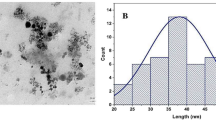
Abstract
A column experiment was conducted to evaluate the effectiveness of nanoscale zerovalent iron (nZVI) for the in situ immobilization of Pb and Zn in an acidic soil. The impact of nZVI on soil was evaluated by monitoring the physicochemical characteristics of the leachates and their ecotoxicological effects on three species, Vibrio fischeri, Artemia franciscana, and Caenorhabditis elegans. Treatment with nZVI resulted in more effective Pb immobilization in comparison to Zn and reduced the leachability by 98 and 72 %, respectively; the immobilization was stable throughout the experiment. Leachates from nZVI-treated soils showed lower toxicity than leachates from untreated ones. The highest toxicity in treated soils was observed in the first leachate, which presented high values of electrical conductivity due to the leachability of soil ions and those provided by the commercial nanoparticle suspension (Na and Fe). V. fischeri and C. elegans were more sensitive to leachates from nZVI-treated soils polluted with Zn than those from soils polluted with Pb; A. franciscana showed the opposite trend.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Soil is both an important reservoir of chemical elements and a living matrix, being a key component of terrestrial ecosystems. In the last several decades, environmental problems associated with soil pollution have attracted the interest of the scientific community. Among other pollutants, metals and metalloids are well known to be toxic to most organisms when exceeding a threshold level (Adriano 2001; USEPA 1992). The main causes of the accumulation of metals and metalloids in soil are anthropogenic activities such as mining, military activities, manufacturing, and the land application of industrial or domestic sludge (Adriano 2001). Metals and metalloids do not degrade like organic pollutants, and for that reason, it is very difficult to eliminate metals from soils. The total concentration of heavy metals in soil provides limited information about their potential mobility and their possible impact to organisms. The main objective of many in situ remediation strategies is to use metal immobilization to reduce their mobility and bioavailability in the soil and minimize potentially adverse effects. Both the mobility and bioavailability of the metal in the soil depend on the physical and chemical soil properties, the metal speciation, and the biological organism (Vangronsveld and Cunningham 1998). The immobilization of metals prevents their transport into deeper soil layers, rivers, and groundwater, an important source of drinking water.
In recent years, the environmental application of nanoscale zerovalent iron (nZVI) has generated a great deal of attention due to its potential for cost reduction compared to other in situ treatments, higher reactivity, and broader applications (Grieger et al. 2010; Karn et al. 2009; Li et al. 2006; Mueller et al. 2012; Zhang 2003). Although most studies have focused on using nZVI to remove metals and metalloids from water and groundwater (Crane and Scott 2012; Liendo et al. 2013; Karn et al. 2009; Klimkova et al. 2011; Mueller et al. 2012; Zhang 2003), metal immobilization in soils using nZVI has recently attracted attention (Fajardo et al. 2012; Singh et al. 2012; Xu and Zhao 2007; Zhang et al. 2010). The direct application of nZVI for soil remediation is a potential entry of nZVI into groundwater. Thus, characterizations of soil leachates as well as their potential toxicity have become crucial in evaluating the impact of ZVI nanoparticles in soil remediation.
The ecotoxicity of the interaction between nanoparticles and soil can be assessed by several tests conducted in species ranging from microorganisms to eukaryotes (Handy et al. 2012). To date, little information exists on the assessment of nZVI–soil interactions. In this sense, Cullen et al. (2011), Fajardo et al. (2012) and Pawlett et al. (2013) have studied the effects of nZVI on typical soil microorganisms. However, ecotoxicological analysis in other species is being increasingly considered (Chen et al. 2011; El-Temsah and Joner 2012; Keller et al. 2012; Li et al. 2009).
The marine bioluminescent bacterium Vibrio fischeri (V. fischeri) has been broadly used in environmental toxicity (Boluda et al. 2002). It is a very powerful tool for screening the toxicity of nanoparticles (Mortimer et al. 2008). The Artemia species have been used to test the acute toxicity of metals because they offer several advantages, including year-round availability, low cost, ease of culture, high offspring production, and a short life cycle (Kokkali et al. 2011; Sánchez-Fortún et al. 1997). The short-term toxicity test with Artemia nauplii was tested by European and American laboratories to determine the degree of standardization of the experimental protocol; they concluded that its repeatability and reproducibility were at least equal to those of the short-term Daphnia test (Vanhaecke and Persoone 1984). Another organism group emerging in the field of environmental sciences is the nematode. The soil-dwelling bacterivorous nematode Caenorhabditis elegans (C. elegans) has been successfully used as a test organism for investigating complex matrices, such as soils, and the toxicity of both metals and engineered nanoparticles (Höss et al. 2009; Roh et al. 2006).
Therefore, the aim of this study was to examine (i) the effectiveness of nZVI for the in situ immobilization of metals (Pb or Zn) in soil column experiments, (ii) the potential leachability of metals and nZVI, and (iii) the physicochemical and ecotoxicological properties of the leachates to determine the environmental risk of this remediation technology.
