Abstract
Accumulation of metals in cultivated crops is considered one of the primary constraints to irrigated agriculture. A greenhouse pot experiment was carried out to study the effects of irrigation with elevated cadmium (Cd) and a combination of cadmium and zinc (Zn) levels on Cd uptake, translocation, and productivity of tomato (Solanum lycopersicum) plants. Tomato seedlings were grown in 3-kg pots irrigated for three months until maturity. Treatments were as follows: pots irrigated with fresh water containing Cd concentrations (0, 0.01, 0.04, 0.16, 0.64, 2.54 mg L−1), and pots irrigated with a combination of Cd + Zn concentrations (0 + 0, 0.01 + 2, 0.04 + 8, 0.16 + 32, 0.64 + 128, and 2.56 + 256 mg L−1). Cadmium and Zn concentration in soil and plant parts (root, shoot, and fruit) increased with increasing metal dose in irrigation water. Results also showed that Cd accumulation in the fruit was much lower than in the shoot indicating lower Cd transfer from soil to the fruit. Tomato biomass was not affected by treatments even at the highest metal dose. The uptake of Cd in tomato fruit ranged from 0.5 to 2.0 and from 0.3 to 1 mg kg−1, in single and combination treatments, respectively. Cadmium in fruit exceeded the permissible limit at 0.04 and 0.16 + 32 mg L−1 in Cd and Cd + Zn treatments, respectively. Therefore these levels could be considered as a threshold for tomato cultivation in clayey soil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Agricultural crops face biotic and abiotic stresses which limit crop production. Heavy metals induce abiotic stress to crops and adversely affect their production through interfering with a number of metabolic processes. Moreover, these metals can contaminate both soil and water mainly through industrial processes and application of fertilizers (Waisberg et al. 2003). If these metals present in irrigation water, even at low levels, they gradually accumulate in soil and in plants to levels that could pose health risks to humans and to animals (Devi et al. 1996; Waisberg et al. 2003; Sinha et al. 2006).
Cadmium (Cd) and zinc (Zn) are among the most risky elements that could contaminate soils and irrigation waters (Baker 1981). Cadmium is very toxic heavy metal, non-essential for plant growth, and it interferes with a number of metabolic processes (Adriano 1986). On the other hand, Zn is an essential micronutrient for plant growth and enzymatic functioning (Cherif et al. 2011). Cd is often associated with Zn in the environment; and this may result in various synergistic and/or antagonistic interactions in plants (Wu and Zhang 2002; Balen et al. 2011).
Accumulation of Cd and Zn in soil and plant is influenced by plant species and cultivar, Cd/Zn ratio, soil Cd and Zn phytoavailability, soil pH, soil sorption surfaces including organic matter, chloride concentration, nitrogen and phosphorus levels, and crop rotation (Yang et al. 2001; Peralta-Videa et al. 2002; Shang and Leung 2003; Liu et al. 2007). Cadmium to Zn ratio plays a major role in Cd toxicity in plants since both metals may compete for the binding sites in proteins (Cakmak et al. 2000). Chaney et al. (1999) reported that Cd/Zn ratio has a strong effect on Cd uptake in lettuce shoots.
Moreover, Zn supplement is reported to reduce Cd uptake (Hart et al. 2002), Hassan et al. 2005 attributed this decrease to possible competition between Cd and Zn for uptake, which suggests that higher Zn concentrations were synergistic to Cd uptake. On the other hand, Nan et al. (2002) observed no antagonistic Cd + Zn interaction at uptake level.
The efficiency of different plants in absorbing metals is generally evaluated by either plant uptake or by soil–plant transfer factors (TF). The TF is defined as the ratio of metal concentration in the edible parts to the total metal concentration in soil (Alloway et al. 1990). The transfer of Cd and Zn is different, since Zn is essential for plant growth, mobile and highly bioavailable whereas Cd is toxic to plants. Cadmium has lower TF compared to Zn and varies according to plant species. Streit and Stumm (1993) explained the higher transfer ratio of Zn compared to Cd to its higher bioavailability.
Tomato (Solanum lycopersicum), one of the healthiest vegetable crops due to high content of lycopene and other health promoting natural compounds, is considered a major and traditional constituent of diet in the Middle East. In Jordan for example, average production of tomato is 50 metric tons ha−1 and 25 % of the total production is produced in greenhouses. Exposure of tomatoes to Cd and Zn in irrigation water has been prevalent in recent years, and effluents of multiple industrial sources were reported to contain these two metals. The knowledge of the threshold concentration of Cd in irrigation water that results in accumulation of Cd in tomato fruits and shoots to unsafe concentrations for human health and animal consumption (fodder) is a concern that should be addressed.
The Zarqa River Basin, an important economical and agricultural area, located in the central north part of Jordan and covering an area of 3,567 km2, is cultivated with many crops. Some of these crops are being illegally irrigated with river water where unreported incidents of toxic waste from industrial sources being illegally dumped into the river consequently metal levels in soils and cultivated crops are expected to increase to levels that might pose health hazards to humans and animals feeding on fodder crops.
