Abstract
The effects of 5 μM cadmium (Cd), a non-essential toxic element and 25 and 50 μM zinc (Zn), an essential micronutrient, were investigated in aquatic plant Lemna minor L. after 4 and 7 days of exposure to each metal alone or to their combinations. Both metals showed tendency to accumulate with time, but when present in combination, they reduced uptake of each other. Cd treatment increased the lipid peroxidation and protein oxidation indicating appearance of oxidative stress. However, Zn supplementation in either concentration reduced values of both parameters, while exposure to Zn alone resulted in elevated level of lipid peroxidation and protein oxidation but only on the 7th day. Enhanced DNA damage, which was found on the 4th day in plants treated with Cd alone or in combination with Zn, was reduced on the 7th day in combined treatments. Higher catalase activity obtained in all treated plants on the 4th day of experiment was reduced in Zn-treated plants, but remained high in plants exposed to Cd alone or in combination with Zn after 7 days. Cd exposure resulted in higher peroxidase activity, while Zn addition prominently reduced peroxidase activity in the plants subjected to Cd stress. In conclusion, Cd induced more pronounced oxidative stress and DNA damage than Zn in applied concentrations. Combined treatments showed lower values of oxidative stress parameters—lipid peroxidation, protein oxidation and peroxidase activity as well as lower DNA damage, which indicates alleviating effect of Zn on oxidative stress in Cd-treated plants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
All non-essential heavy metals, as well as essential ones when present in higher concentrations than optimal, affect different cellular components thereby interfering with the normal metabolic functions of plant cell. Reactive oxygen species (ROS), which include superoxide radical (O •−2 ), hydroxyl radicals (OH•) and hydrogen peroxide (H2O2), are commonly generated under different stress conditions, including presence of heavy metals (Arora et al. 2002). ROS have strong oxidizing activities that can attack all types of biomolecules leading to membrane peroxidation, ion leakage, inactivation and damage of proteins, and even DNA strand breaks (Mithöfer et al. 2004). Cells are equipped with enzymatic and non-enzymatic mechanisms to eliminate or reduce harmful effects caused by ROS. These protective mechanisms include antioxidant enzymes such as superoxide dismutase (SOD, E.C. 1.15.1.1), catalase (CAT, E.C. 1.11.1.6), peroxidases like ascorbate (APX, E.C. 1.11.1.11) and guaiacol peroxidase (PPX, E.C. 1.11.1.7) and other antioxidant compounds such as ascorbate, glutathione, carotenoids, etc. (Mittler 2002). Heavy metals can cause oxidative damage by two mechanisms: they enhance production of ROS and slow down or inhibit removal of ROS (Shaw et al. 2004).
As an essential element, zinc (Zn) is of great chemical and metabolic significance in plant systems (Aravind et al. 2009). It plays a fundamental role in several cellular functions such as protein metabolism, gene expression, chromatin structure, photosynthetic carbon metabolism and indole acetic acid metabolism (Aravind and Prasad 2005). Zn is involved in the catalytic function of many enzymes and structural stability of various cell proteins (Vallee and Falchuk 1993). Moreover, it is known to have a stabilizing and protective effect on the biomembranes against oxidative and peroxidative damage, loss of plasma membrane integrity and alteration of the permeability of the membrane (Aravind and Prasad 2003). Zn deficiency can cause an increase in membrane permeability (Cakmak 2000). In the soil solution concentration of Zn needed for optimal plant growth of most plant species is in the range of 1 × 10−5–1 × 10−4 μM (Welch 1995). However, when present in excessive concentration, Zn can limit cell growth (Vaillant et al. 2005; Muschitz et al. 2009) and induce a strong toxicity (Rout and Das 2003).
On the other hand, cadmium (Cd) is very toxic heavy metal, without any metabolic significance (Das et al. 1997). Cd pollution is increasing due to excessive mining, industrial usage and other anthropogenic activities (Aravind and Prasad 2005). When released into the environment Cd can pollute soil, sediments and natural waters and become available to plants. Cd can affect the photosynthetic apparatus, as well as the respiratory and nitrogen metabolism, which results in growth retardation, leaf chlorosis as well as water and nutrient imbalances. It has also been found that Cd causes oxidative stress in many plant species (Singh et al. 2006; Gratão et al. 2008).
Generally, the effect of heavy metals on plant metabolism and physiological processes depends not only on their concentrations but also on relationships to other mineral elements. Since environment is often contaminated with more than one metal in potentially toxic concentration, bioassays in which each chemical is applied separately are inadequate for assessing the potential toxic effect of complex metal mixtures. Due to chemical similarity between Cd and Zn, they both can be taken by plants as divalent cations and therefore Cd can inhibit most of the Zn-dependent processes (Aravind and Prasad 2005). So far, investigations of Zn effect on few plant species suggest antagonistic interaction between Cd and Zn. Wu and Zhang (2002) found that the physiological damage caused by Cd could be alleviated by application of Zn. Moreover, Aravind and Prasad (2003) reported that Cd uptake was suppressed by Zn, while Hassan et al. (2005) found that Zn supplementation can decrease Cd-induced oxidative stress.
Duckweed (Lemna minor L.) is an aquatic plant living in many types of water ecosystems, including lakes, streams and ponds. It has been commonly used as a test organism in ecotoxicological and environmental studies (Horvat et al. 2007; Khellaf and Zerdaoui 2010) due to its high sensitivity to various chemicals, small size, rapid vegetative reproduction and easy handling in laboratory conditions. As a free-floating plant, it takes mineral nutrients through lower frond surface (Lewis 1995).