2 Materials and Methods
2.1 Zerovalent Iron Nanoparticles
A commercial stabilized water dispersion of nZVI NANOFER 25S (NANO IRON Rajhrad, Czech Republic) was used in these column experiments. The Fe(0) percentage in the commercial product was between 14 and 18 %, according to the commercial specifications (http://www.nanoiron.cz/en/nanofer-25s). Klimkova et al. (2011) presented a complete characterization of the commercial nanoparticles that were used. In brief, the nZVI particles are coated by polyacrylic acid (3 %), which stabilizes the product, prevents sedimentation and agglomeration, and maintains its accessibility to reactions; the nZVI particles show an average size of 60 nm and an active surface area of 20 m2 g−1. Before use, the solution was covered with aluminum foil to prevent light degradation and stirred for 1 h. The pH of the nZVI suspension was alkaline (12.2 ± 0.1). A concentration of 10 % nZVI, representing 14–18 g Fe (0) per kg of soil, was used in this experiment.
2.2 Soil
The soil used in this study was collected from an untreated agricultural field located in Talamanca del Jarama (Madrid, Spain); this field had no agricultural activities at least for the last 10 years. Bulk soil samples were collected from surface layer A (0–30-cm depth). The soil samples were air-dried and sieved (<2 mm) prior to experimental use. The soil properties were analyzed according to the official Spanish methodology for soil analysis (MAPA 1994), and results are shown in Table 1. In brief, the electrical conductivity and pH were measured at a 1:2.5 soil-to-water ratio; the organic matter and total nitrogen content were determined using the Walkley–Black and Kjeldahl methods, respectively. The percentage of carbonates was measured using the Bernard calcimeter; available phosphorus was evaluated using sodium bicarbonate at a pH of 8.5. Available nutrients were extracted with 0.1 N NH4Ac and assessed using atomic absorption spectrometry (AAS) (AA 240 FS, Varian, Victoria, Australia). The soil texture was analyzed using a Bouyoucos densimeter.
Air-dried soil samples (2.5 kg) were spiked with 800 mL of solution of Pb(NO3)2 or Zn(NO3)2 (625 mg L−1). They were prepared from commercial solutions of nitrate of Pb(II) and Zn(II) in nitric acid (1,000 mg L−1) purchased from Scharlab (Sentmenat, Spain). After soil spiking, a decrease of soil pH was observed (pH = 3). Spiked acid solutions were used to simulate mining activities that often induce acid mine drainage containing toxic metals (Klimkova et al. 2011). Soil was properly mixed during metal addition to make sure that the metal was evenly distributed in the soil. Spiked soils were consolidated for 40 days. After this 40-day period, the soils were sieved (<2 mm) to homogenize the mixtures. The original soils were left unpolluted. The total Pb and Zn levels in the soil samples were determined by AAS after acid digestion (0.5 g) with a mixture of 6 mL of nitric acid (69 % purity) and 2 mL of hydrochlorhydric acid (37 % purity), in a microwave reaction system (Multiwave 3000, Anton Paar GmbH, Graz, Austria); after the solutions were cooled to room temperature, they were filtered and diluted to 50-mL with Milli-Q water. The total Pb and Zn contents of the spiked soils were 192 ± 10 and 250 ± 30 mg kg−1, respectively. The limits of quantitation were 0.01 and 0.1 mg L−1 for Zn and Pb, respectively. The iron concentration in the extract was also measured, and the quantitation limit was 0.06 mg L−1. All analytical determinations were performed in duplicate.
2.3 Column Experiments
The leaching experiments were performed at room temperature (23 ± 1 °C). Glass columns with a length of 30 cm and inner diameter of 4.5 cm were used in the present study. The columns were closed at the bottom with plastic mesh (0.2 mm), and 75 g (4 cm) of gravel was added to prevent loss of the soil. To quantify the effectiveness of nZVI for the in situ immobilization of Pb or Zn and the leaching of Fe from nanoparticles, three parallel column systems were utilized: columns with unpolluted soil (original soil), columns with polluted soil (Pb or Zn), and columns with polluted soil treated with nZVI at 10 % (called Pb-nZVI or Zn-nZVI). Three independent columns were used per condition. The columns filled with unpolluted soil provide information about the potential impact of the remediation technique on soil characteristics. The columns with original soil and columns with polluted soil were uniformly packed with 400 g of air-dried soil. To obtain the columns with polluted soil treated with nZVI, 400 g of air-dried soil was mixed with nZVI suspension and then introduced into the column. Deionized water was applied through the soil column to saturate the soil (141 mL). Then, 100 mL of de-ionized water was added everyday for 10 days by gravity flow. After irrigation, the leachates were collected over a 24-h period in a 250-mL Erlenmeyer flask with a funnel and Whatman paper (grade 40, 125 mm diameter). The volume of leachate collected was in the range of 83–103 mL. The total amount of water applied to each column was 1,120 mL, simulating 2 years of typical annual rainfall from central Spain.
The total Pb or Zn in the leachates was determined by AAS after acidification. To quantify the transportability of the Fe nanoparticles through the soil column, the total Fe concentration in the leachates was monitored by AAS. The physicochemical and ecotoxicological characteristics of the leachates were also analyzed.
Finally, the soil was removed from the column and air-dried. Metal availability (Pb, Zn) was studied in the soils after applying a sequential extraction procedure to determine the most available fractions (exchangeable and linked to carbonates). The metal concentrations were measured by AAS in all the extracts obtained in the sequential extraction procedure described below.