Therefore, the main objectives of this study were: (1) to study the response/productivity of tomato to irrigation water containing elevated concentrations of Cd only (single treatments) and a combinations of Cd + Zn (combination treatments), (2) to determine Cd uptake and transfer in tomato parts, and (3) to study the threshold level of Cd and Cd + Zn in irrigation water which causes accumulation of Cd in tomato fruits and shoots grown in clayey soils to unsafe levels for human and animal consumption.
2 Materials and Methods
2.1 Soil Sample Collection and Physicochemical Characterization
Composite soil sample was collected from the surface (0–20 cm) of an agricultural field (elevation 130 m) along Zarqa River (about 25 km north of Amman). The area is cultivated with vegetable crops, and some parts are being irrigated infrequently from Zarqa River.
The soil sample was air dried, sieved through 2-mm sieve and stored for further analysis. Soil electrical conductivity (EC) and pH were determined in saturated paste extract using Beckman Coulter 650 pH-salinity meter following the methods by Rhoades (1996) and Thomas (1996), respectively. Calcium carbonate (CaCO3) was determined by the method described by Loeppert and Suarez (1996). Cation exchange capacity (CEC) was determined by method described by Sumner and Miller (1996). Organic carbon content (OC) was determined by Walkley-Black method (dichromate oxidation) as described by Nelson and Sommers (1996). Soil texture was determined by the hydrometer method (Gee and Or 2002), and total metal content (Cd, Zn, Cu, Pb, and Cr) were measured after acid digestion with HNO3 using inductively coupled plasma (ICP-AES). Soil physicochemical properties are shown in (Table 1).
2.2 Irrigation Water
Irrigation water was obtained from a groundwater well located in Jordan University of Science and Technology (JUST) campus. Irrigation water characterized according the standard methods of water analysis. The pH and EC of water sample was analyzed using Beckman Coulter 650 pH-salinity meter. Magnesium (Mg2+) and calcium (Ca2+) were measured by the EDTA method, sodium (Na+) and potassium (K+) were measured by flame photometer (Jenway Clinical PFP7), chloride (Cl−) and sulphate (SO4 2−) concentrations were measured by Dionex DX-120 ion chromatograph, and metal concentrations (Cd, Zn, Cu, Pb, and Cr) were measured using inductively coupled plasma (ICP-AES). All results are shown in (Table 2).
2.3 Pot Experiment
A greenhouse pot experiment was carried out by planting tomato seedlings (Solanum lycopersicum) var. GS12 in 3-kg plastic pots. Plants were grown in a clayey soil for 90 days. Pots were arranged in a complete randomized design with three replicates. Treatments were divided into (1) control; (2) pots irrigated with the following single Cd levels: 0.01, 0.04, 0.16, 0.64, and 2.56 mg L−1, in the remainder of this paper, these treatments are referred to as: SL1, SL2, SL3, SL4, and SL5; and (3) pots irrigated with a combination of Cd + Zn levels: 0.01 + 2, 0.04 + 8, 0.16 + 32, 0.64 + 128, and 2.56 + 256 mg L−1, in the remainder of this paper, these treatments are referred to as: CL1, CL2, CL3, CL4, and CL5. Metal levels in irrigation water prior to spiking were <0.0003 Cd and 0.02 Zn mg L−1 (Table 2). Metal levels in irrigation water were chosen to represent a wide range of Cd and Zn that could be found in river water as affected by increased pollution incidents from the surrounding factories. The lowest Cd and Zn concentrations used in the experiment in CL1 correspond to the recommended maximum concentrations of metals in irrigation water suggested by Ayers and Westcot (1985). Other levels were chosen to represent a wide range of increased metal concentrations by a factor of 4, except for the highest Zn level (256 mg L−1), which represents a worst case scenario for metal level in irrigation water.
Prior to irrigation, water was spiked with required volume of Cd and Cd + Zn solutions; the volume required for each level was pipetted into a glass beaker and made up by required volume of irrigation water to provide the desired metal level. Each week, Cd and Zn stock solutions were prepared using reagent grade cadmium nitrate tetrahydrate (Cd(NO3)2.4H2O) and zinc nitrate hexahydrate (Zn(NO3)2.6H2O) reagent grade chemicals (Sigma-Aldrich). Irrigation was performed twice a week and the applied water amount was determined from the weight loss of fiverepresentative pots. The total amount of irrigation water varied according to growth stage ranging from 100 to 300 mm in each irrigation event and totaling 7.5 L pot−1. The temperature in the greenhouse was held at approximately 25 °C and 65 % relative humidity.
The general design of the experiment closely mimics the general irrigation practices of farmers in that area, where increased levels of metals are expected to contaminate both irrigated soil and cultivated crops.
2.4 Chemical Analysis of Plant Tissue and Soil
Tomato plants were harvested after 3 months and separated into roots, shoots, and fruits. Tomato fruits were hand harvested at vine-ripened stage, cut into two halves, placed face up in aluminum cans and oven dried for four days at 70 ° C. Plant roots were carefully taken out from pots and rinsed with distilled water. Tomato roots and shoots were placed in paper bags, oven dried for 2 days at 70 ° C, ground to <0.25 mm using a rotating plant mill (ZM 100, Retsch, Titanium ring sieve) and stored in paper bags for metal analysis. After roots were taken out, soils were air dried, sieved through a 2-mm sieve, oven dried for 24 h at 105 ° C, and ground with an agate ball mill (Fritsch pulverisette 7 premium) to <0.25 mm.