The objective of present investigation was to assess the oxidative stress caused by Zn and Cd applied separately or in combinations and to find if Zn addition can influence Cd-induced oxidative stress in Lemna minor. For that purpose oxidative damage of cell macromolecules (lipids, proteins, DNA) and activities of antioxidative enzymes (CAT and PPX) was evaluated.
Materials and methods
Materials and instrumentation
CdCl2, CuCl2, HNO3, H2O2, TCA, FeCl2 and K3Fe(CN)6 were obtained from Kemika (Zagreb, Croatia), while Tris, TBA, 2,4-DNPH, guaiacol, PVP and EDTA were purchased from Sigma (Steinheim, Germany). The set of standards applied for preparation of calibration curve for metal concentration determination was obtained from Merck (Darmstadt, Germany). Determination of Cd and Zn content was performed using ICP-OES instrument type IRIS INTREPID II XSP from Thermo Elemental (MA, USA). For nuclei analysis, a Zeiss Axioplane fluorescent microscope from Carl Zeiss MicroImaging (Jena, Germany) was used. Komet version 5 image analysis system from Kinetic Imaging Ltd. (Liverpool, UK) was used to measure percentage of tail DNA. For determination of protein content, MDA and carbonyl content as well as CAT and PPX activities, UV VIS spectrophotometer Specord 40 (Analytik Jena, Germany) was used. For CAT and PPX isoenzyme analysis Bio-Rad vertical gel electrophoresis system (PROTEAN II) was used.
Plant material and culture conditions
Lemna minor L. stock culture was maintained on a Pirson and Seidel nutrient medium (Pirson and Seidel 1950). For the experiment, duckweed plants were subcultured from stock cultures and grown under axenic conditions on Steinberg’s nutrient solution (ISO 2006) at 24 ± 1°C with 16/8 light/dark cycle and light intensity of 40 μE m−2 s−1.
Cd and Zn treatment
Approximately 10 colonies of healthy duckweed plants were transferred into a 300 ml Erlenmeyer flask containing 100 ml Steinberg’s nutrient solution with required concentration of metals. Cd treatment in concentration of 5 μM was prepared by the addition of the stock solution of CdCl2 in the duckweed nutrient medium. Zn was added as ZnCl2 to the nutrient medium in amounts appropriate to achieve concentrations 25 and 50 μM. Duckweed was also exposed to the combinations of metals (5 μM Cd with 25 or 50 μM Zn). After 4 and 7 days of treatment with tested metal solutions, Cd and Zn content as well as lipid peroxidation, carbonyl content, comet assay, CAT and PPX activities of duckweed fronds were determined.
Determination of Cd and Zn content
Plants were oven-dried at 80°C for 24 h until a constant weight was recorded. Plant tissue was then microwave digested in two steps. The first step was digestion in 10 ml of 16 mM HNO3 at 70°C for 5 min, then at 130°C for another 5 min and finally at 150°C for 4 min. The second step was digestion in 1 ml of H2O2 at 85°C for 5 min and then at 130°C for 4 min. After cooling, the samples were diluted with 1% (v/v) HNO3 up to the total volume of 50 ml. Cd and Zn were analysed using Inductively Coupled Plasma-Optical Emission Spectroscopy (ICP-OES, IRIS INTREPID II XSP) according to the HRN EN ISO 11885:1998 standard. The results were processed using TEVA software. Metal concentrations were calculated according to the calibration curve obtained with a set of standards of known concentrations. For Cd we used a lower concentration range of 1–50 μg kg−1, and for Zn a higher concentration range of 50–5000 μg kg−1. Detection limits for Cd and Zn were 0.5 and 10 μg kg−1, respectively. Limit of quantification (LOQ) was <1 and <20 μg kg−1 for Cd and Zn, respectively.
Malondialdehyde and carbonyl content
The level of lipid peroxidation was determined by measuring the amount of malondialdehyde (MDA), a product of lipid peroxidation, according to the modified method of Heath and Packer (1968). Treated plants (50 mg fresh weight) were homogenized in 1 ml of 0.25% (w/v) 2-thiobarbituric acid (TBA) in 10% (w/v) trichloroacetic acid (TCA) and incubated at 95°C for 30 min. The tubes were transferred into an ice bath and then centrifuged at 15 000×g for 10 min and +4°C. The absorbance of the supernatant was recorded at 532 nm and corrected for non-specific turbidity by subtracting the absorbance at 600 nm. As a blank 0.25% TBA in 10% TCA solution was used. The content of lipid peroxides was expressed as total 2-thiobarbituric acid reactive metabolites (TBARS), mainly MDA, as μmol/g of fresh weight using a molar absorption coefficient of 155 mM−1 cm−1.
For carbonyl quantification, the reaction with 2,4-dinitrophenylhydrazine (DNPH) was used basically as described by Levine et al. (1994). Fresh plant tissue (80 mg) was homogenized in 1 ml of 10 mM potassium phosphate buffer (pH 7.4) which contained 1 mM ethylenediaminetetraacetic acid (EDTA) along with polyvinylpyrrolidone (PVP). After centrifugation at 20 000×g and +4°C for 20 min, the supernatants (200 μl) were combined with 300 μl of 10 mM DNPH in 2 M HCl. After 1 h incubation at room temperature, the proteins were precipitated with 500 μl of cold 10% (w/v) TCA. Samples were cooled at −20°C and then centrifuged for 10 min at 12 000×g and +4°C. The pellets were washed three times with 500 μl of ethanol/ethylacetate (1/1 v/v) to remove excess reagent. The precipitated proteins were finally dissolved in 6 M urea in 20 mM potassium phosphate buffer (pH 2.4) in an ultrasonic bath and the absorbance at 370 nm was measured. Protein recovery was estimated for each sample by measuring the absorbance at 280 nm. Carbonyl content was calculated using a molar absorption coefficient for aliphatic hydrazones of 22 mM−1 cm−1 and expressed as μmol/mg of proteins.