Exchangeable (EX)
Soil samples (2.5 g) were extracted with 25 mL of MgCl2 (1 M, pH 7) for 1 h.
Linked to carbonates (CB)
The residue from exchangeable fraction was extracted with 25 mL of buffer CH3COONa/CH3COOH (1 M, pH 5) for 5 h.
After each successive extraction, separation was done by centrifugation (Beckman Model J2-21) at 13,000 rpm for 30 min. The supernatants were removed with pipette and filtered through 0.45-μm regenerated cellulose membrane filters (Phenomenex, Torrance, CA).
The residue from carbonate fraction was air-dried and grinded with an agate mortar. The grinded residue (0.5 g) was digested in a microwave reaction system (Multiwave 3000) as was described previously. This fraction includes the metal content linked to the less available fraction of the soil (called LA).
2.4 Physicochemical Properties of Leachates
The conductivity and pH of the leachates were monitored; the available nutrients Ca2+, Mg2+, Na+, and K+ were also determined by AAS, and the anions Cl−, NO2 −, SO4 2−, and PO4 3− were determined by ionic chromatography with a conductivity detector (Dionex ICS-1100). The chromatography conditions were an analytical column, Dionex Ion Pac AS9-HC (4 × 250 mm); a guard column, Dionex AG9-HC; a suppressor column Anion Micromembrane Suppresor III, AMMS III (4 mm); the eluent, 9.0 mM sodium carbonate; the flow rate, 1.0 mL min−1; and the injection volume, 25 μL.
2.5 Ecotoxicological Analysis of Leachates
2.5.1 Vibrio fischeri (Microtox® Test)
The Microtox® Basic Test was carried out using the following protocol from the manufacturer (Microbics Corporation, Carlsbad, USA). Briefly, a range of culture filtrate dilutions from 45 to 0.56 % was made in solvent supplied by the manufacturer. For the reactions, freeze-dried V. fischeri were reconstituted with 0.01 % sodium chloride, and 10 mL was mixed with 500 mL of each culture filtrate dilution.
A Microtox® Model 500 Analyzer (AZUR Environmental, Carlsbad, CA, USA) was used to measure the luminosity from the reconstituted bacteria after 5 and 15 min of exposure to the culture filtrate. Because there were no significant differences between the two measurements, the luminescence inhibition after a 5-min exposure was taken as the endpoint (Froehner et al. 2000). A 2 % sodium chloride solution was used for bacterial regeneration, sample dilution, and control. The osmotic control was made using an Osmotic Adjusting Solution (OAS) of 22 % sodium chloride.
In the Microtox® test, the inhibition of light emission was measured in relative units of luminescence. The data were used to calculate the leachate volume required to induce full luminescence inhibition for each of the samples tested. The behavior of the bacteria was tested according to normative AFNOR T90-320 (AFNOR 1991).
2.5.2 Artemia franciscana
The Artemia cysts were purchased from Argent (Argentemia Silver Grade, Argent Chemical Lab., WA, USA). To obtain Artemia for the test, the method of Persoone et al. (1989) was modified according to the following procedure. Encysted A. franciscana were hydrated in distilled water at 4 °C for 12 h, followed by washing to separate the cysts that float from those that sink. The sinkers were collected in a Buchner funnel and washed with cold distilled water followed by synthetic seawater. Synthetic seawater was prepared by mixing 35 ‰ Synthetica Sea salts (Waterlife Research Ltd., UK) with distilled and deionized (Milli-Q) water, stirring for 24 h with suitable aeration and filtering through 30-μm Millipore cellulose filters.
The cysts were incubated in a graduated glass cylinder in 100 mL of seawater medium at 25 °C and pH 8.6. A photon irradiance of 16 μmol m−2 s−1 over the wave band 400–700 nm was employed, and slight aeration was maintained by a small tube in contact with the bottom of the cylinder. Under these conditions, the time required for the cysts to hatch was approximately 24 h.
The standard environmental conditions for all acute toxicity bioassays were as follows: temperature 25 °C, salinity 35 ‰, and pH 8.6. All tests were performed in darkness and conducted in sterile 24-well polystyrene tissue culture plates.
To confirm the nZVI toxicity assays, preliminary 24-h static toxicity tests were performed to define the range of concentrations covering 0 to 100 % mortality. The test concentrations used, chosen on the basis of preliminary range-finding tests, were 1,000–5,000 mg L−1. To determine the toxic effect of leachates from soil polluted with Pb or Zn, samples from these leachates had been previously lyophilized and resuspended in an equal volume of seawater.
In all toxicity assays, each nZVI concentration or leachate sample was replicated four times and involved ten organisms per well; at least four replicate test series were performed. All plates were placed in an incubator under standard conditions for 24 h. Larvae were considered dead if they showed neither internal nor external movement over a 10-s observation period.
2.5.3 Caenorhabditis elegans
The C. elegans wild-type strain N2 from the Caernorhabditis Genetic Center (University of Minnesota, St. Paul, MN, USA) was used for the bioassay. The strain was maintained on nematode growth medium (NGM) plates seeded with Escherichia coli strain OP50 at 20 °C (Brenner 1974). The NGM consisted of agar, peptone, cholesterol, KH2PO4 and K2HPO4 buffer, NaCl, and MgSO4. E. coli was cultured overnight in sterilized Luria Bertani (LB) medium (5 g L−1 of yeast extract, 10 g L−1 of tryptone, and 10 g L−1 NaCl).
