, none of the sites in the invaded area had Hg pools larger than the mean of the Hg pools in the uninvaded area, which suggests that erosional losses of Hg-enriched surficial sediments were probably of minor importance when compared to Hg volatilization.
4 Conclusions
The loss of soil organic C due to earthworm invasions has been confirmed by other studies; however, no prior studies have utilized a mass balance approach to assess the fate of Hg in earthworm invaded soils. Using two mass balance approaches to quantitatively assess earthworm perturbation of soil Hg cycling and the fate of Hg formerly contained in the forest floor, we showed that a fraction (approximately 35–65 %) of the forest floor Hg is incorporated into the mineral surface soil as earthworms consume OM-rich forest floor and mix within the A horizon. A potentially larger proportion of forest floor Hg is missing from the mass balance calculations. A similar trend is observed for organic C loss with approximately 32–67 % of C missing from the soil system. The most likely explanation of the missing Hg is that the majority has been released to the atmosphere during forest floor consumption and microbially induced volatilization. A small portion of this missing Hg is undoubtedly bioaccumulated within earthworms; however, mass balance calculations show that earthworm accumulation cannot account for more than a few percent (1–3 %) of the missing Hg. Other potential, but less likely, explanations include loss of Hg by leaching to depths beyond those sampled in this study or erosion of Hg-enriched surficial sediments from barren soil surfaces.
Notes
Definitions for the O, A, and E master soil horizons are provided in Chapter 3 of: Soil Survey Division Staff. 1993. USDA NRCS soil survey manual. Soil Conservation Service. U.S. Department of Agriculture Handbook 18.
References
Alban, D. H., & Berry, E. C. (1994). Effects of earthworm invasion on morphology, carbon and nitrogen of a forest soil. Applied Soil Ecology, 1, 243–49.
Andersson, A. (1979). Mercury in soils. In J. O. Nriagu (Ed.), The biogeochemistry of mercury in the environment (pp. 79–122). Amsterdam New York, Oxford: Elsevier/North Holland Biomedical Press.
Barkay, T., Miller, S. M., & Summers, A. O. (2003). Bacterial mercury resistance from atoms to ecosystems. FEMS Microbiology Reviews, 27, 355–384.
Barkay, T., Kritee, K., Boyd, E., & Geesey, G. (2010). A thermophilic bacterial origin and subsequent constraints by redox, light and salinity on the evolution of the microbial mercuric reductase. Environmental Microbiology, 12(11), 2904–2917.
Binkley, D., & Fisher, R.F. (2012). Ecology and management of forest soils. 4th Edition. John Wiley & Sons
Bloom, N. S., & Crecelius, E. A. (1983). Determination of mercury in seawater at sub-nanogram per liter levels. Marine Chemistry, 14, 49–59.
Bohlen, P. J., Groffman, P. M., Fahey, T. J., Fisk, M. C., Suarez, E., Pelletier, D. M., & Fahey, R. T. (2004a). Ecosystem consequences of exotic earthworm invasion of north temperate forests. Ecosystems, 7, 1–12.
Bohlen, P. J., Pelletier, D. M., Groffman, P. M., Fahey, T. J., & Fisk, M. C. (2004b). Influence of earthworm invasion on redistribution and retention of soil carbon and nitrogen in northern temperate forests. Ecosystems, 7, 13–27.
Brimhall, G. H., & Dietrich, W. E. (1987). Constitutive mass balance relations between chemical composition, volume, density, porosity, and strain in metasomatic hydrochemical systems: results on weathering and pedogenesis. Geochimica et Cosmochimica Acta, 51, 567–587.
Burtelow, A. E., Bohlen, P. J., & Groffman, P. M. (1998). Influence of exotic earthworm invasion on soil organic matter, microbial biomass and denitrification potential in forest soils of the northeastern United States. Applied Soil Ecology, 9, 197–202.
Eisenhauer, N., Partsch, S., Parkinson, D., & Scheu, S. (2007). Invasion of a deciduous forest by earthworms: changes in soil chemistry, microflora, microarthropods and vegetation. Soil Biology and Biochemistry, 39(5), 1099–1110.
Ernst, G., & Frey, B. (2007). The effect of feeding behavior on Hg accumulation in the ecophysiologically different earthworms Lumbricus terrestris and Octolaseon cyaneum: a microcosm experiment. Soil Biology & Biochemistry, 39, 386–390.
Ernst, G., Zimmermann, S., Christie, P., & Frey, B. (2008). Mercury, cadmium and lead concentrations in different ecophysiological groups of earthworms in forest soils. Environmental Pollution, 156, 1304–1313.
Friedli, H. R., Radke, L. F., Payne, N. J., McRae, D. J., Lynham, T. J., & Blake, T. W. (2007). Mercury in vegetation and organic soil at an upland boreal forest site in Prince Albert National Park, Saskatchewan, Canada. Journal of Geophysical Research, 112, G01004.
