Abstract
This study investigates the use of sericite beads and microalgae for the removal of heavy metals from acid mine drainage (AMD) and the simultaneous enhancement of biomass productivity. The experiment was conducted over a period of 6 days in a hybrid system containing sericite beads and microalgae Chlorella sp. The results show that the biomass production increased to ~8.04 times its initial concentration of 0.367 g/L as measured by an optical panel photobioreactor (OPPBR) and had a light transmittance of 95 % at a 305-mm depth. Simultaneous percent removal of Fe, Cu, Zn, Mn, As, and Cd from the AMD effluent was found to be 97.78 to 99.26 %. Biomass production was significantly enhanced by removal of heavy metal ions. We thus found that our hybrid system of sericite beads and microalgae was highly effective in removing heavy metal and in enhancing biomass production and could be a useful alternative treatment of AMD.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Acid mine drainage (AMD) is a serious environmental problem when it contaminates water bodies in and around the abandoned mines (Johnson and Hallberg 2005). Several methods have been described for the treatment of AMD. Adsorption is one of the most commonly used methods and is efficient, particularly in the removal of toxic pollutants from effluent waters (An et al. 2007; Ata and Koldas 2006). Even though sorbents are highly effective in metal removal compared to other removal treatments, the cost of sorbents such as activated carbon or zeolite is high compared to that of other sorbent (Coulton et al. 2003). To overcome this high sorbent cost, the identification and development of low-cost and eco-friendly adsorbents are currently gaining increasing attention for effective and useful AMD treatment (Genc et al. 2003; Johnson and Hallberg 2003). A modified sericite and microalgae hybrid system can meet this need. The biosorbent sericite is much less costly than other biosorbents and is readily available anywhere in Korea.
The use of natural clays as an alternative sorbent in AMD treatment is being widely studied because clays have several advantages: low cost, wide natural availability, lack of toxicity, high specific surface area, and an affinity for charged pollutants (Kim et al. 2014; Kim and Lee 2010). Sericite is a natural fine powder of muscovite-type clay having nanosized layer structure. Sericite is a clay mineral whose major constituents are SiO2, Al2O3, Fe2O3, CaO, and MgO and is a cryptocrystalline form of muscovite (Reddy et al. 2013). It is a fine lustrous powder. Sericite is a petrographic term used to indicate highly refractive and fine-grained mica, found in hydrothermally altered rocks (Lee and Tiwari 2014). Typically, sericite consists of a three-layered unit cell, consisting of two silicon-oxygen tetrahedrons and one aluminum/magnesium-oxygen octahedron sheet. Within the interspace, potassium, sodium, or magnesium ionic bonds hold the layers together and compensate the excess charge of unit cell (Tiwari and Lee 2012). It is very abundant in Korea. The presence of hydroxyl groups on the surface of sericite makes it a useful material for the removal of various pollutants (Reddy et al. 2013; Kwon and Jeon 2013).
Microalgae can sequester heavy metal ions by the same adsorption and absorption mechanisms as other microbial biomass. As living organisms, microalgae require traces of heavy metals for some of their enzymatic activity. However, high concentrations of heavy metals are toxic to them because at such concentrations, the metals severely inhibit some metabolic reactions (Ajjabi and Chouba 2009). Some authors have studied the influence of pH on the toxicity of heavy metals for microalgae growth (Mendoza-Co et al. 2005; Liu et al. 1996). Microalgae have an affinity for polyvalent ions, enabling them to remove heavy metal ions from the environment (Romera et al. 2007; Choi 2014). This aspect is very important from the environmental protection point of view.
There are several biological, chemical, and physical processes for heavy metal removal from AMD, but no one has reported on the use of a sericite beads and microalgae hybrid system in the treatment of AMD. We investigated such an application in this study.
2 Materials and Methods
2.1 Characteristics of AMD
The chemical characteristics of the AMD used for this investigation are shown in Table 1. The raw AMD was collected from the Young-Dong mine, located in Gangneung city, in Korea. This AMD is contained excesses of Fe, Cu, Zn, Mn, As, and Cd and was low in nutrients compared to municipal wastewaters. The average pH and electrical conductivity of the AMD were measured to be 2.41 and 2295 μS/cm, respectively.