Comet assay
For the genotoxicity assessment, we used an alkaline version of the cellular comet assay (Single Cell Gel Electrophoresis) following the protocol by Gichner et al. (2004) with a slight modification (10 min denaturation, 10 min electrophoresis at 0.72 V cm−1 and 300 mA). Duckweed plants were placed in a 60 mm diameter Petri dish containing 200 ml of ice-cold 400 mM Tris–HCl buffer (pH 7.5) and cut into small pieces with a sterile razor blade to isolate the nuclei. Partially frosted microscope slides were briefly dipped in the solution of 1% (w/v) normal melting point agarose prepared with deH2O and left to dry over night. The mixture of 120 μl of the nuclear suspension and 120 μl of 1% (w/v) low melting agarose in phosphate-buffered saline, pH 7.5, was prepared. 100 μl of the mixture was gently mixed, and spread onto each slide. Prior to electrophoresis, the slides were put in an electrophoresis buffer (1 mM EDTA and 300 mM NaOH, pH > 13) for 10 min at +4°C to allow the DNA to unwind. Following denaturation, electrophoresis was performed in the same buffer and in the same conditions at 0.72 V cm−1 and 300 mA. After electrophoresis, slides were gently rinsed 3 times for 5 min with neutralisation buffer (400 mM Tris buffer, pH 7.5). Prior to analysis slides were dehydrated and stained with 70 μl ethidium bromide (10 μg ml−1) for 5 min, dipped in cold water to remove the excess of ethidium bromide and finally covered with a coverslip. Fifty randomly chosen nuclei per treatment from two independent experiments were analysed under a fluorescent microscope equipped with an excitation filter BP 520/09 nm and a barrier filter of 610 nm. A computerised image analysis system (Komet version 5) was used to measure percentage of tail DNA (% tDNA).
CAT and PPX enzyme assays
Plants (80 mg) were homogenized in 1 ml of cold 50 mM potassium phosphate buffer (pH 7.0), containing 0.1 mM EDTA and polyvinylpyrrolidone (PVP). The homogenate was centrifuged at 20 000×g for 30 min at +4°C and the supernatant was used for the CAT and PPX enzyme assays.
The soluble protein content was determined according to Bradford method (Bradford 1976) using bovine serum albumin as a standard.
For the isoenzyme analysis of the both investigated antioxidative enzymes (CAT and PPX), polyacrylamide gel electrophoresis (PAGE) without sodium dodecylsulfate (SDS) was performed on 10% (w/v) polyacrylamide gels (Laemmli 1970).
CAT (EC 1.11.1.6) activity was evaluated spectrophotometrically according to Aebi (1984) by measuring the decrease in H2O2 absorbance at 240 nm (ε = 36 mM−1 cm−1) every 10 s during 2 min. The reaction mixture consisted of 50 mM potassium phosphate buffer (pH 7.0), 10 mM H2O2, and 50 μl enzyme extract. CAT activity was expressed as μmol of decomposed H2O2 per min per mg of proteins. For CAT in-gel detection, the gels were incubated in distilled water for 45 min and then in H2O2 solution (0.003% v/v) for 10 min. The gels were then washed in distilled water and stained in a 1:1 mixture of 2% (w/v) FeCl3 and 2% (w/v) K3Fe(CN)6 for 10 min (Woodbury et al. 1971).
PPX activity was determined spectrophotometrically by measuring the increase in absorbance at 470 nm (ε = mM−1 cm−1) every 15 s during 2.5 min. The reaction mixture consisted of 50 mM potassium phosphate buffer (pH 7.0), 18 mM guaiacol, 5 mM H2O2 (Chance and Maehly, 1955) and 50 μl enzyme extract. PPX activity was expressed as μmol of formed tetraguiaicol per min per mg of proteins. For PPX in-gel detection, the gels were equilibrated with 50 mM potassium phosphate buffer (pH 7.0) for 30 min, then incubated in 50 mM potassium phosphate buffer (pH 7.0) containing 20 mM guaiacol and 4 mM H2O2, until brown bands appeared (Chance and Maehly 1955).
Statistical analysis
The results of each assay were compared by analysis of variance (ANOVA), Newman–Keuls test using the STATISTICA 8.0 (Stat Soft Inc., USA) software package. Differences between corresponding controls and exposed samples were considered statistically significant at p < 0.05. Each data point is the average of six replicates unless stated otherwise.
Results
Cd and Zn content
Heavy metal concentrations (μg g−1 DW) in Lemna minor plants were measured 4 and 7 days after exposure to Cd and/or Zn (Table 1). On the 4th day of the experiment plants exposed to Cd alone exhibited the highest Cd content, which significantly decreased after exposure to combination of Cd and both examined concentrations of Zn. On the 7th day the highest Cd concentration was obtained again in plants exposed to Cd alone. Combination of Cd and higher examined concentration of Zn (50 μM) significantly decreased the Cd content in comparison to combined treatment with Cd and lower tested Zn concentration (25 μM) and particularly to treatment with Cd alone. In comparison to the 4th day of experiment the longer time of exposure (7 days) resulted in significantly higher Cd accumulation in each treatment.
In plants which were not exposed to Cd (control and Zn-treated plants), detected Cd content was below the instrument LOQ (<0.001 μg g−1).