Chunks of agar with worms from a stock of dauer larvae (an alternative juvenile stage of C. elegans that occurs with a lack of food) were transferred to fresh NGM plates seeded with OP50. After 3 days, many gravid hermaphrodites, as well as L1 and L2 stage juveniles, were found on the NGM.
Toxicity testing was conducted in 24-well microtiter plates (Nunclon Delta SI, Nunc, Roskilde, Denmark). Each test well was loaded with an adult gravid nematode; 1 mL of the test leachate recovered at 48-h intervals and E. coli (OP50 15 mg mL−1 suspended in K-medium as the food supply plus cholesterol 5 μg mL−1). Wells in which leachates were substituted with 1 mL of K-medium were used as the internal controls. Leachates from soils without Pb, Zn, or nanoparticles were also tested as controls.
The plates were then incubated in darkness at 20 °C. Three days later, the content of each well was mixed with 0.5 mL of an aqueous solution of Rose Bengal (0.3 g L−1) to stain the worms (Höss et al. 2009). The assay was stopped by heat-killing the worms at approximately 80 °C after 10 min. The content of each well was removed with tap water, transferred to a centrifuge tube, and poured into a Petri dish. The nematode reproduction was quantified by counting the offspring per test organism under a stereomicroscope. For each solution, the average number of progeny from four wells was obtained for each test replicate, and the testing was replicated three times.
2.6 Statistical Analysis
The physicochemical properties of leachates are shown graphically in the form of breakthrough curves, i.e., concentration versus time (days), and indicate the means ± standard deviation. Statistical calculations of toxicological endpoints were performed using Graphpad Prism 5 (San Diego, CA, USA) software. One-way ANOVA was carried out followed by a post hoc multiple comparison of means using the Tukey test (p < 0.05).
3 Results and Discussion
3.1 Metal Immobilization
To test the effectiveness of nZVI in enhancing Pb and Zn immobilization, column experiments were performed, and the leachability of untreated and nZVI-treated soils was compared. As shown in Fig. 1a, the concentrations of Pb and Zn in leachates from columns containing soils treated with nZVI were significantly lower than those from the untreated soils. Most of the nonimmobilized metal was leached on the first day. Lead-polluted soils treated with nZVI only leached 1.5 % of the total Pb. In the case of Zn, treated soils leached 21 % of the total Zn, as the immobilization was significantly less effective than that for Pb. Successive water irrigations did not increase the metal availability, suggesting that metal immobilization remained stable through the experiment. This fact is of great interest because of the need for applying long-term immobilization technologies. The observed differences in the immobilization percentages between Pb and Zn can be explained in terms of the different chemical characteristics between both metals. Sorption and partial chemical reduction are the removal mechanisms for metal ions such as Pb(II), which has a standard potential for reduction to a metallic state (E° = −0.14 V) that is slightly more electropositive than iron (−0.41 V). In the case of Zn(II), whose E° (−0.76 V) is more negative than iron, its reduction is thermodynamically unfavorable. Zn ions are attracted to the iron surface by sorption or surface complex formation, which may include electrostatic interactions and specific surface bonding (Li and Zhang 2007). Regarding the mobility of nZVI, as expected, higher amounts of iron were found in leachates from soil columns with nanoparticles than those from untreated ones (Fig. 1b). According to the commercial specifications, the mean amount of iron in the nZVI suspension added to the soil column was approximately 6,400 mg. In the first leachate, the highest concentration of iron was detected, 127 mg for columns with Pb and 195 mg for columns with Zn (the mean volume of leachate was 103 ± 7 and 97 ± 1 mL, respectively). In the following leachates, the amounts of iron leached from treated and untreated soils were similar. The total percentages of iron leached throughout the column experiment were 2.1 and 3.1 % of the total iron added, for Pb and Zn experiments, respectively. Subsequently, the use of nZVI to immobilize metals in soils could produce an initial input of Fe to the groundwaters that was less than 3.5 % of the total Fe added.
After the leaching experiments, soils were removed from the columns, and the metal availability was analyzed according a sequential extraction procedure. Soils treated with nZVI showed a significantly higher percentage of metal in the less available fractions than those from untreated soils (Fig. 2), which agrees with the rate of leachability. In treated soils, the percentages of metal in the less available fractions were 65.6 % for Pb and 45.4 % for Zn, confirming the greater effectiveness in the immobilization of Pb compared to that of Zn. The obtained results suggest that metal immobilization would be stable after simulating 2 years of rainfall from Central Spain, which is a critical factor in determining the effectiveness and the stability of a remediation procedure.