2.2 Microalgae Cultures and Medium
Seed cultures of Chlorella sp. (KMMCC-1468) were cultivated in Jaworski’s medium (JM) under light-emitting diode (LED) lamps at ambient temperature. JM consisted of 4.0 g Ca(NO3)2 · H2O, 2.48 g KH2PO4, 10.0 g MgSO4 · 7H2O, 3.18 g NaHCO3, 0.45 g EDTAFeNa, 0.45 g EDTANa2, 0.496 g H3BO3, 0.278 g MnCl2 · 4H2O, 0.20 g (NH4)6Mo7O24 · 4H2O, 0.008 g cyanocobalamin, 0.008 g thiamine HCl, 0.008 g biotin, 16.0 g NaNO3, and 7.2 g Na2HPO4 · 12H2O in 200 mL deionized water. The microalgae were cultured in a 200-mL conical flask containing 100 mL of JM, pH (7.2 ± 0.3). The inoculums consisted of 10 mL of Chlorella sp. culture. The culture was maintained in a dark and light cycle of 8 and 16 h, respectively.
2.3 Experimental Design
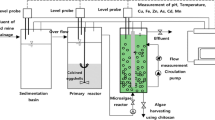
The reactor is contained with two compartments. The first compartment is filled with the sericite beads, and the AMD water is introduced first here. The sericite beads technology is primarily relying on the fixed-bead suspension where the influent AMD is intimately interacting with the sericite beads. This enables to neutralize the excess acid and could remove simultaneously the heavy metals from the AMD effluents. Thereafter, the sericite-pretreated liquid is introduced to the optical panel (OP) photobioreactor for the posttreatment of AMD effluent. This step may enable to remove further the heavy metals along with the other impurities from the AMD effluent water. The hydraulic retention time (HRT) is prepared 3 h for sericite beads reactor and 6 days for microalgae reactor for neutralization and removal of heavy metal ions from the AMD. This integrated system is having an additional advantage of pretreatment of AMD effluent which effectively neutralizes the acidity of AMD water and partly removes the heavy metals from effluent water. Therefore, this enhances the performance and efficiency of optical panel photobioreactor (OPPBR) for AMD treatment.
2.3.1 Sericite Beads and Operation
Sericite is a muscovite-type natural clay mineral (cryptocrystalline form of muscovite). It forms a three dioctahedral silicate layers and shows a close relationship to the micas. It often arises from rock-forming aluminosilicates by diagenetic or hydrothermal processes and forms the main component of many types of clay (Lee and Tiwari 2014; Reddy et al. 2013). Sericite’s negative charge comes from Si–Al substitution in the tetrahedral by replacement of Al3+ by Mg2+ and Fe2+ in the octahedral. When sericite particles break, additional positive edge charges are formed. The binding of inorganic substances of sericite usually takes place through several mechanisms involving physical adsorption and chemisorption (Kwon and Jeon 2013; Reddy et al. 2013). Sericite contains various metal oxides. The elemental percent composition of sericite is reported as in Table 2. Sericite powder is hydrophilic substance. Kim et al. (2014) reported that the modified sericite shows potential material for the efficient and effective treatment of wastewaters comparing to the unmodified sericite. Therefore, the experiments are planned to introduce the modified sericite bead for neutralization of acidity and adsorption of heavy metals in the AMD.
Sericite obtained from the Keumnam deposit in Samcheok City, Korea, was used as the clay mineral. To remove any adhesion and interference materials, such as organics and salts, the sample was rinsed several times with deionized water. After cleaning, sericite was dried in an oven at 105 °C for 24 h and crushed into fine powder (325 mesh) using a mortar and pestle. The sericite bead was prepared by mixing the sericite powder (95 %) and MgO (5 %) in water, and it was performed at 750–900 °C for 2 h in the oven. This gives a light porous foamed sericite bead (Fig. 1b). Sericite bead (Fig. 1a), with small block pieces 4–5-cm length, about 1-mm pore size having the specific gravity of ca. 0.4, and water absorbance of ca. Twenty percent are then employed for further experimentation. The sericite beads process is operated with a 40-L working volume and used 10 g/L sericite beads.