On the 4th day of the experiment the combination of Cd and lower Zn concentration (25 μM) resulted in higher Zn accumulation in comparison to control and Cd-treated plants. Both Zn treatments and combination of Cd and higher Zn concentration (50 μM) exhibited similarly high values. The highest Zn concentration was obtained in plants exposed 7 days to the higher Zn concentration alone (50 μM), while the treatment with 25 μM Zn as well as with the combination of Cd and 50 μM Zn resulted in a lower Zn accumulation. The combination of Cd and 25 μM Zn showed even lower values, but significantly higher in comparison to control and Cd-treated plants, in which the lowest Zn concentration was measured on both investigated days.
Effect on lipid peroxidation and protein oxidation
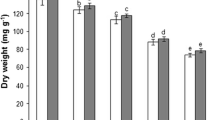
On the 4th day of the experiment the highest MDA content was measured in plants treated with 5 μM Cd, which was the only treatment exhibiting significantly higher MDA content in comparison to control plants (Fig. 1a). Plants exposed to 50 μM Zn had significantly lower MDA content in comparison to those treated with 25 μM Zn either alone or in combination with Cd. On the 7th day of the experiment the highest MDA content was measured again in plants treated with Cd alone, although in comparison to control plants, significantly higher values were also obtained in plants treated with 25 and 50 μM Zn. No significant difference was observed between control plants and plants treated with combination of 25 or 50 μM Zn and Cd neither on the 4th nor on the 7th day of the experiment. Almost all treatments showed significant decrease in MDA content on the 7th day in comparison to 4th day, except for the 50 μM Zn, where significantly higher values were obtained. The values obtained seven days after exposure to the combined treatment with 50 μM Zn and Cd were lower when compared with values measured after 4 days, although they showed no significant difference.
Lipid peroxidation and protein oxidation in Lemna minor L. on days 4 and 7 of exposure to Cd (5 μM), Zn (25 and 50 μM) and their combinations are shown as: a MDA content; b protein carbonyl content. Values are means ± SE based on six replicates. If columns are marked with different letters, the treatments (mean values) are significantly different (p < 0.05) according to Newman–Keuls test
On the 4th day of the experiment significantly elevated content of carbonyl was found in plants exposed to combination of 50 μM Zn with Cd in comparison to control plants (Fig. 1b). Seven days after exposure significantly higher value was measured in plants treated with Zn alone at both concentrations when compared to control plants as well as to plants from other treatments. The carbonyl content was significantly higher on the 7th day of experiment, in plants exposed to 25 or 50 μM Zn in comparison to the day 4.
Effect on DNA
In the comet test, fragmented DNA forms the structure resembling the comet tail and therefore percentage of tail DNA is used as a measure of DNA damage. On the 4th day of experiment, treatment with 5 μM Cd alone as well as in combination with 25 μM Zn exhibited significantly longer DNA tails in comparison to control plants, while the highest DNA damage was obtained in the treatment with 50 μM Zn in combination with Cd. However, on the 7th day of experiment significantly longer DNA tail was observed only in treatment with 5 μM Cd. Interestingly, the DNA damage significantly decreased in the plants exposed to 25 or 50 μM Zn in combination with Cd on the 7th day when compared with the results from the 4th day (Fig. 2).
DNA damage after 4 and 7 days of exposure to Cd (5 μM), Zn (25 and 50 μM) and their combinations. Differences in % tail DNA are presented as mean value ± SE based on six replicates. If columns are marked with different letters, the treatments (mean values) are significantly different (p < 0.05) according to Newman–Keuls test
Effect on antioxidant enzyme activity
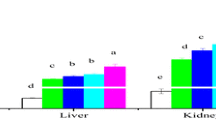
On the 4th day of experiment significantly higher CAT activity was observed in all treatments in comparison to the control. On the 7th day, plants treated with combination of Cd and both concentrations of Zn exhibited the highest activity. In plants exposed to Cd alone obtained activity was lower in comparison to combined Cd/Zn treatments, but significantly higher when compared to control plants and those exposed to Zn alone in both concentrations. All treatments showed significant decrease in CAT activity on the 7th day of experiment in comparison to the 4th day (Fig. 3a).
Catalase (CAT) activity. Differences in a CAT activity on days 4 and 7 of experiment. Values are means ± SE based on six replicates. If columns are marked with different letters, the treatments (mean values) are significantly different (p < 0.05) according to Newman–Keuls test; b Isoenzyme pattern of CAT on days 4 and 7 of the treatments. Equal amounts of proteins (100 μg) were loaded on the gel. 1 control, 2 5 μM Cd, 3 25 μM Zn, 4 50 μM Zn, 5 5 μM Cd + 25 μM Zn, 6 5 μM Cd + 50 μM Zn
Electrophoretic analysis revealed two CAT isoenzymes which were marked as CAT 1 and CAT 2 according to their rising mobility. Both isoforms were present in control as well as in all investigated treatments (Fig. 3b).
On the 4th day of the experiment significantly higher PPX activity was obtained in plants treated with either Cd alone or in combination with 25 μM Zn in comparison to control and other treatments. However, on the 7th day only in plants exposed to Cd alone PPX activity remained high, and it was even higher than the values obtained in Cd-treated plants on the 4th day of experiment (Fig. 4a).