3.2 Physicochemical Properties of Leachates
Because nZVI applied to the soil can potentially reach groundwater, the physicochemical characterization of leachates is an important endpoint to be considered; however, little information in this area is available. As shown in Fig. 3a, leachates from soils polluted with Pb or Zn presented pH values that are significantly more acidic than both the original (unpolluted) and nZVI-treated soils from the beginning to the end of the experiments. Furthermore, in all of the cases, the pH values tended to stabilize at 4–5 days. The electrical conductivity was dramatically high in leachates collected from all soils except the unpolluted ones as a consequence of contamination and the nanoparticle treatment. The artificial soil contamination resulted, on the one hand, a supply of ions, and on the other hand, a decrease of the soil pH, which favors soil salt solubilization. However, after 24–48 h, conductivity significantly decreased to the level of the unpolluted soil where it remained until the end of the experiments (Fig. 3b).
The influence of soil remediation with nZVI on cation and anion leaching was also studied. The amount of ions leached depends primarily on the amounts of soluble ions present in the soil and also on the amount of leachate (Terman 1977). Thus, the extent of anion and cation leaching depends on both the chemical and physical soil properties as well as other factors, such as the amount and rate of drainage. The monitoring of the available nutrients K+, Na+, Ca2+, and Mg2+ is shown in Fig. 4. In general, the original soil showed low levels of ion leaching, which was expected because of its chemical properties, an acidic soil with low levels of nutrients. Metal-contaminated soils (Pb, Zn) demonstrated a low pH, contributing to an increase in the ion mobilization. The treated soils presented different behaviors depending on the ion; in general, significant mobilization of cations was observed at the early stages of the experiment. These results are in strong agreement with those related to electrical conductivity. In the first leachate, Pb-polluted soils leached significantly (p < 0.05) higher amounts of K+ and Mg2+ than nZVI-treated ones, while in the case of Zn, the concentration of these cations was similar in both the treated and untreated soil leachates. Soils treated with nZVI showed higher elution of Ca2+ than untreated ones, most likely due to exchangeable processes with Fe2+, which are favored by the excess of iron in the soil. Regarding Na+, the original and contaminated soils released significantly lower concentrations (p < 0.05) than the approximate concentration of 1,400 mg L−1 released by soils treated with nZVI. In these soils, Na+ originates from the commercial nanoparticles because it is a by-product of nZVI synthesis. Monitoring the soil cations shows that iron nanoparticle soil treatment seems to release cations from positions that were unavailable in the polluted soil.
In addition, the concentrations of the Cl−, NO2 −, SO4 2−, and PO4 3− anions in the leachates were analyzed (Fig. 4e–h). Nitrate was not quantified because metal contamination was carried out with the respective nitrate salt; so, it should be the dominant anion in the leachates. The analyzed anion concentrations in leachates from the unpolluted, polluted, and treated soils were moderate, with SO4 2− showing the highest amounts. The breakthrough curve of Cl− was similar to those of the cations because the Cl− in soils results from potassium, sodium, calcium, and magnesium salts. The highest amounts of Cl− were eluted at the beginning of the leaching experiment and were significantly higher (p < 0.05) in the nZVI-treated soils than in the polluted and original (unpolluted) ones. Chloride is a mobile anion; it is barely adsorbed and moves with water in the soil. Under these experimental conditions, the application of nZVI favored Cl− availability, although the leached amount was lower than the level allowed by Spanish Water Regulations. The results obtained for NO2 − show that the use of iron nanoparticles results in an important mobility and these values were significantly higher (p < 0.05) than those from the original and polluted soils throughout the column experiment. The ZVI nanoparticles favored the reduction of nitrate to nitrite (it should be noted that metal contamination was carried out with the nitrate salts, as explained above) which is in agreement with the results obtained by Chen et al. (2004). Treatment with nZVI induced the immobilization of the PO4 3− anion because of the high binding strength of PO4 3− to ferric oxides (Ryden et al. 1977). Under the experimental conditions of acidic soil with high concentrations of iron, iron phosphate minerals, such as strengite (FePO4 · 2H2O) and vivianite (Fe3(PO4)2 · 8H2O), and complexes are dominant (Reddy et al. 1999). The binding strength of phosphate to ferric oxides is higher than that of the inorganic anions SO4 2−, Cl−, and NO2 − (Ryden et al. 1977). However, metal-polluted soils showed an increase in the elution of PO4 3−, which could produce eutrophication problems. Sulfate behavior was contrary to PO4 3−; substantial leaching of SO4 2− was observed after soil remediation with nZVI. Both anions, SO4 2− and PO4 3−, compete for the active sites that are more specific to the latter (Rhue and Kamprath 1973).
3.3 Ecotoxicological Analysis of Leachates
To determine if leachates from soils polluted with Pb or Zn and treated with nZVI induce ecotoxicological effects, select toxicity endpoints were evaluated for V. fischeri, A. franciscana larvae, and C. elegans.
The marine bioluminescent bacterium V. fischeri, through the specific Microtox® bioassay, has been used broadly in ecotoxicology. This bioassay tests the decrease in V. fischeri bioluminescence in the presence of pollutants or other toxic agents (Boluda et al. 2002). The mean results obtained by the Microtox® tests are presented in Fig. 5. The leachates from the soils polluted with Pb or Zn exhibited a time-dependent decrease in luminescence inhibition. In the soil contaminated with Pb, leachates from the treated and untreated soil induced similar toxicity on V. fischeri on day 1. With Zn, the leachates from nZVI-treated soils showed significantly lower toxicity than the leachates from untreated soils. The application of nZVI to polluted soils decreased the leachate toxicity, reaching values similar to those from the original (unpolluted) soil on days 2 and 4 for Pb-NP and Zn-NP, respectively. These differences could be due to the lower effectiveness of Zn immobilization (Fig. 1a), resulting in a higher metal concentration in leachates on days 2 and 3.