To avoid cross contamination, soil and plant grinding was done separately in ascending order of concentration. The ball mill was cleaned after each grinding step with clean quartz sand, while the plant mill was brushed and cleaned with pressurized air. The finely ground materials (250 mg) were weighed into digestion tubes, 10 mL of concentrated nitric acid were added, covered, and left to digest overnight. In the next day, the tubes were digested at 180 °C using microwave pressure method (Mars Xpress, CEM GmbH, Kamp-Lintfort, Germany), filtered through acid washed filter paper no. 640, and transferred into 50-mL polyethylene bottle for metal analysis (Chander et al. 2008). Total Cd and Zn concentrations of soil and plant materials were analyzed using ICP-AES (Ciros, Spectro Analytical Instruments GmbH, Kleve, Germany).
2.5 Estimation of Transfer Factors (TF) and Transfer Ratios (TR)
The TF describes the amount of an element expected to enter a plant from soil (Sheppard and Sheppard 1985). The TF values were determined for: soil–root, soil–shoot, and soil–fruit. The TF from soil to tomato parts was calculated as follows:
Where C plant part is Cd concentration (in milligram per kilogram) in plant tissue (e.g. root, shoot, and fruit), and Csoil is Cd concentration (in milligram per kilogram) in soil.
The distribution of Cd between plant parts was also represented by the transfer ratio (TR). The TR is defined as the ratio of metal concentration (in milligram per kilogram) in upper plant part (e.g., shoot and fruits) to the metal concentration in lower plant part (e.g., root).
Where C Upper plant part is the concentration of Cd (in milligram per kilogram) in plant tissue (e.g. shoot, and fruit), and C lower plant part is the concentration of Cd (in milligram per kilogram) in respective lower plant parts. Three TRs were calculated, root–shoot, root–fruit, and shoot–fruit.
2.6 Statistical Analysis
Significance was tested using two-way ANOVA between treatments with subsequent mean separation by student’s LSD test using statistical package SigmaPlot 12.3. Probability was used to indicate significant treatments and interaction effects, means were separated according to the Fisher’s Least Significant Difference (LSD) at 0.05 probability level.
3 Results and Discussion
3.1 Soil and Water Characterization
The used soil has medium alkaline (pH= 8.15) reaction with low EC level of 0.5 dS m−1. The soil has clayey texture (66 % clay), CEC of 30 cmol(+) kg−1, 0.2 % organic carbon (OC), 31.6 % CaCO3, Cu, Pb, Cr, and Ni < 1 mg L−1, Cd and Zn 2.4 and 99.0 mg kg−1, respectively (Table 1). Clayey soils are commonly found in studied area and their utilization for pot trials can be considered a case study due to their expected high metal sorption capacity. Cd and Zn concentrations in the original soil fall within the European maximum allowable limits for Cd (5 mg kg−1) and Zn (300 mg kg−1) (Kabata-Pendias and Mukherjee 2007).
Irrigation water has low salinity level with an EC of 0.6 dS m−1 and low SAR of 0.7. Water chemical analysis was determined as follows: 22.9 Na+, 25.1 Ca2+, Mg2+ 25.1, 3.5 K+, 51.3 Cl−, 10 HCO3 −, and 20 SO4 2− mg L−1. Metal concentrations in irrigation water (Table 2) prior to metal additions (control) fall below the FAO recommended maximum concentrations limits for Cd (0.01 mg L−1) and Zn (2 mg L−1) (Ayers and Westcot 1985).
3.2 Cadmium Concentrations in Soils After Harvest
Cadmium in soils increased with the increase in metal levels in irrigation water. Treatments, metal levels, and their interaction significantly affected soil Cd concentrations (P < 0.05). Soil Cd in CL3-CL5 were significantly increased compared to SL3–SL5 treatments. For example, soil Cd in CL4 and CL5 was increased by 23 and 26 % compared to SL4 and SL5 treatments, respectively (Table 3). Metal levels in all treated soils (Except for CL4 and CL5) fall below the European maximum allowable limits for Cd and Zn (Kabata-Pendias and Mukherjee 2007).
3.3 Metal Concentration in Tomato Shoots and Roots
Shoot Cd increased significantly with increasing metal level in irrigation water (Table 3). Treatments, metal level, and their interaction significantly affected shoot Cd concentrations (P < 0.05). Shoot Cd in CL3 and CL4 was decreased by 36 and 52 % compared to SL3 and SL4 treatments, respectively. The decrease may be attributed to the presence of Zn which reduced Cd uptake in combination treatments. These results are in agreement with Mohammad and Moheman (2010) who reported that increased zinc application significantly reduced Cd accumulations in all parts of tomato plants.