Peroxidase (PPX) activity. Differences in a PPX activitiy on days 4 and 7 of experiment. Values are means ± SE based on six replicates. If columns are marked with different letters, the treatments (mean values) are significantly different (p < 0.05) according to Newman–Keuls test; b Isoenzyme pattern of PPX on days 4 and 7 of the treatments. Equal amounts of proteins (100 μg) were loaded on the gel. 1 control, 2 5 μM Cd, 3 25 μM Zn, 4 50 μM Zn, 5 5 μM Cd + 25 μM Zn, 6 5 μM Cd + 50 μM Zn
In-gel PPX activity exhibited six PPX isoforms which were marked as PPX 1–PPX 6 according to their rising mobility (Fig. 4b). Isoenzymes PPX 1–PPX 5 were common for all investigated plants. On the 4th day of experiment higher staining intensity of all isoforms was obtained in plants exposed to Cd alone. Moreover, these plants exhibited the additional PPX 6 isoform, which was missing in control and other treatments. On the 7th day of the experiment stronger staining intensity of PPX 2 isoform was present in all samples, while the PPX 6 isoform was not detected.
Discussion
Interaction between mineral nutrients and non-essential elements may be important for understanding, analyzing and improving plant responses to metal mixtures which could appear in realistic environment. Since Cd and Zn are both taken by plants as divalent cations, and both belong to the same group (II b) of transition metals, Cd can readily inhibit most of the Zn-dependent processes by binding to the crucial and functional membranes and enzyme active sites, inactivating their functions (Shaw et al. 2004). Hence, increased Zn concentration is able to replace a physiologically unimportant metal like Cd and accordingly reduce its toxic effects (Aravind and Prasad 2005).
The present investigation has shown that both metals tend to accumulate in Lemna minor which is in accordance with data about Lemna species as metal accumulators (Mohan and Hosetti 1997; Khellaf and Zerdaoui 2010). In plants treated with Cd and Zn alone, metal uptake increased with duration of treatment, although the relationship was not linear. Kwan and Smith (1991) found that after 10 days Cd uptake reached steady state concentration probably due to Cd-binding to cell constituents from which it can be slowly removed. Contrary to Cd, Zn was detected in plants grown on control medium as well as in those treated with Cd. It reflects the presence of Zn as a micronutrient which was added in Steinberg medium in concentration of 0.63 μM.
Investigated metals, when present in combination, influenced uptake of each other. At the 4th day of experiment, both applied concentrations of Zn had a significant effect on reduction of Cd content. Moreover, higher concentration of Zn (50 μM) was even more effective in reducing Cd uptake than lower Zn concentration (25 μM) after longer exposure (7 days). Our findings are in accordance with results reported for Ceratophyllum demersum, a free floating freshwater macrophyte, where the suppression in Cd uptake by increased Zn concentration was observed (Aravind and Prasad 2003; Aravind and Prasad 2005; Aravind et al. 2009). Furthermore, Hassan et al. (2005) reported a significant decrease in Cd concentration by Zn application in rice roots. On the other hand, Cd reduced Zn accumulation in plants grown on combined treatments in comparison to plants treated with Zn alone, especially after longer exposure (7 days). Reduced concentration of Zn in Cd-treated plants was already reported by Cakmak (2000) and Cd has been described as an antimetabolite of Zn due to the observed Zn deficiency in various Cd-treated plants (Peraza et al. 1998). However, in our experiment, Zn content was still much higher in combined treatments than in control Lemna plants, therefore Zn deficiency can be excluded.
Obtained results suggest a strong competition between Zn and Cd uptake in the plant system, particularly after prolonged exposure (7 days). Generally, there are specific metal ion uptake systems in cells, especially for essential micronutrients. On the other hand, transport of non-essential elements like Cd is most likely to occur via membrane transporters of essential cations, for example ZIP proteins, known to transport Zn, or iron transporters (Aravind and Prasad 2005). Also, uptake of metals is depended on the H+ gradient generated by H+-ATPase located in the plasma membrane. There are some evidences for the regulation of the H+-ATPase-mediated proton pumping mechanism by Zn, through which Zn can indirectly antagonize Cd entry into the plant cell (Aravind and Prasad 2005 and references therein).
The main site of any redox active metal attack in a plant cell is usually the cell membrane. Heavy metals induce severe lipid peroxidation, which can be initiated by activated oxygen species such as O •−2 , OH• and 1O2 or by the action of lipoxygenase (Singh et al. 2006). The highest MDA content was found in Lemna plants exposed to Cd on both 4th and 7th day of the experiment, which indicates appearance of the metal-caused oxidative damage. Our findings that Cd toxicity induces oxidative stress are in accordance with the reports of other authors (Singh et al. 2006; Gratão et al. 2008; Tkalec et al. 2008). However, from the results obtained on both 4th and 7th day of experiment it was evident that Zn supplementation in either concentration significantly reduced lipid peroxidation, which is in consistence with findings reported for C. demersum (Aravind and Prasad 2003). Zn can reduce lipid peroxidation and protect plants from ROS. In C. demersum Cd increased free radical production by mediating enhanced NADPH oxidation. It was found that Zn inhibited NADPH oxidation induced by Cd, thereby preventing ROS formation (Aravind and Prasad 2005). In our experiment, in plants treated with Zn only, MDA content did not show any significant change on the 4th day in comparison to the control. Nevertheless, on the 7th day MDA values for both Zn concentrations were elevated, although not as much as for Cd treatment. Our results are contrary to those obtained in C. demersum where Zn, even in the concentration of 200 μM, did not show any toxic effects (Aravind and Prasad 2005). However, it was reported that Zn enhanced lipid peroxidation in 10-day-old bean plants (Chaoui et al. 1997) and induced ROS production in Phaseolus vulgaris when applied as 50 μM Zn (Cuypers et al. 2001) as well as in Oryza sativa in concentrations between 0.2 and 25 μM (Lin et al. 2005). Zn, based on its chemical properties, cannot directly induce formation of ROS. Therefore, toxic forms of oxygen, which cause lipid peroxidation, probably arise as a consequence of Zn interaction with lipid membrane which could produce conformational changes able to activate the plasma membrane-localised NADPH-oxidase and generate ROS (Bueno and Piqueras 2002). However, mechanisms of this oxidative burst and its relation to oxidative stress are still poorly understood.