2.3.2 OPPBR Construction and Operation
The OPPBR is operated with a 40-L working volume and is equipped with an optical panel (OP). The initial concentration of the inoculated microalgae Chlorella sp. is 0.367 ± 0.6 g/L. The experiments are conducted at neutral pH (7.2 ± 0.3) under dark and light cycles of 8 and 16 h, respectively. The temperature is maintained at 23 ± 1 °C using LEDs for 20 days. The OPPBRs are aerated continuously at an aeration rate of 0.5 L/min. CO2 at the equivalent aeration rate of 0.02 vvm is used for cultivation. CO2 with a flow rate of 0.74 L/min is introduced into the reactor. The OPPBR is designed such that the light source (22 LEDs), an LED panel (bar type), is placed in the OPPBR. A v-grooved OP is inserted underneath in the photobioreactor (PBR). The OP exhibits 93 % transmittance and 1.19 g/cm2 specific gravity. The OP dimensions are 210 mm (L) × 290 mm (H) × 6 mm (W) and are constructed from a transparent panel of pure polymethylmethacrylate (PMMA). This material is fairly transparent, and it has almost negligible light absorption in the visible region (Choi 2014; Choi and Lee 2014). The incident light is uniformly distributed across both sides of the OP in the reactor and provides greater functionality. The LED light source is used because it is efficient and provides a required wavelength of light from 430 to 670 nm, which is selective for microalgal growth. The light intensity, which represents the amount of light used for photosynthesis, is 200 ~ 250 μmol photons/m2/s. The OPPBR is operated for 6 days, and the grown microalgae are harvested using chitosan.
2.4 Analytical Methods
To determine the biomass concentration, a sample of microalgae in growth medium was centrifuged for 10 min at 628g, washed with distilled water, and then dried in an oven at 105 °C for 24 h to a constant weight. The biomass productivity (g/L/day) was calculated from the amount of biomass (X 1−X 0) produced in a given time interval (t 1−t 0) according to the following equation:
The specific growth rate μ (in days) was calculated using Eq. (2)
where X 1 and X 0 are the biomass concentrations (g/L) on days t 1 and t 0, respectively.
Uniformity is calculated using Eq. (3)
where L min is the minimum light intensity and L max is the maximum light intensity. All samplings and measurements were conducted at the same time every day.
The Fe, Cu, Zn, Mn, As, and Cd were analyzed using an inductively coupled plasma optical emission spectrometry (ICP-OES: Optima 3300XL, PerkinElmer, MA, USA), following the standard method of measurements (vide APHA 2012). The Eh and EC were measured using pH/ORP meter (ISTEK, pH-20N) and EC/TDS/salinity meter (ISTEK, EC-40N), respectively.
3 Results
3.1 Sericite Beads Process
3.1.1 Neutralization of pH in the AMD
Figure 2 depicts the change in AMD effluent pH in presence of sericite beads. We found that a fast initial increase of pH occurred for AMD effluents in the presence of sericite beads. The pH rose from 2.41 ± 0.11 to 6.8 ± 0.76 within just 90 min of contact with sericite beads. After 90 min, the pH increased slowly and reached a constant value of 7.3 ± 0.87 after 3 h of contact.
3.1.2 Heavy Metal Removal in the AMD
The adsorption of several heavy metals by the sericite beads in the AMD is represented in Table 3, which shows that the heavy metal adsorption efficiency was 78.915, 82.04, 83.46, 80.29, 82.22, and 85.19 % for Fe, Cu, Zn, Mn, As, and Cd, respectively, with the sericite beads. A greater percentage of the Cd than of the Fe was adsorbed. The sericite beads were thus not only able to increase the pH of the AMD but simultaneously to adsorb significantly the toxic heavy metal ions.
3.2 OPPBR Process
3.2.1 Growth of Biomass
Light is one of the most vital factors that influences the growth and productivity of microalgae. The uniformity of the light in the OPPBR as defined by Eq. 3 was 95 % at a depth of 305 mm, indicating that the light intensity was fairly uniform over the depth of the reactor. The OP radiates light with adequate uniformity, prevailing from the top to the bottom of the PBR. The results clearly demonstrate that enhanced light intensity is achieved even deep inside of the PBR using an OP. Moreover, the performance of PBR containing OP is better than the PBR that does not have an OP unit.
The average biomass growth in the AMD is shown in Fig. 3. The biomass of Chlorella sp. grew to a concentration of 2.95 g/L in 6 days from an initial concentration of 0.367 ± 0.6 g/L, representing an 8.04-fold increase. The growth curve in Fig. 3 shows that the biomass increase in the OPPBR was greatest in the initial 3 days and then slowed. The biomass concentration was constant after 5–6 days.
The specific biomass growth rate is known as the increase in cell mass per unit time. This was calculated using the Eq. (2). This rate is also considered to be an indicator of the photosynthetic efficiency of the microalgae. The specific growth rate from the initial cell concentration is found to be significant as 1.20 ± 0.015 1/day. This is because the OP affects the high transparent surfaces, high illumination surfaces, and high mass transfer rates, which enable an increased specific growth rate in the OPPBR.