Gabriel, M. C., & Williamson, D. G. (2004). Principal biogeochemical factors affecting the speciation and transport of mercury through the terrestrial environment. Environmental Geochemistry and Health, 26, 421–434.
Gates, G. E. (1982). Farewell to North American megadriles. Megadrilogica, 4, 12–77.
Grigal, D. F., Kolka, R. K., Fleck, J. A., & Nater, E. A. (2000). Mercury budget of an upland-peatland watershed. Biogeochemistry, 50, 95–109.
Gustafsson, J. P., Pechová, P., & Berggren, D. (2003). Modeling metal binding to soils: the role of natural organic matter. Environmental Science and Technology, 37, 2767–2774.
Hale, C. M., Frelich, L. E., & Reich, P. B. (2005a). Exotic European earthworm invasion dynamics in northern hardwood forests of Minnesota, U.S.A. Ecological Applications, 15, 848–860.
Hale, C. M., Frelich, L. E., & Reich, P. B. (2005b). Effects of European earthworm invasion on soil characteristics in northern hardwood forests of Minnesota. Ecosystems, 8, 911–927.
Hale, C. M., Frelich, L. E., & Reich, P. B. (2006). Changes in hardwood forest understory plant communities in response to European earthworm invasions. Ecology, 87(7), 1637–1649.
Hinton, J. J., & Veiga, M. M. (2002). Earthworms as bioindicators of mercury pollution from mining and other industrial activities. Geochemistry: Exploration, Environment, Analysis, 2, 269–274.
Jersak, J., Amundson, R., & Brimhall, G., Jr. (1997). Trace metal geochemistry in Spodosols of the northeastern United States. Journal of Environmental Quality, 26, 511–521.
Kolka, R. K., Grigal, D. F., Nater, E. A., & Verry, E. S. (2001). Hydrologic cycling of mercury and organic carbon in a forested upland-bog watershed. Soil Science Society of America Journal, 65(3), 897–905.
Kolka, R., Sturtevant, B., Townsend, P., Miesel, J., Wolter, P., Fraver, S., & DeSutter, T. (2014). Post-fire comparisons of forest floor and soil carbon, nitrogen, and mercury pools with fire severity indices. Soil Science Society of America Journal, 78, S58–S65.
Lyttle, A., Yoo, K., Hale, C., Aufdenkampe, A., & Sebestyen, S. (2011). Carbon–mineral interactions along an earthworm invasion gradient at a Sugar Maple Forest in Northern Minnesota. Applied Geochemistry, 26, S85–S88.
Lyttle, A., Yoo, K., Hale, C., Aufdenkampe, A., Sebestyen, S., Resner, K., & Blum, A. (2014). Impact of exotic earthworms on organic carbon sorption on mineral surfaces and soil carbon inventories in a northern hardwood forest. Ecosystems, 18, 16–29.
Mason, R. P., Fitzgerald, W. F., & Morel, F. M. M. (1994). The biogeochemical cycling of elemental mercury: anthropogenic influences. Geochimica et Cosmochimica Acta, 58(15), 3191–3198.
Merritts, D. J., Chadwick, O. A., Hendricks, D. M., Brimhall, G. H., & Lewis, C. J. (1992). The mass balance of soil evolution on late quaternary marine terraces, northern California. Geological Society of America Bulletin, 104, 1456–1470.
Nater, E. A., & Grigal, D. F. (1992). Regional trends in mercury distribution across the Great Lakes states, north central USA. Nature, 358, 139–141.
National Cooperative Soil Survey. (2006). United States Department of Agriculture. Warba Soil Series Description. https://soilseries.sc.egov.usda.gov/OSD_Docs/W/WARBA.html. Accessed March 20 2015.
Obrist, D., Pokharel, A. K., & Moore, C. (2014). Vertical profile measurements of soil air suggest immobilization of gaseous elemental mercury in mineral soil. Environmental Science & Technology, 48(4), 2242–2252.
Oh, N. H., & Richter, D. D. (2005). Elemental translocation and loss from three highly weathered soil-bedrock profiles in the southeastern United States. Geoderma, 126, 5–25.
Oregaard, G., & Sorensen, S. J. (2007). High diversity of bacterial mercuric reductase genes from surface and sub-surface floodplain soil (Oak Ridge, USA). International Society for Microbial Ecology Journal, 1, 453–467.
Osborn, A. M., Bruce, K. D., Strike, P., & Ritchie, D. A. (1997). Distribution, diversity and evolution of the bacterial mercury resistance (cp) operon. FEMS Microbiology Reviews, 19, 239–262.
Resner, K., Yoo, K., Hale, C. M., Aufdenkampe, A., & Sebestyen, S. (2011). Elemental and mineralogical changes in soils due to bioturbation along an earthworm invasion chronosequence in Northern Minnesota. Applied Geochemistry, 26, S127–S131.