However, In L5 treatments (the extreme case), shoot Cd in CL5 was significantly higher than SL5treatment. As shown in Table 3, shoot Zn in CL5 was 1845.8 mg kg−1 compared to 33.9 mg kg−1 in SL5. Higher Cd in combination treatment could be attributed to Zn toxicity which could result in breakdown of the defense mechanism that allowed higher shoot Cd accumulation. These results are in agreement with Takijima and Katsumi (1973) and Simmons et al. (2003) who showed that extremely high Zn levels increased Cd levels in rice leaves, however rice grain Zn was not increased.
It is worthy to note that tomato shoots are commonly cut and used for animal feed in Jordan. It was observed that shoot Cd concentrations in SL1-SL3, and CL1-CL3 treatments were lower than the FAO/WHO (2001) permissible limits for fodder crops (Cd: <1.0 mg kg−1 fresh weight (FW), equivalent to <6.7-8.3 mg kg−1 dry weight (DW) at 85-88 % water content). For Zn, no regulatory limits exist, however the observed Zn concentrations of 200-500 mg kg−1 are of no concern in animal feed (Begerow et al. 2008).
For roots, Cd concentration increased significantly with increasing metal levels (P < 0.05) in irrigation water, however no significant differences were observed between metal levels in single and combination treatments. A similar finding was reported by Mohammad and Moheman (2010) where Cd uptake in roots was higher, but not significant, in single treatments compared to combination treatments. Apparently, the presence of Zn in combination treatments reduced root Cd, however Cd translocation to the shoots was higher than root uptake itself. These results are contradictory to the results obtained by McKenna et al. (1993)) who reported that Zn inhibits Cd uptake by roots and its transport to shoots.
Comparing shoot to root uptake, the following was observed: Shoot contained higher Cd than root in L3-L5 in both treatments. However, the percentage of increase (between shoot to root) was higher in single compared to combination treatments. For example, shoot Cd was 130 and 42 % higher than root Cd in SL3 and CL3, respectively. At the lower levels, the opposite was observed only in single treatments, where shoot Cd was 37 % lower than root Cd in both SL1 and SL2 treatments. This could be attributed to the following: at the lower metal levels in both treatments, roots may have effective protection mechanisms that reduce metal uptake. This may be supported by the presence of certain cations, such as iron (Fe) or magnesium (Mg). At high metal concentrations, these protective mechanisms can break down due to direct toxic effect of Zn or due to insufficient concentrations of competing cations such as Fe or Mg (Marschner 1995).
3.4 Metal Concentration in Tomato Fruits
Metal concentrations in tomato fruit increased significantly (P < 0.05) with metal level in irrigation water (Table 3). Combination treatments significantly reduced fruit Cd compared to single ones. Fruit accumulated lower Cd than shoot and root. Both treatments showed significantly higher fruit Cd in L5 than all other treatments (except for SL4). This could be attributed to metal toxicity which resulted in breaking down of the transfer mechanism at the highest metal levels as discussed earlier in the previous section (Marschner 1995).
Except for the control and SL1 treatment, which represents the maximum allowable Cd in irrigation water, all fruit Cd in SL2–SL5 exceeded the safe limit for human consumption (>0.1 mg kg−1 FW or 0.7 mg kg−1 DW) (Begerow et al. 2008). On the other hand, fruit Cd in combination treatments exceeded the safe limit for human consumption only in CL4 and CL5 treatments (Cd concentrations > 0.64 mg L−1).
It is interesting to note the following observations: (1) in all combination treatments fruit Zn did not exceed 50 mg kg−1 indicating that Zn translocation to fruits was at minimum. Since Zn is an essential micronutrient for plants and Cd is not, for food products no maximum permissible guidelines for Zn exist. (2) Cd uptake in tomato roots and shoots was clearly inhibited when soil Cd exceeded 8 mg kg−1. This trend was not observed in tomato fruits, where Cd uptake was inhibited at a lower soil Cd concentration. (3) Zn additions clearly resulted in higher inhibition of Cd uptake in fruits compared to the roots and shoots of tomato plants.
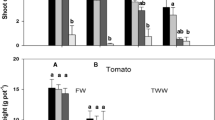
3.5 Biomass Yields
Biomass shoot yields slightly decreased with increasing metal levels in irrigation water. Tomato yields decreased from 6.5 g in the control to 4.3 and 5 g in SL5 and CL5, respectively (Table 4). It is worthy to note that no apparent chlorosis or physical injury was observed in all plants regardless of metal level and treatment. All plants produced tomato fruits; however the size and the amount of produced fruit were inconsistent. Statistical analysis showed no significant differences between SL5 and CL5; however, the biomass was significantly reduced in these treatments compared to the control.
3.6 Transfer Factors of Cd from Soil to Different Tomato Parts
The TF reflects the relative concentration of transferred metal in plant parts to its concentration in soil. The TF values from soil to tomato root, shoot, and fruit are shown in Fig. 1. The TF can be evaluated only when a linear relationship between soil and metal plant content is observed (Chojnacka et al. 2005). The linearity of the relationship is rarely observed between total metal in soil and plants since metals come from a very complex and heterogeneous matrix (soil solution). In this study, the availability of metals is hypothetically high since they were freshly added and directly applied to tomato plants prior to irrigation. In this study, a linear relationship between soil metal and plant tissues was observed. Correlation coefficients (r 2) between Cd in soil and in fruit, shoot, and root are shown in Fig. 3.