3.2.2 Heavy Metal Removal in the AMD
The removal percentage of heavy metal ions in AMD using Chlorella sp. is shown in Fig. 4. It seems that the average percent of investigated heavy metal removal is 94.89, 95.60, 94.19, 89.22, 87.50, and 95.00 % for Fe, Cu, Zn, Mn, As, and Cd, respectively, in the OPPBR using microalgae (Table 3). The removal of As and Mn ions in the presence of the microalgae was less than that of the other heavy metal ions. Microalgae require heavy metal ions as trace elements for growth. However, large amounts of heavy metal ions have an inhibitory effect on the microalgae growth (Chen et al. 2011).
3.2.3 The Relationship Between Biomass Productivity and Removal of Heavy Metal Ions
Figure 5 shows the linear relationship of investigated heavy metal ions and biomass productivity. The coefficient (R 2) of determination is an important tool for determining the degree of linear correlation of variables in regression analysis. In this experiment, a relatively high correlation coefficient (R 2) of 0.9195 for Fe, 0.9079 for Cu, 0.9933 for Zn, 0.995 for Mn, 0.9366 for As, and 0.9577 Fe is obtained. The investigated heavy metal concentration in the AMD is decreased linearly with increasing the biomass productivity. Especially, the removal percentage of As (87.50 %) with microalgae was obtained lower than that of other heavy metal ions. However, the R 2 of As concentration is linearly related. This result indicates that the biomass productivity in the AMD is strongly influenced by the removal of heavy metal ions.
3.3 Total Heavy Metal Removal by Sericite and Microalgae Hybrid System
The experimental results indicate that the total percent removal of the investigated heavy metal ions from the AMD effluents using the sericite beads and microalgae hybrid system is 98.92, 99.21, 99.04, 97.87, 97.78, and 99.26 % for Fe, Cu, Zn, Mn, As, and Cd, respectively (Fig. 6). These results clearly demonstrate that the sericite beads and microalgae hybrid process is effective for heavy metal removal and comparable to the individual application of sericite beads or microalgae process. Moreover, an enhanced growth of Chlorella sp. could further be utilized as a potential biodiesel source.
4 Discussion
A silica feedstock in sericite beads is used to neutralize the acidity in AMD systems by removing free hydrogen ions from the AMD, thereby increasing the effluent pH (Lee and Tiwari 2012). Since the silicate anion captures H+ ions from bulk solution (i.e., increase of bulk pH), it forms readily the monosilicic acid (H4SiO4), a neutral solute. Monosilicic acid remains in the solution to play an important role in correcting the adverse effects of acidic conditions. In the solution, the silicate anion is very active in neutralizing H+ cations (Reddy et al. 2013). While its mode of action is quite different from sericite, the ability of silica to neutralize acid solutions is equivalent to sericite. Once the pH of AMD increases in presence of sericite, several heavy metals seemingly tend to be precipitated to its metal hydroxides (with extremely low solubility) (Lee and Tiwari 2014). In the silica aggregate, as silicic acid species are absorbed onto the metal surface, the development of silica layers (monolayer and bilayer) leads to the formation of colloidal complexes with neutral or negative surface charges (Genc et al. 2003). These negatively charged colloids create an electrostatic repulsion with each other (as well as with the negatively charged silica granules), and the sequestered metal colloids are stabilized and remain in a dispersed state—effectively. Moreover, the surface of sericite provides active sites capable of adsorbing several heavy metal toxic ions from aqueous solutions which is further enhanced at elevated solution pH conditions (Tiwari et al. 2009).
Sericite is a nontoxic, noncorrosive, and safe-to-handle material, hence, has wider potential applications in wastewater treatment technologies. It is biocompatible and has attractive adsorption properties and polyelectrolicity; additionally, it can be regenerated in a number of applications. The high cationic charge density of sericite allows it to strongly adsorb negative regions on other particles. An et al. (2007) reported 18.78 and 8.70 % Mn removal at pH 6.2 using dolomite and sand, respectively. Dolomite has less content of SiO2 and Al2O3 compared to the sericite; however, the structure of dolomite is very similar to sericite. Therefore, the removal rate of Mn using dolomite was obtained similar to this experiment. However, major component of sand was quartz, and it is less porous in the layer than sericite. Thus, the sand has lower adsorption rate than sericite.