Luminescence inhibition induced by leachates from nZVI-treated and untreated soils polluted with a Pb or b Zn on Vibrio fischeri. Bars represent mean ± SD (n = 3, 4 replicates each) of leachates from soils treated with nZVI (black bars) and untreated soils (white bars) and expressed as percent (v/v). Asterisk on bars corresponding to leachates treated with nZVI means significant differences (p < 0.05) compared to its untreated equivalent
The lethal effect on A. franciscana nauplii induced by selected leachates is shown in Fig. 6. The results showed that there are no significant differences between the time-dependent Pb and Zn leachate decreases. However, when nZVI is added to the soil, the nauplii lethality was significantly different between Pb-NP and Zn-NP during the first 24 h of exposure. At 24 h, leachates from the Zn-NP soils induced 63.30 ± 6.16 % of the nauplii lethality, whereas Pb-NP soils induced 100 % of the lethality. From the second day, both leachates induced a lethal effect of less than 10 %, demonstrating practically any toxicological risk to A. franciscana nauplii.
Lethal effect induced by leachates from nZVI-treated and untreated soils polluted with a Pb or b Zn on Artemia franciscana nauplii. Bars represent mean ± SD (n = 3, 4 replicates each) of leachates from soils treated with nZVI (black bars) and untreated soils (white bars) and expressed as percent (v/v). Asterisk on bars corresponding to leachates treated with nZVI means significant differences (p < 0.05) compared to its untreated equivalent
Although several endpoints have been proposed for assessing the toxicity of soils contaminated with C. elegans, reproduction is one of the most commonly used (Höss et al. 2009). Reproduction determination, as described here, can only be performed using adult-stage worms. The advantages are the use of few worms (one per well) and a simple technique; however, this endpoint requires a relatively long exposure time and manual scoring (Anderson et al. 2001).
The average number of offspring obtained (53 ± 9) when leachates from unpolluted soils were tested was not significantly different from the number obtained in internal controls (56 ± 8) (wells containing K-medium, see “Methods”). Therefore, they were used as controls for comparison. As shown in Fig. 7, leachates collected on the first day from columns containing soil polluted with Zn or Pb significantly inhibited the reproduction of C. elegans. After 5 days, leachates from soils did not affect the reproduction of C. elegans in any instances. Notably, when ZVI nanoparticles were added to the soils, they prevented the inhibitory effect of the leachates on nematode reproduction on the third day of exposure to leachates from Pb-NP. In soils polluted with Zn, the effect lasted 2 more days; nevertheless, nZVI significantly reverted the inhibitory effect of the leachate on the third day (Fig. 7b).
Effect of collected leachates, at 48-h interval, from columns containing soils polluted with Pb (a) or Zn (b) in the presence or absence of nZVI on Caenorhabditis elegans reproduction. Results are expressed as mean ± SEM (n = 3, 4 replicates each). Comparisons of leachate effects with respect to original soil (unpolluted), as well as between them, were performed. **p < 0.01; ***p < 0.001. τ p < 0.01, with respect to leachates from soil with Zn
Taken together, the results of the ecotoxicological analysis indicate a lower toxicity in nZVI-treated soils than in untreated ones. In the case of the treated soils, three species showed the highest toxicity effects on the first day (Figs. 5–7), and the toxicity dramatically decreased after that. This agrees with the characteristics of the first leachate: more acidic pH; high conductivity due to the leachability of soil ions, especially Na and Fe from commercial nanoparticles; and higher concentration of Pb or Zn in comparison to the following leachates (Figs. 1, 3, and 4). V. fischeri and C. elegans showed a longer effect from Zn when compared to Pb (nZVI-treated and untreated soil leachates), and A. franciscana was more sensitive to Pb-NP leachates than to Zn-NP leachates. This could be explained by the different sensitivity of the species to heavy metals (van der Oost et al. 2003; Wright and Welbourn 2002). In addition, the lower effectiveness of Zn immobilization by nZVI would allow a higher concentration of this metal to be available in the leachates; thus, a higher sensitivity of V. fischeri and C. elegans to Zn leachates compared to Pb ones can be expected.
4 Conclusions
The application of nZVI to remediate an acidic soil polluted with Pb or Zn significantly decreased the soil metal availability. Metal immobilization was stable after simulating 2 years of typical annual rainfall from central Spain, which is very interesting because of the need to apply long-term immobilization technologies. The first leachate from the nZVI-treated soils showed a high conductivity due to the elution of soil ions and those provided by the commercial nanoparticle suspension (Na and Fe), resulting to the highest toxicity levels. Toxicity from nZVI-treated soils was lower than that from untreated ones. The impact of nZVI on the soil–water system should be taken into account before the application of this remediation strategy, particularly in the more permeable soils. Toxicity assays using the selected test organisms could be considered suitable tools to analyze soil toxicity after the application of nZVI. The results obtained in the present study suggest that the use of nZVI to remediate soils polluted with Pb or Zn is a promising in situ strategy.