The lowest TF values were observed in soil–fruit and the highest were in soil–shoot indicating lower Cd transfer to the fruit compared to the other parts (Gallego et al. 2012).
The addition of Zn in irrigation water (combination treatments) reduced the TF values compared to single ones. The TF of soil–fruit, soil–shoot, and soil–root in CL4 was reduced by 37, 18, and 22 % compared to SL4, respectively. Moreover, soil–root, soil–shoot, and soil–fruit TF values were reduced by 37, 60, and 50 % in CL3 compared to SL3, respectively.
The reduction may be due to the inhibition of Cd uptake as a result of high Zn levels in irrigation water (antagonistic effect of Zn). Our results are in agreement with Adriano (1986), who reported that Zn application reduced Cd uptake in roots and aerial parts of spring wheat (Triticum aestivum L.) and Corn (Zea mays L.).
Moreover, possible reduction in TF value could be attributed to the presence of an exclusion mechanism at the higher metal levels (Marschner 1995). Exclusion mechanisms may include pH modification in the rhizosphere, excretion of organic acids capable of binding metals (Marschner 1995), and thickening of root tips (Pallavi and Shanker 2005). Other suggested mechanisms may involve metal immobilization in the cell wall and the soluble cell fraction in roots (Lozano Rodriguez et al. 1997). However, Rauser (1986) and Hall (2002) suggested that Cd remains in the roots and minimum Cd transport to the shoots occurs.
At higher concentrations, roots could be overloaded with Cd and a significant mobilization to the shoot may occur (Nan et al. 2002). This was in agreement with the findings of (López-Millán et al. 2009) who reported that Cd transfer at low Cd concentrations relies on accumulating Cd at the root level. However, Dong et al. (2006) observed no such changes in tomato roots with low Cd supply.
A possible prevention mechanism in roots and shoots could be the reason to which enabled physiologically tomato plants to tolerate excessive Cd and Zn accumulation in the edible parts. This mechanism might be responsible for reduced Cd which could be complexed as Cd-binding peptides in tomato roots (Mckenna et al. 1993).
3.7 Transfer Ratios of Cd Between Plant Parts
The distribution of Cd between plant parts (Fig. 2) was also represented by the transfer ratio (TR). Three TRs were calculated, root–shoot, root–fruit, and shoot–fruit (Fig. 2). The lowest TR values were observed in shoot–fruit CL3–CL5, and the highest were in root–shoot SL4–SL5 treatments, indicating lower Cd transfer to the fruits. Moreover, with increasing metal levels, shoot–fruit, and root–fruit TF value was decreased in all combination treatments (CL1–CL5), and only in SL3–SL5. For root–shoot, inconsistent trends were observed. Significant differences between treatments are shown in (Fig. 2).
A couple of mechanisms might be responsible for the decrease in TF values; the first is the antagonistic effect of Zn (on Cd uptake), and the second the toxicity effect of increased levels of Cd or both metals on root metal uptake (Marschner 1995).
3.8 Accumulation of Cd in Tomato Parts in Relation to Cd Concentration in Soil
The relationships between Cd concentration in soil and in tomato parts (root, shoot, and fruit) are shown in (Fig. 3). The relation was linear and shows that plant metal uptake relative to soil concentration was lower in combination treatments compared to single ones. Moreover, Cd concentration in plant parts relative to soil concentration was generally higher in shoot and root compared to tomato fruit.
Many scientists reported that tomato plants vary greatly in their ability to accumulate Cd from the soil (Cui et al. 2004; Wang et al. 2009). The interaction between Cd and Zn has been reported to be antagonistic (Li et al. 1990; Long et al. 2003) and synergistic by others (Adriano 1986; Piotrowska et al. 1994; Nan et al. 2002). Great differences occur among species and even between different varieties of the same species (Grant and Bailey 1997). Results of this study are in agreement with previous findings reported by Lund et al. (1981) and Jinadasa et al. (1997) where vegetable leafs and crop root generally accumulated higher Cd concentrations than fruits or seeds.
It is reported that most plant species tend to sequester metals in their roots, with small amounts of metals being translocated to the aerial plant parts (Kim et al. 1988; Moreno-Caselles et al. 2000). The ability to absorb Cd in different species depends on differences in root capacity to secrete citric acid (Duarte et al. 2007). It has been also indicated that increased concentrations of divalent metals in root could be partially explained by Cd interference in nutrient uptake by affecting the permeability of plasma membranes (Dong et al. 2006). Other studies showed synergistic effects between high Cd concentrations and Zn (Liu et al. 2003; Larbi et al. 2002).
This study showed that reduced Cd uptake in combination treatments may be attributed to the interaction between the two metals (Cd and Zn) revealing mostly antagonistic interactions in uptake, therefore reducing Cd uptake in tomato parts (shoot, fruit, and root).