The biomass production increased from 0.367 to 2.95 g/L in 6 days of operation. The OP of PBR increases the illumination surface to volume ratio as well, providing efficient utilization of light radiation for biomass production. The use of OP provides an equal amount of light energy passing through the reactor, and the irradiance profile is redistributed due to diffuse light from the OP (Choi 2014; Choi and Lee 2014). The redistributed irradiance profile results in the higher photosynthetic efficiency of microalgae and leads to effective light utilization. Consequently, the photobioreactor with OP not only increases the illumination area for cultivation but also provides conditions for the effective utilization of light energy to increase the growth rate of microalgae. Previously, it was reported that in an 11-L undular row tubular photobioreactor for Arthrospira platensis, the productivity was found to be 2.7 g/L/day (Carlozzi 2003). Hsieh and Wu (2009) reported the biomass production of 0.340 g/L in a transparent rectangular chamber PBR which is much less than the one reported here. Furthermore, about 0.27 g/L is obtained in 440-L outdoor flat-plate photobioreactor, which is used for cultivation of Nannochloropsis (Chen et al. 2011). Garcia-Malea Lopez et al. (2006) reported a 55-L bubble column photobioreactor (for the outdoor cultivation of Haematococcus pluvialis), and the biomass productivity obtained is 0.06 g/L/day. It should be noted that aside from volumetric productivity (productivity per unit of reactor volume per unit of time), algal biomass productivity can be evaluated in photobioreactors based on areal productivity (productivity per unit of occupied land area per unit of time), photosynthetic efficiency, or biomass yield (g-biomass per unit of solar radiation).
Microalgae are one of the most important biosorbents. Metals are taken up by algae through adsorption. First, the metal ions are quickly adsorbed by the cell surface; this process is called physical adsorption. Then, these ions are transported slowly into the cytoplasm in a process called chemisorptions (Ajjabi and Chouba 2009). In the present investigation, by increasing the exposure time, the rate of the percent of metal ion removal slowed down gradually and an apparent equilibrium was established. After the equilibrium period, the metal ion sorption by the microalgae biomass was not changed significantly, further increasing the operation time. The results are in agreement with those obtained by Shanab et al. (2012). Various studies have been carried out to show the role of microalgae in the bioremediation of heavy metals. Johnson and Hallberg (2005) reported that microalgae adsorbed 70.26, 70.21, 73.48, 71.26, 60.87, and 60.12 % for Fe, Cu, Zn, Mn, As, and Cd, respectively. Similarly, Ajjabi and Chouba (2009) reported the removal from aqueous solutions of 70.24 % Cu and 68.42 % Zn by Chaetomorpha linum.
In summary, this study investigated the heavy metal removal and biomass productivity in an AMD using sericite beads and microalgae hybrid system. Sericite beads effectively neutralized the acidity and partially removed the heavy metals from the AMD effluents. The biomass increased 8.04-fold during the AMD treatment. The total removal rate of Fe, Cu, Zn, Mn, As, and Cd was 98.92, 99.21, 99.04, 97.87, 97.78, and 99.26 %, respectively, from the AMD effluent using sericite beads and microalgae hybrid system. A fairly good linear relationship was achieved between the investigated heavy metal removal and the biomass productivity because a high value of correlation coefficient (R 2) was obtained. The sericite beads and microalgae hybrid system was highly effective for heavy metal removal and for comparing it to the conventional biological process in the AMD. Therefore, the sericite beads and microalgae system is a useful alternative for a large-scale treatment of effluent waters emanating from the AMDs.
References
Ajjabi, L. C., & Chouba, L. (2009). Biosorption of Cu and Zn from aqueous solutions by dried marine green macroalga Chaetomorpha linum. Journal of Environmental Management, 90, 3485–3489.
An, J. H., Kim, C. C., Choi, S. B., Kim, S. R., Jung, J. Y., Lee, W. A., & Lee, T. S. (2007). A study on removal effect of heavy metals in mine wastewater by adsorbents. Report Institute of Health & Environment, 18, 138–149.
APHA (2012). Standard methods for the examination of water and wastewater. 22nd edition, Washington DC, USA.
Ata, A., & Koldas, S. (2006). Acid mine drainage (AMD): causes, treatment and case studies. Journal of Cleaner Production, 14(12–13), 1139–1145.
Carlozzi, P. (2003). Dilution of solar radiation through “culture” lamination in photobioreactor rows facing south–north: a way to improve the efficiency of light utilization by cyanobacteria (Arthrospira platensis). Biotechnology and Bioengineering, 81(3), 305–315.
Chen, C. Y., Yeh, K. L., Aisyah, R., Lee, D. J., & Chang, J. S. (2011). Cultivation, photobioreactor design and harvesting of microalgae for biodiesel production: a critical review. Bioresource Technology, 102(1), 71–81.