References
Adriano, D. C. (2001). Trace elements in terrestrial environments: biogeochemistry, bioavailability and risk of metals (2nd ed.). Heidelberg: Springer-Verlang New York Berlin.
AFNOR (1991). Détermination de l’inhibition de la luminescence de V. fischeri. NF T90-320, Paris. p. 331.
Anderson, B. S., Hunt, J. W., Phillips, B. M., Fairey, R., Roberts, C. A., Oakden, J. M., et al. (2001). Sediment quality in Los Angeles Harbor, USA: a triad approach. Environmental Toxicology and Chemistry, 20, 359–370.
Boluda, R., Quintanilla, J. F., Bonilla, J. A., Sáez, E., & Gamón, M. (2002). Application of the Microtox® test and pollution indices to the study of water toxicity in the Albufera Natural Park (Valencia, Spain). Chemosphere, 46, 355–369.
Brenner, S. (1974). The genetics of Caenorhabditis elegans. Genetic, 77, 71–94.
Chen, S. S., Hsu, H. D., & Li, C. W. (2004). A new method to produce nanoscale iron for nitrate removal. Journal of Nanoparticle Research, 6, 639–647.
Chen, P. J., Su, C. H., Tseng, C. Y., Tan, S. W., & Cheng, C. H. (2011). Toxicity assessments of nanoscale zerovalent iron and its oxidation products in medaka (Oryzias latipes) fish. Marine Pollution Bulletin, 63, 339–346.
Crane, R. A., & Scott, T. B. (2012). Nanoscale zero-valent iron: future prospects for an emerging water treatment technology. Journal of Hazardous Materials, 211–212, 112–125.
Cullen, L. G., Tilston, E. L., Mitchell, G. R., Collins, C. D., & Shaw, L. J. (2011). Assessing the impact of nano- and micro-scale zerovalent iron particles on soil microbial activities: particle reactivity interferes with assay conditions and interpretation of genuine microbial effects. Chemosphere, 82, 1675–1682.
El-Temsah, Y. S., & Joner, E. J. (2012). Ecotoxicological effects on earthworms of fresh and aged nano-sized zero-valent iron (nZVI) in soil. Chemosphere, 89, 76–82.
Fajardo, C., Ortiz, L. T., Rodríguez-Membibre, M. L., Nande, M., Lobo, M. C., & Martín, M. (2012). Assessing the impact of zero-valent iron (ZVI) nanotechnology on soil microbial structure and functionality: A molecular approach. Chemosphere, 86, 802–808.
Froehner, K., Backhaus, T., & Grimme, L. H. (2000). Bioassays with Vibrio fischeri for the assessment of delayed toxicity. Chemosphere, 40, 821–828.
Grieger, K. D., Fjordbøge, A., Hartmann, N. B., Eriksson, E., Bjerg, P. L., & Baun, A. (2010). Environmental benefits and risks of zero-valent iron nanoparticles (nZVI) for in situ remediation: risk mitigation or trade-off? Journal of Contaminant Hydrology, 118, 165–183.
Handy, R. D., van den Brink, N., Chappell, M., Mühling, M., Behra, R., Dušinská, M., et al. (2012). Practical considerations for conducting ecotoxicity test methods with manufactured nanomaterials: what have we learnt so far? Ecotoxicology, 21, 933–972.
Höss, S., Jänsch, S., Moser, T., Junker, T., & Römbke, J. (2009). Assessing the toxicity of contaminated soils using the nematode Caenorhabditis elegans as test organism. Ecotoxicology and Environmental Safety, 72, 1811–1818.
Karn, B., Kuiken, T., & Otto, M. (2009). Nanotechnology and in situ remediation: a review of the benefits and potential risks. Environmental Health Perspectives, 117, 1823–1831.
Keller, A. A., Garner, K., Miller, R. J., & Lenihan, H. S. (2012). Toxicity of nano-zero valent iron to freshwater and marine organisms. PLoS ONE, 7(8), e43983.
Klimkova, S., Cernik, M., Lacinova, L., Filip, J., Jancik, D., & Zboril, R. (2011). Zero-valent iron nanoparticles in treatment of acid mine water from in situ uranium leaching. Chemosphere, 82, 1178–1184.
Kokkali, V., Katramados, I., & Newman, J. D. (2011). Monitoring the effect of metal ions on the mobility of Artemia salina nauplii. Biosensors, 1, 36–45.
Li, X., & Zhang, W. (2007). Sequestration of metal cations with zerovalent iron nanoparticles—a study with high resolution X-ray photoelectron spectroscopy (HR-XPS). Journal of Physical Chemistry C, 111, 6939–6946.
Li, X., Elliott, D. W., & Zhang, W. (2006). Zero-valent iron nanoparticles for abatement of environmental pollutants: materials and engineering aspects. Critical Reviews in Solid State and Materials Sciences, 31, 11–22.
Li, H., Zhou, Q., Wu, Y., Fu, J., Wang, T., & Jiang, G. (2009). Effects of waterborne nano-iron on Medaka (Oryzias latipes): antioxidant enzymatic activity, lipid peroxidation and histopathology. Ecotoxicology and Environmental Safety, 72, 684–692.