4 Conclusions
This work showed that Cd concentration in tomato parts was largely dependent on Zn level supplied in irrigation water. Although tomato plants accumulated considerable Cd concentrations in shoots and roots, fruit metal concentrations were still below the safe limits for human consumption. No growth depression was detected even at the highest supplied metal levels. Cadmium uptake was reduced as a result of Zn addition indicating higher tolerance, competitive absorption, and transport interaction between the two metals. For clay soils; 0.16–32 mg L−1 of Cd + Zn in irrigation water could be considered as a threshold level for tomato cultivation in clay soil.
References
Adriano, D. C. (1986). Bioavailability of trace metals. In D. C. Adriano (Ed.), Trace elements in the terrestrial environment (pp. 61–89). Berlin: Springer-Verlag.
Alloway, B. J., Jackson, A. P., & Morgan, H. (1990). The zccumulation of cadmium by vegetables grown on soils contaminated from a variety of sources. The Science of the Total Environment, 91, 223–236.
Ayers, R. S., & Westcot, D. W. (1985). Water quality for agriculture (FAO Irrigation and Drainage Paper 29, p. 174). Rome: FAO.
Baker, A. J. M. (1981). Accumulators and excluders-strategies in the response of plants to heavy metals. Journal of Plant Nutrition, 3, 643–654.
Balen, B., Tkalec, M., Sikić, S., Tolić, S., Cvjetko, P., Pavlica, M., & Vidaković-Cifrek, Z. (2011). Biochemical responses of Lemna minor experimentally exposed to cadmium and zinc. Ecotoxicology, 20(4), 815–26.
Begerow, J., Crößmann, G., Ewers, U., & Finck, M. (2008). Standards and regulations regarding metals and their compounds in environmental materials, drinking water, food, feeding-stuff, consumer products, and other materials. In E. Merian, M. Anke, M. Ihnat, & M. Steoppler (Eds.), Elements and their compounds in the environment (pp. 1498–1524). Weinheim: Wiley-VCH Verlag GmbH.
Cakmak, I., Welch, R. M., Hart, J., Norvell, W. A., Ozturk, L., & Kochian, L. V. (2000). Uptake and retranslocation of leaf-applied cadmium (Cd-109) in diploid, tetraploid and hexaploid wheats. Journal of Experimental Botany, 51, 221–226.
Chander, K., Hartmann, G., Joergensen, R. G., Khan, K. S., & Lamersdorf, N. (2008). Comparison of three methods for measuring heavy metals in soils contaminated by different sources. Archives of Agronomy and Soil Science, 54, 413–422.
Chaney, R. L., Ryan, J. A., Li, Y. M., & Brown, S. L. (1999). Soil cadmium as a threat to human health. In M. J. McLaughlin & B. R. Singh (Eds.), Cadmium in soils and plants (pp. 219–256). Dordrecht: Kluwer Academic Publishers.
Cherif, J., Mediouni, C., Ammar, W. B., & Jemal, F. (2011). Interactions of zinc and cadmium toxicity in their effects on growth and in antioxidative systems in tomato plants (Solanum lycopersicum). Journal of Environmental Sciences, 23(5), 837–844.
Chojnacka, K., Chojnacki, A., Górecka, H., & Górecki, H. (2005). Bioavailability of heavy metals from polluted soils to plants. Science of the Total Environment, 337(1–3), 175–182.
Cui, Y. J., Zhu, Y. G., Zhai, R. H., Chen, D. Y., Huang, Y. Z., Qui, Y., & Liang, J. Z. (2004). Transfer of metals from soil to vegetables in an area near a smelter in Nanning, China. Environmental International, 30(6), 785–791.
Devi, M., Thomas, D. A., Barber, J. T., & Fingerman, M. (1996). Accumulation and physiological and biochemical effects of cadmium in a simple aquatic food chain. Ecotoxicology and Environmental Safety, 33(1), 38–43.
Dong, J., Wu, F. B., & Zhang, G. P. (2006). Influence of cadmium on antioxidant capacity and four microelement concentrations in tomato seedlings (Lycopersicon Esculentum). Chemosphere, 64, 1659–1666.
Duarte, B., Delgado, M., & Caçador, I. (2007). The role of citric acid in cadmium and nickel uptake and translocation, in Halimione portulacoides. Chemosphere, 69, 836–840.
FAO/WHO (2001). Codex Alimentarius Commission Food Additives and Contaminants. Joint FAO/WHO Food Standards Program, ALINORM 01/12A:1–289.
Gallego, S. M., Pena, L. B., Barcia, R. A., Azpilicueta, C. A., Iannone, M. F., Rosales, E. P., Zawoznik, M. S., Groppa, M. D., & Benavides, M. P. (2012). Unravelling cadmium toxicity and tolerance in plants: insight into regulatory mechanisms. Environmental and Experimental Botany, 83, 33–46.
Gee, G. W., & Or, D. (2002). Particle-size analysis. In J. H. Dane & C. G. Topp (Eds.), Methods of soil analysis: part 4—physical methods (pp. 255–293). Madison: ASA and SSSA.