Choi, H. J. (2014). Effect of optical panel distance in a photobioreactor for nutrient removal and cultivation of microalgae. World Journal of Microbiology and Biotechnology, 30, 2015–2023.
Choi, H. J., & Lee, S. M. (2014). Effect of optical panel thickness for nutrient removal and cultivation of microalgae in the photobioreactor. Bioprocess and Biosystems Engineering, 37(4), 697–705.
Coulton, R., Bullen, C., & Hallet, C. (2003). The design and optimization of active mine water treatment plants. Land Contamination and Reclamation, 11, 273–279.
García-Malea López, M. C., Del Río Sánchez, E., Casas López, J. L., Acién Fernández, F. G., Rivas, J., Guerrero, M. G., & Molina, G. E. (2006). Comparative analysis of the outdoor culture of Haematococcus pluvialis in tubular and bubble column photobioreactors. Journal of Biotechnology, 123(3), 329–342.
Genc, H., Tjell, C. J., McConchie, D., & Schuiling, O. (2003). Adsorption of arsenate from water using neutralized red mud. Journal of Colloid and Interface Science, 264, 327–334.
Hsieh, C. H., & Wu, W. T. (2009). A novel photobioreactor with transparent rectangular chambers for cultivation of microalgae. Biochemical Engineering Journal, 46(3), 300–305.
Johnson, D. B., & Hallberg, K. B. (2003). The microbiology of acidic mine waters. Research in Microbiology, 154, 466–473.
Johnson, D. B., & Hallberg, K. B. (2005). Acid mine drainage remediation options: a review. Science of the Total Environment, 338, 3–14.
Kim, M. N., & Lee, S. M. (2010). Organo-sericite for the removal of phenol and Cu2+ from aqueous solution. Korean Society of Water Science and Technology, 18(5), 29–35.
Kim, J. O., Lee, S. M., & Jeon, C. (2014). Adsorption characteristics of sericite for cesium ions for an aqueous solution. Chemical Engineering Research and Design, 92(2), 368–374.
Kwon, T. N., & Jeon, C. (2013). Adsorption characteristics of sericite for nickel ions from industrial waste water. Journal of Industrial and Engineering Chemistry, 25, 68–72.
Lee, S. M., & Tiwari, D. (2012). Organo and inorgano-organo-modified clays in the remediation of aqueous solutions: an overview. Applied Clay Science, 59–60, 84–102.
Lee, S. M., & Tiwari, D. (2014). Organo-modified sericite in the remediation of aquatic environment contaminated with As(III) or As(V). Environmental Science and Pollution Research, 21, 407–418.
Liu, C. B., Lin, L. P., & Su, Y. C. (1996). Utilization of Chlorella vulgaris for uptake of nitrogen, phosphorus and heavy metals. Journal of the Chinese Agricultural Chemical Society, 34, 331–343.
Mendoza-Co, L., Zatl, D. G., & Moreno-Sa, L. R. (2005). Cd2+ transport and storage in the chloroplast of Euglena gracilis. Biochimica et Biophysica Acta, 1706, 88–97.
Reddy, D. H. K., Lee, S. M., & Kim, J. O. (2013). A review on emerging applications of natural sericite and its composites. World Applied Sciences Journal, 27(11), 1514–1523.
Romera, E., Gonzáalez, F., Ballester, A., Bláazquez, M. L., & Muñnoz, J. A. (2007). Comparative study of biosorption of heavy metals using different types of algae. Bioresource Technology, 98(17), 3344–3353.
Shanab, S., Essa, A., & Shalaby, E. (2012). Bioremoval capacity of tree heavy metals by some microalgae species (Egyptian Isolates). Plant Signaling & Behavior, 7(3), 392–399.
Tiwari, D., & Lee, S. M. (2012). Novel hybrid materials in the remediation of ground waters contaminated with As(III) and As(V). Chemical Engineering Journal, 204–206, 23–31.
Tiwari, D., Kim, H. Y., & Lee, S. M. (2009). Application of sericite in wastewater treatment: removal of Cu(II) and Pb(II) from aqueous solutions. Environmental Engineering Research, 11(6), 303–310.
Acknowledgments
This study was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2013006899).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Choi, HJ. Biosorption of Heavy Metals from Acid Mine Drainage by Modified Sericite and Microalgae Hybrid System. Water Air Soil Pollut 226, 185 (2015). https://doi.org/10.1007/s11270-015-2433-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-015-2433-3