Resner, K. (2013). Impacts of earthworm bioturbation on elemental cycles in soils: an application of a geochemical mass balance to an earthworm invasion chronosequence in a sugar maple forest in Northern Minnesota. Master’s thesis: Department of Soil, Water and Climate, University of Minnesota, St. Paul, Minnesota, United States.
Resner, K., Yoo, K., Sebestyen, S., Aufdenkampe, A., Hale, C., Lyttle, A., & Blum, A. (2014). Invasive earthworms deplete key soil inorganic nutrients (Ca, Mg, K, and P) in a northern hardwood forest. Ecosystems, 18, 89–102.
Reynolds, J. W. (1994). The distribution of the earthworms (Oligochaeta) of Indiana: a case for the post quaternary introduction theory for megadrile migration in North America. Megadrilogica, 5, 13–32.
Richardson, J. B., Gorres, J. H., Jackson, B. P., & Friedland, A. J. (2015). Trace metals and metalloids in forest soils and exotic earthworms in northern New England, USA. Soil Biology & Biochemistry, 85, 190–198.
Riebe, C., Kirchener, J. W., & Finkel, R. C. (2003). Long-term rates of chemical weathering and physical erosion from cosmogenic nuclides and geochemical mass balance. Geochimica et Cosmochimica Acta, 67, 4411–4427.
Rieder, S. R., Brunner, I., Horvat, M., Jacobs, A., & Frey, B. (2011). Accumulation of mercury and methylmercury by mushrooms and earthworms from forest soils. Environmental Pollution, 159, 2861–2869.
Rieder, S. R., Brunner, I., Daniel, O., Liu, B., & Frey, B. (2013). Methylation of mercury in earthworms and the effects of mercury on the associated bacterial communities. PLoS ONE, 8(4), e61215.
Robinson, J. B., & Tuovinen, O. H. (1984). Mechanisms of microbial resistance and detoxification of mercury and organomercury compounds: physiological, biological, and genetic analyses. Microbiological Reviews, 48(2), 95–124.
Schuster, E. (1991). The behavior of mercury in the soil with special emphasis on complexation and adsorption processes—a review of the literature. Water, Air, and Soil Pollution, 56, 667–680.
Selin, N. E., Jacob, D. J., Yantosca, R. M., Strode, S., Strode, S., Jaeglé, L., & Sunderland, E. M. (2008). Global 3-D land-oceanatmosphere model for mercury: present-day versus preindustrial cycles and anthropogenic enrichment factors for deposition. Global Biogeochemical Cycles, 22, GB2011.
Skyllberg, U., Qian, J., Frech, W., Xia, K., & Bleam, W. F. (2003). Distribution of mercury, methyl mercury and organic sulphur species in soil, soil solution and stream of a boreal forest catchment. Biogeochemistry, 64, 53–76.
Slemr, F., Schuster, G., & Seiler, W. (1985). Distribution, speciation, and budget of atmospheric mercury. Journal of Atmospheric Chemistry, 3(4), 407–434.
St. Louis, V., Rudd, J. W. M., Kelly, C. A., Hall, B. D., Rolfhus, K. R., Scott, K. J., Lindberg, S. E., & Dong, W. (2001). Importance of the forest canopy to fluxes of methyl mercury and total mercury to boreal ecosystems. Environmental Science and Technology, 35, 3089–3098.
Sutherland, R. A. (1998). Loss-on-ignition estimates of organic matter and relationships to organic carbon in fluvial bed sediments. Hydrobiologia, 389, 153–167.
Talmage, S. S., & Walton, B. T. (1993). Food chain transfer and potential renal toxicity of mercury to small mammals at a contaminated terrestrial field site. Ecotoxicology, 2, 243–256.
Tipping, E., Poskitt, J. M., Lawlor, A. J., Wadsworth, R. A., Norris, D. A., & Hall, J. R. (2011). Mercury in United Kingdom topsoils; concentrations, pools, and critical limit exceedances. Environmental Pollution, 159, 3721–3729.
Woodruff, L. G., & Cannon, W. F. (2010). Immediate and long-term fire effects on total mercury in forests soils of northeastern Minnesota. Environmental Science & Technology, 44, 5371–5376.
Zagury, G. J., Neculita, C.-M., Bastien, C., & Deschenes, L. (2006). Mercury fractionation, bioavailability, and ecotoxicity in highly contaminated soils from chlor-alkali plants. Environmental Toxicology and Chemistry, 25(4), 1138–1147.
Acknowledgments
We thank Kit Resner for providing subsamples of the 2009 soil she collected for her Master’s thesis research and Chris Mahr for his assistance with fieldwork.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM. 1
(PDF 772 kb)
Rights and permissions
About this article
Cite this article
Psarska, S., Nater, E.A. & Kolka, R.K. Impacts of Invasive Earthworms on Soil Mercury Cycling: Two Mass Balance Approaches to an Earthworm Invasion in a Northern Minnesota Forest. Water Air Soil Pollut 227, 205 (2016). https://doi.org/10.1007/s11270-016-2885-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-016-2885-0