Grant, C. A., & Bailey, L. D. (1997). Effects of phosphorous and zinc fertilizer management on cadmium accumulation in flaxseed. Journal of the Science of Food and Agriculture, 73, 307–314.
Hall, J. L. (2002). Cellular mechanisms for heavy metal detoxification and tolerance. Journal of Experimental Botany, 53(366), 1–11.
Hart, J. J., Welch, R. M., Norvell, W. A., & Kochian, L. V. (2002). Transport interactions between cadmium and zinc in roots of bread and durum wheat seedlings. Physiologia Plantarum, 116, 73–78.
Hassan, M. J., Zhang, G., Wu, F., Wei, K., & Chonghua, C. (2005). Zinc alleviates growth inhibition and oxidative stress caused by cadmium toxicity in rice. Journal of Plant Nutrition and Soil Science, 168, 256–261.
Jinadasa, K., Milham, C., Hawkins, P., Cornish, P., Williams, Kaldor, C., & Conroy, J. (1997). Survey of cadmium levels in vegetables and soils of Greater Sydney. Australian Journal of Environment, 26, 924–933.
Kabata-Pendias, A., & Mukherjee, A. B. (2007). Trace elements of group 12 (Previously group IIb). In A. Kabata-Pendias, & A. B. Mukherjee (Eds.). Trace elements from soil to human (pp. 283-319). Springer Berlin Heidelberg.
Kim, S. J., Chang, A. C., & Page, A. L. (1988). Relative concentration of cadmium and zinc in tissue of selected food crops grown on sludge treated soil. Journal of Environmental Quality, 17, 568–573.
Larbi, A., Morales, F., Abadda, A., Gogorcena, Y., Lucena, J. J., & Abadda, J. (2002). Effects of Cd and Pb in sugar beet plants grown in nutrient solution: induced Fe deficiency and growth inhibition. Functional Plant Biology, 29, 1453–1464.
Li, S. L., Wang, H. X., & Wu, Y. S. (1990). Antagonistic effect of zinc on cadmium in water hyacinth. Acta Scientiae Circumstantiae, 10, 244–249.
Liu, J., Li, K. Q., Xu, J. K., Liang, J. S., Lu, X. L., Yang, J. C., & Zhu, Q. S. (2003). Interaction of Cd and five mineral nutrients for uptake and accumulation in different rice cultivars and genotypes. Field Crops Research, 83, 271–281.
Liu, J., Qian, M., Cai, G., Yang, J., & Zhu, Q. (2007). Uptake and translocation of Cd in different rice cultivars and the relation with Cd accumulation in rice grain. Journal of Hazardous Materials, 143, 443–447.
Loeppert, R. H., & Suarez, D. L. (1996). Carbonate and gypsum. In D. L. Sparks, A. L. Page, P. A. Helmke, & R. H. Loeppert (Eds.), Methods of soil analysis: part 3—chemical methods (pp. 437–474). Madison: ASA and SSSA.
Long, X. X., Yang, X. E., Ni, W. Z., Ye, Z. Q., He, Z. L., Calvert, D. V., & Stoffela, J. P. (2003). Assesing zinc thresholds for phytotoxic and potential dietary toxicity in selected vegetable crops. Communications in Soil Science and Plant Analysis, 34, 1421–1434.
López-Millán, A.-F., Sagardoy, R., Solanas, M., Abadía, A., & Abadía, J. (2009). Cadmium toxicity in tomato (Lycopersicon esculentum) plants grown in hydroponics. Environmental and Experimental Botany, 65, 376–385.
Lozano Rodriguez, E., Hernandez, L. E., Bonay, P., & CarpenaRuiz, R. O. (1997). Distribution of cadmium in shoot and root tissues of maize and pea plants: Physiological disturbances. Journal of Experimental Botany, 48, 123–128.
Lund, L. J., Betty, E. E., Page, A. L., & Elliott, R. A. (1981). Occurrence of naturally high cadmium levels in soils and its accumulation by vegetation. Journal of Environmental Quality, 10, 551–556.
Marschner, H. (1995). Mineral nutrition of higher plants (2nd ed., p. 889). London: Academic Press.
McKenna, I. M., Chaney, R. L., & Williams, F. M. (1993). The effects of cadmium and zinc interactions on the accumulation and tissue distribution of zinc and cadmium in lettuce and spinach. Environment Pollution, 79, 113–120.
Mohammad, A., & Moheman, A. (2010). The effects of cadmium and zinc interactions on the accumulation and tissue distribution of cadmium and zinc in tomato (Lycopersicon esculentum Mill.). Archives of Agronomy and Soil Science, 56, 551–561.
Moreno-Caselles, J., Moral, R., Perez-Espinosa, A., & Perez-Murcia, M. D. (2000). Cadmium accumulation and distribution in cucumber plant. Journal of Plant Nutrition, 23, 250–243.
Nan, Z., Li, J., Zhang, J., & Cheng, G. (2002). Cadmium and zinc interactions and their transfer in soil crop system under actual field conditions. Science of the Total Environment, 285, 187–195.