Proteins can also be affected by ROS, either by oxidation of amino acid side chains or by reactions with aldehydic products of lipid peroxidation. Both of these reactions can introduce carbonyl-groups into proteins, and the appearance of such groups is taken as an evidence of oxidative stress (Sandalio et al. 2001). In this work, on the 4th day of experiment significantly elevated carbonyl content was observed only in plants treated with combination of Cd and 50 μM Zn. Seven days after exposure to both Zn concentrations, significantly higher carbonyl content was measured in comparison to control and other treatments. These results are in accordance with elevated MDA values in abovementioned treatments and could be explained by the findings that Zn in higher concentrations can also induce oxidative stress in various plant species (Drost et al. 2007; Źróbek-Sokolnik et al. 2009). Moreover, exposure to combinations of Cd and Zn in our experiment resulted in lower carbonyl content in comparison to plants treated with Zn alone, probably reflecting the reduced concentration of Zn in plants. Cd-treated plants exhibited only a slight increase in carbonyl content, which was not significantly different in comparison to control plants, but still indicates induced ROS production. Obtained results are somewhat surprising because enhancement of carbonyl content has been reported in C. demersum treated with 10 μM Cd (Aravind and Prasad 2005) as well as in Zea mays exposed to 6 μM Cd (Rellán-Álvarez et al. 2006). However, in pea plants grown in presence of Cd significant increase of carbonyl content was found only at higher Cd concentrations (40 and 50 μM), while lipid peroxidation was noticed at lower Cd concentration (Sandalio et al. 2001).
Besides lipids and proteins, nucleic acids are also highly susceptible to metal-catalyzed oxidations in which both nucleoside bases and sugar moieties are targets of ROS (Aravind and Prasad 2005). The mediation of metal toxicity on DNA damage may be direct (Hossain and Huq 2002) or indirect (Gichner et al. 2004). Alkaline protocol of the plant comet assay has already been reported as fast, reliable, simple and widely used method for estimation of the extent of induced DNA damage (Gichner et al. 2008) and for evaluation of the genotoxicity of chemicals in the environment (Lin et al. 2007). In our study, elevated DNA damage was recorded on the 4th and 7th day of experiment in plants treated with Cd which indicates its genotoxic effect. The genotoxic effect of Cd has already been found in several plant species (Gichner et al. 2004; Ünyayar et al. 2006; Tuteja et al. 2001). The possible pathway(s) of Cd induced genotoxicity are still unknown, but may involve the interaction of this metal with DNA, either directly or indirectly via the induction of oxidative stress (Valverde et al. 2001). Interestingly, elevated DNA damage was also found in plants treated with combination of Cd and both Zn concentrations on the 4th day. However, on the 7th day values obtained in those treatments corresponded to control plants, which indicates DNA damage repair in plants exposed to both Cd and Zn combinations after 7 days of experiment. Moreover, our results are in accordance with reports on aquatic plant C. demersum in which was suggested that Zn probably modulates the protection of DNA from Cd-induced damage either by inhibition of Ca2+/Mg2+-dependent endonuclease or by inhibiting metal-catalyzed oxidative damage on either the DNA or protein (Aravind and Prasad 2005; Aravind et al. 2009). In our study, increased level of DNA damage can be correlated with increased MDA and protein carbonyl contents, suggesting that the overproduction of ROS could induce DNA damage (Gichner et al. 2004). Therefore, it can be concluded that DNA damage caused by Cd-induced oxidative stress contributes to Cd-phytotoxicity in Lemna minor plants.
In response to the oxidative stress, plants employ antioxidative defence system to scavenge ROS and prevent destructive oxidative reactions (Arora et al. 2002). In this study, the antioxidative enzymes, CAT and PPX, were investigated to estimate the efficiency of antioxidative mechanism of the plants in response to Cd, Zn or their combinations. Metal treatments can have different effects on enzymes—enhancement caused by increased level of ROS or suppression by binding of metals on important groups of enzymes (Arora et al. 2002). Significantly higher CAT as well as PPX activity was obtained in plants after Cd exposure on both treatment times, which is in agreement with previous findings for Lemna (Tkalec et al. 2008; Razinger et al. 2008) as well as for other plants species treated with Cd (Shah et al. 2001; Gratão et al. 2008). However, opposite effect or unchanged activity has also been reported (Aravind and Prasad 2005; Cho and Seo 2005) especially at higher Cd concentrations, which probably indicates inhibitory effects of metals on enzymes (Shaw et al. 2004). Interestingly, an elevated CAT activity was also found in plants exposed to Zn either alone or in combination with Cd on the 4th day of experiment. Aravind and Prasad (2005) reported that Zn treatment enhanced SOD, CAT and peroxidase activity for 3–5 times in C. demersum, which could be associated with possible role of Zn in stimulating the biosynthesis of antioxidant enzymes (Cakmak 2000). However, in our case PPX activity was not significantly changed after treatment with Zn alone; it was even reduced after longer exposure to 50 μM Zn. Moreover, addition of 25 μM Zn dramatically decreased PPX activity in the plants subjected to Cd stress, which is in accordance with results found by Hassan et al. (2005) in rice, but in contrast with findings reported by Aravind and Prasad (2005) for C. demersum. Obtained results could indicate different response of PPX to applied Zn in Lemna plants or possible involvement in cellular processes other than scavenging ROS. It is known that plant PPXs participate in lignification, suberization, auxin catabolism and wound healing (Hiraga et al. 2001), and even in ROS generation (Kawano 2003). After 7 days CAT activity was reduced in Zn-treated plants, but remained high in plants exposed to both combinations of Cd and Zn. Aravind and Prasad (2003) also reported a high increase in the CAT activity in C. demersum plants exposed to combined treatments with Cd and Zn as compared to plants treated with Cd or Zn alone. This indicates the efficient antioxidative and ROS scavenging activity by Zn against Cd-induced free radicals and oxidative stress.