Liendo, M. A., Navarro, G. E., & Sampaio, C. H. (2013). Nano and micro ZVI in aqueous media: copper uptake and solution behavior. Water, Air, & Soil Pollution, 224, 1541.
MAPA (1994). Métodos Oficiales de Análisis, vol. III, Spain.
Mortimer, M., Kasemets, K., Heinlaan, M., Kurvet, I., & Kahru, A. (2008). High throughput kinetic Vibrio fischeri bioluminescence inhibition assay for study of toxic effects of nanoparticles. Toxicology in Vitro, 22, 1412–1417.
Mueller, N. C., Braun, J., Bruns, J., Černík, M., Rissing, P., Rickerby, D., et al. (2012). Application of nanoscale zero valent iron (NZVI) for groundwater remediation in Europe. Environmental Science and Pollution Research, 19, 550–558.
Pawlett, M., Ritz, K., Dorey, R. A., Rocks, S., Ramsden, J., & Harris, J. A. (2013). The impact of zero-valent iron nanoparticles upon soil microbial communities is context dependent. Environmental Science and Pollution Research, 20, 1041–1049.
Persoone, G., Van de Vell, A., Van Steertegem, M., & Nayer, B. (1989). Predictive value for laboratory tests with aquatic invertebrates: influence of experimental conditions. Aquatic Toxicology, 14, 149–166.
Reddy, K. R., Kadlec, R. H., Flaig, E., & Gale, P. M. (1999). Phosphorus retention in streams and wetlands-a review. Critical Reviews in Environmental Science and Technology, 29, 86–146.
Rhue, R. D., & Kamprath, E. J. (1973). Leaching losses of sulfur during winter months when applied as gypsum, elemental S or prilled S. Agronomy Journal, 65, 603–605.
Roh, J. Y., Lee, J., & Choi, J. (2006). Assessment of stress-related gene expression in heavy metal-exposed nematode Caenorhabditis elegans: a potential biomarker for metal-induced toxicity monitoring and environmental risk assessment. Environmental Toxicology & Chemistry, 25, 2946–2956.
Ryden, J. C., McLaughlin, J. R., & Syers, J. K. (1977). Mechanisms of phosphate sorption by soils and hydrous ferric oxide gel. Journal of Soil Science, 28, 72–92.
Sánchez-Fortún, S., Sanz, F., Santa-María, A., Ros, J. M., De Vicente, M. L., Encinas, M. T., et al. (1997). Acute sensitivity of three age classes of Artemia salina larvae to seven chlorinated solvents. Bulletin of Environmental Contamination and Toxicology, 59, 445–451.
Singh, R., Misra, V., & Singh, R. P. (2012). Removal of Cr(VI) by Nanoscale zero-valent iron (nZVI) from soil contaminated with tannery wastes. Bulletin of Environmental Contamination and Toxicology, 88, 210–214.
Terman, G. L. (1977). Quantitative relationships among nutrients leached from soils. Soil Science Society of America Journal, 41, 935–940.
USEPA (1992). Ground Water Issue. Behavior of metals in soils. EPA/540/S-92/018.
van der Oost, R., Beyer, J., & Vermeulen, N. P. E. (2003). Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environmental Toxicology and Pharmacology, 13, 57–149.
Vangronsveld, J., Cunningham, S.D. (1998). Introduction to the concepts. In: Metal-contaminated soils. In situ inactivation and phytorestoration. (eds. J. Vangronsveld , S.D. Cunningham), Springer, New York, pp. 1-15.
Vanhaecke, P., Persoone, G. (1984) The ARC-Test: A standardized short-term routine toxicity test with Artemianauplii. Methodolgy and evaluation. In: G. Persoone, J. Jaspers and C. Claus (Eds.). Ecotoxicological Testing for the Marine Environment. State Univ. of Ghent and Inst. Mar. Scient. Res., Bredene, Belgium vol. 2.
Wright, D. A., & Welbourn, P. (2002). Environmental toxicology. Cambridge, UK: Cambridge University Press.
Xu, Y., & Zhao, D. (2007). Reductive immobilization of chromate in water and soil using stabilized iron nanoparticles. Water Research, 41, 2010–2108.
Zhang, W. (2003). Nanoscale iron particles for environmental remediation: an overview. Journal of Nanoparticle Research, 5, 323–332.
Zhang, M., Wang, Y., Zhao, D., & Pan, G. (2010). Immobilization of arsenic in soils by stabilized nanoscale zero-valent iron, iron sulfide (FeS), and magnetite (Fe3O4) particles. Chinese Science Bulletin, 55, 365–372.
Acknowledgments
The authors thank Ministerio de Educación y Ciencia (Spain) for supporting Project CTM 2010-20617-C02-02 and Consejería de Educación from Comunidad de Madrid for supporting Project S2009/AMB-1478 (EIADES, www.eiades.org).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gil-Díaz, M., Ortiz, L.T., Costa, G. et al. Immobilization and Leaching of Pb and Zn in an Acidic Soil Treated with Zerovalent Iron Nanoparticles (nZVI): Physicochemical and Toxicological Analysis of Leachates. Water Air Soil Pollut 225, 1990 (2014). https://doi.org/10.1007/s11270-014-1990-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-014-1990-1