Nelson, D. W., & Sommers, L. E. (1996). Total carbon, organic carbon, and organic matter. In D. L. Sparks, A. L. Page, P. A. Helmke, & R. H. Loeppert (Eds.), Methods of soil analysis: part 3—chemical methods (pp. 961–1010). Madison: ASA and SSSA.
Pallavi, S., & Shanker, D. R. (2005). Cadmium toxicity in plants. Brazilian Journal of Plant Physiology, 17(1), 21–34.
Peralta-Videa, J. R., Gardea-Torresday, J. L., Gomez, E., Tiemann, K. J., Parsons, J. G., & Carrillo, G. (2002). Effect of mixed cadmium, copper, nickel, and zinc at different pHs upon alfalfa growth and heavy metal uptake. Environmental Pollution, 119, 291–301.
Piotrowska, M., Dudka, S., & Chilopecka, A. (1994). Effect of elevated concentrations of Cd and Zn in soil on spring wheat yield and metal contents of plants. Water Air Soil Pollution, 76, 333–341.
Rauser, W. E. (1986). The amount of cadmium associated with Cd-binding protein in roots of Agrostis gigantea, maize and tomato. Plant Science, 43, 85–91.
Rhoades, J. D. (1996). Salinity: electrical conductivity and total dissolved solids. In D. L. Sparks, A. L. Page, P. A. Helmke, & R. H. Loeppert (Eds.), Methods of soil analysis: part 3—chemical methods (pp. 417–435). Madison: ASA and SSSA.
Shang, Z. R., & Leung, J. K. (2003). 110mAg root and foliar uptake in vegetables and its migration in soil. Journal of Environmental Radioactivity, 65, 297–307.
Sheppard, M. I., & Sheppard, S. C. (1985). The plant concentration concept as applied to natural uranium. Health Physics, 48, 494–500.
Simmons, R. W., Pongsakul, P., Chaney, R. L., Saiyasitpanich, D., Klinphoklap, S., & Nobuntou, W. (2003). The relative exclusion of zinc and iron from rice grain in relation to rice grain cadmium as compared to soybean: implications for human health. Plant and Soil, 257, 163–170.
Sinha, S., Gupta, A. K., Bhatt, k., Pandey, K., Rai, U. N., & Singh, K. P. (2006). Distribution of metals in the edible plants grown at Jajmau, Kanpur (India) receiving treated tannery wastewater: relation with physicochemical properties of the soil. Environmental Monitoring and Assessment, 115, 1–22.
Streit, B., & Stumm, W. (1993). Chemical properties of metals and the process of bioaccumulation in terrestrial plants. In B. Market (Ed.), Plants as bio-monoitors: indicators for heavy metals in terrestrial environment (pp. 31–62). Weinheim: VCH.
Sumner, M. E., & Miller, W. P. (1996). Cation exchange capacity and exchange coefficients. In D. L. Sparks, A. L. Page, P. A. Helmke, & R. H. Loeppert (Eds.), Methods of soil analysis: part 3—chemical methods (pp. 1201–1229). Madison: ASA and SSSA.
Takijima, Y., & Katsumi, F. (1973). Cadmium contamination of soils and rice plants caused by zinc mining. l. Production of high-cadmium rice on the paddy fields in lower reaches of the mine station. Soil Science and Plant Nutrition, 19, 29–38.
Thomas, G. W. (1996). Soil pH and soil scidity. In D. L. Sparks, A. L. Page, P. A. Helmke, & R. H. Loeppert (Eds.), Methods of soil analysis: part 3—chemical methods (pp. 475–490). Madison: ASA and SSSA.
Waisberg, M., Joseph, P., Hale, B., & Beyersmann, D. (2003). Molecular and cellular mechanisms of cadmium carcinogenesis. Toxicology, 192, 95–117.
Wang, C., Zhang, S. H., Wang, P. F., Hou, J., Zhang, W. J., Li, W., & Lin, Z. P. (2009). The effect of excess Zn on mineral nutrition and antioxidative response in rapeseed seedlings. Chemosphere, 75(11), 1468–1476.
Wu, F., & Zhang, G. (2002). Alleviation of cadmium-toxicity by application of zinc and ascorbic acid in barley. Journal of Plant Nutrition, 25, 2745–2761.
Yang, H., Wong, J. W., Yang, Z. M., & Zhou, L. X. (2001). Ability of Agrogyron elongatum to accumulate the single metal of cadmium, copper, nickel and lead and root exudation of organic acids. Journal of Environmental Science (China), 13, 368–375.
Acknowledgments
The authors would like to acknowledge the financial support of the Scientific Research Deanship at Jordan University of Science and Technology, grant no. 7-2013. The authors also acknowledge the support from Department of Soil Science/Soil Ecology, Institute of Geography, Ruhr-University Bochum, Germany, for using lab facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gharaibeh, M.A., Albalasmeh, A.A., Marschner, B. et al. Cadmium Uptake and Translocation of Tomato in Response to Simulated Irrigation Water Containing Elevated Concentrations of Cadmium and Zinc in Clayey Soil. Water Air Soil Pollut 227, 133 (2016). https://doi.org/10.1007/s11270-016-2829-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-016-2829-8