In conclusion, obtained results showed that Lemna plants accumulated high concentration of Cd and Zn, while combined treatments showed reduced uptake of both metals. Cd induced more pronounced oxidative stress and DNA damage than Zn in applied concentrations. Moreover, Zn supplementation resulted in decreased lipid peroxidation, protein oxidation and PPX activity as well as in lower DNA-damage, whereas CAT activity was increased. Our results indicate that Zn alleviates oxidative stress in Cd-treated plants probably through suppressed Cd uptake and increased antioxidative and ROS scavenging capacity.
References
Aebi M (1984) Catalase in vitro. Method Enzymol 105:121–126
Aravind P, Prasad MNV (2003) Zinc alleviates cadmium induced toxicity in Ceratophyllum demersum, a fresh water macrophyte. Plant Physiol Biochem 41:391–397
Aravind P, Prasad MNV (2005) Cadmium-zinc interactions in a hydroponic system using Ceratophyllum demersum L.: adaptive ecophysiology, biochemistry and molecular toxicology. Braz J Plant Physiol 17:3–20
Aravind P, Prasad MNV, Malec P, Waloszek A, Strzałka K (2009) Zinc protects Ceratophyllum demersum L. (free-floating hydrophyte) against reactive oxygen species induced by cadmium. J Trace Elem Med Biol 23:50–60
Arora A, Sairam RK, Srivastava GC (2002) Oxidative stress and antioxidative system in plants. Curr Sci 82:1227–1234
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Bueno P, Piqueras A (2002) Effect of transition metals on stress, lipid peroxidation and antioxidant enzyme activities in tobacco cell cultures. Plant Growth Regul 36:161–167
Cakmak I (2000) Possible roles of zinc in protecting plant cells from damage by reactive oxygen species. New Phytol 146:185–205
Chance B, Maehly AC (1955) Assay of catalases and peroxidases. Method Enzymol 2:764–775
Chaoui A, Mazhoudi S, Ghorbal MH, El Ferjani E (1997) Cadmium and zinc induction of lipid peroxidation and effects on antioxidant enzyme activities in bean (Phaseolus vulgaris L.). Plant Sci 127:139–147
Cho U-H, Seo N-H (2005) Oxidative stress in Arabidopsis thaliana exposed to cadmium is due to hydrogen peroxide accumulation. Plant Sci 168:113–120
Cuypers A, Vangronsveld J, Clijsters H (2001) The redox status of plant cells (AsA and GSH) is sensitive to zinc imposed oxidative stress in roots and primary leaves of Phaseolus vulgaris. Plant Physiol Biochem 39:657–664
Das P, Samantaray S, Rout GR (1997) Studies on cadmium toxicity in plants: a review. Environ Pollut 98:29–36
Drost W, Matzke M, Backhaus T (2007) Heavy metal toxicity to Lemna minor: studies on the time dependence of growth inhibition and the recovery after exposure. Chemosphere 67:36–43
Gichner T, Patková Z, Száková J, Demnerová K (2004) Cadmium induces DNA damage in tobacco roots, but no DNA damage, somatic mutations or homologous recombination in tobacco leaves. Mutat Res 559:49–57
Gichner T, Patková Z, Száková J, Žnidar I, Mukherjee A (2008) DNA damage in potato plants induced by cadmium, ethyl methanesulphonate and γ-rays. Environ Exp Bot 62:113–119
Gratão PL, Monteiro CC, Antunes AM, Peres LEP, Azevedo RA (2008) Acquired tolerance of tomato (Lycopersicon esculentum cv. Micro-Tom) plants to cadmium-induced stress. Ann Appl Biol 153:321–333
Hassan MJ, Zhang G, Wu F, Wie K, Chen Z (2005) Zinc alleviates growth inhibition and oxidative stress caused by cadmium in rice. J Plant Nutr Soil Sci 168:255–261
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Hiraga S, Sasaki K, Ito H, Ohashi Y, Matsui H (2001) A large family of class III plant peroxidases. Plant Cell Physiol 42:462–468
Horvat T, Vidaković-Cifrek Ž, Oreščanin V, Tkalec M, Pevalek-Kozlina B (2007) Toxicity assessment of heavy metal mixtures by Lemna minor L. Sci Total Environ 384:229–238
Hossain Z, Huq F (2002) Studies on the interaction between Cd2+ ions and DNA. J Inorg Biochem 90:85–96
ISO - International Organization for Standardization (2006) Determination of the toxic effect of water constituents and wastewater on duckweed (Lemna minor)—Duckweed growth inhibition test. ISO norm 20079
Kawano T (2003) Role of the reactive oxygen species generating peroxidase reactions in plant defence and growth induction. Plant Cell Rep 21:829–837
Khellaf N, Zerdaoui M (2010) Growth response of the duckweed Lemna gibba L. to copper and nickel phytoaccumulation. Ecotoxicology 19:1363–1368
Kwan KHM, Smith S (1991) Some aspects of the kinetics of cadmium uptake by fronds of Lemna minor L. New Phytol 117:91–102
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Levine RL, Williams JA, Stadtman ER, Shacter E (1994) Carbonyl assay for determination of oxidatively modified proteins. Method Enzymol 233:346–357
Lewis MA (1995) Use of freshwater plants for phytotoxicity testing: A review. Environ Pollut 87:319–336
Lin CW, Chang HB, Huang HJ (2005) Zinc induces mitogen-activated protein kinase activation by reactive oxygen species in rice roots. Plant Physiol Biochem 43:963–968
Lin A, Zhang X, Chen M, Cao Q (2007) Oxidative stress and DNA damages induced by cadmium accumulation. J Environ Sci 19:596–602
Mithöfer A, Schultze B, Boland W (2004) Biotic and heavy metal stress response in plants: evidence for common signals. FEBS Lett 566:1–5
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Mohan BS, Hosetti BB (1997) Potential phytotoxicity of lead and cadmium to Lemna minor grown in sewage stabilization ponds. Environ Pollut 98:233–238
Muschitz A, Faugeron C, Morvan H (2009) Response of cultured tomato cells subjected to excess zinc: role of cell wall in zinc compartmentation. Acta Physiol Plant 31:1197–1204
Peraza MA, Fierro FA, Barber DS, Casarez E, Rael LT (1998) Effects of micronutrients on metal toxicity. Environ Health Persp 106:203–216
Pirson A, Seidel F (1950) Zell- und stoffwechselphysiologische Untersuchungen an der Wurzel von Lemna minor unter besonderer Berücksichtigung von Kalium- und Calciummangel [Cell metabolism and physiology in Lemna minor root deprived of potassium and calcium, in German]. Planta 38:431–473
Razinger J, Dermastia M, Dolenc Koce J, Zrimec A (2008) Oxidative stress in duckweed (Lemna minor L.) caused by short-term cadmium exposure. Environ Pollut 153:687–694
Rellán-Álvarez R, Ortega-Villasante C, Álvarez-Fernández A, Del Campo FF, Hernández LE (2006) Stress responses of Zea mays to cadmium and mercury. Plant Soil 279:41–50
Rout GR, Das P (2003) Effect of metal toxicity on plant growth and metabolism: I. Zinc. Agronomie 23:3–11
Sandalio LM, Dalurzo HC, Gómez M, Romero-Puertas MC, del Río LA (2001) Cadmium-induced changes in the growth and oxidative metabolism of pea plants. J Exp Bot 52:2115–2126
Shah K, Kumar RG, Verma S, Dubey RS (2001) Effect of cadmium on lipid peroxidation, superoxide anion generation and activities of antioxidant enzymes in growing rice seedlings. Plant Sci 161:1135–1144
Shaw BP, Sahu SK, Mishra RK (2004) Heavy metal induced oxidative damage in terrestrial plants. In: Prasad MNV (ed) Heavy metal stress in plants. From biomolecules to ecosystems. Springer, Berlin, Heidelberg, pp 84–126
Singh S, Eapen S, D’Souza SF (2006) Cadmium accumulation and its influence on lipid peroxidation and antioxidative system in an aquatic plant, Bacopa monnieri L. Chemosphere 62:233–246
Tkalec M, Prebeg T, Roje V, Pevalek-Kozlina B, Ljubešić N (2008) Cadmium-induced responses in duckweed Lemna minor L. Acta Physiol Plant 30:881–890
Tuteja N, Singh MB, Misra MK, Bhalla PL, Tuteja R (2001) Molecular mechanisms of DNA damage and repair: progress in plants. A review. Crit Rev Biochem Mol Biol 36:337–397
Ünyayar S, Celik A, Cekic OF, Gozel A (2006) Cadmium induced genotoxicity, cytotoxicity and lipid peroxidation in Allium sativum and Vicia faba. Mutagenesis 21:77–81
Vaillant N, Monnet F, Hitmi A, Sallanon H, Coudret A (2005) Comparative study of responses in four Datura species to a zinc stress. Chemosphere 59:1005–1013
Vallee BL, Falchuk KH (1993) The biochemical basis of zinc physiology. Physiol Rev 73:79–118
Valverde M, Trejo C, Rojas E (2001) Is the capacity of lead acetate and cadmium chloride to induce genotoxic damage due to direct DNA-metal interaction? Mutagenesis 16:265–270
Welch RM (1995) Micronutrient nutrition of plants. Crit Rev Plant Sci 14:49–82
Woodbury WA, Spencer K, Stahlmann MA (1971) An improved procedure using ferricyanide for detecting catalase isozymes. Anal Biochem 44:301–305
Wu F, Zhang G (2002) Alleviation of cadmium-toxicity by application of zinc and ascorbic acid in barley. J Plant Nutr 25:2745–2761
Źróbek-Sokolnik A, Asard H, Górska-Koplińska K, Górecki RJ (2009) Cadmium and zinc-mediated oxidative burst in tobacco BY-2 cell suspension cultures. Acta Physiol Plant 31:43–49
Acknowledgments
This work was supported by the Ministry of Science, Education and Sports of the Republic of Croatia, projects no. 119-1191196-1202 and 119-0982934-3110.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Balen, B., Tkalec, M., Šikić, S. et al. Biochemical responses of Lemna minor experimentally exposed to cadmium and zinc. Ecotoxicology 20, 815–826 (2011). https://doi.org/10.1007/s10646-011-0633-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-011-0633-1