Abstract
Lake water calcium (Ca) decline has recently been recognized as a stressor impacting softwater lake districts that have experienced long-term patterns of acid deposition and/or timber harvesting. Declining aqueous Ca levels may impact the survival of aquatic biota, particularly Ca-rich cladoceran taxa such as daphniids. Daphnia pulex are sensitive to laboratory Ca levels below 1.5 mg l−1; however, responses of cladoceran communities to Ca decline in natural environments require further study. Dickie Lake (Ontario, Canada) is the site of an inadvertent natural experiment, providing insight into the effects of changing aqueous Ca availability upon cladoceran communities, as the lake has a history of acidification, followed by recent (1990s) Ca additions to the watershed via applications of calcium-rich road dust suppressants. Paleolimnological analyses were used to examine changes in cladoceran community structure (with a focus upon Ca-rich daphniids) from pre-industrial times to present day. Three distinct temporal stages were apparent in Dickie Lake’s daphniid community: 1870–1950, 1950–1990, and 1990–present. The daphniid community of the pre-industrial assemblages was dominated by members of the Daphnia longispina species complex, but shifted in the late 1950s to more acid- and Ca-insensitive members of the D. pulex species complex. During the most recent stage, coincident with dust suppressant applications, both daphniid complexes are well represented. Observed transitions between daphniid species complexes provide further evidence of the influence of Ca availability upon cladoceran community structure, indicating the potential importance of the controlled addition of Ca to freshwater systems (i.e., liming) as a mitigation/recovery strategy as Ca declines continue.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Lake ecosystems are subject to a variety of environmental stressors including fluctuations in pH, climate, nutrient availability, and the spread of invasive species (Yan et al. 2008b). Acidification stress associated with acid deposition received much public attention in North America during the late 1970s and 1980s, resulting in political action to reduce sulphate emissions (Jeffries et al. 2003a). Following large reductions in sulphate emissions in the 1980s and 1990s, the widespread recovery of lake ecosystems in North America from acidification was anticipated (Jeffries et al. 2003a). However, expected increases in pH were relatively rare in the early stages of recovery (Jeffries et al. 2003b). The slow recovery of acidified lakes on the Canadian Shield has been attributed principally to long-term declines in exchangeable base cations (in particular, calcium (Ca); Stoddard et al. 1999; Keller et al. 2001; Jeffries et al. 2003b; Molot and Dillon 2008). Depletion of exchangeable stores of Ca from soils has been accelerated by both acid deposition and tree harvesting practices (Watmough et al. 2005), which initially enhance Ca release from the soil (Lawrence et al. 1999), but deplete the exchangeable pool over time (Likens et al. 1996; Stoddard et al. 1999), especially when compounded by low weathering inputs of Ca (Likens et al. 1996). Consequently, long-term soil Ca depletions have resulted in Ca declines in lakes across the Canadian Shield (Stoddard et al. 1999; Jeziorski et al. 2008), with growing concern regarding the impacts of ongoing Ca declines upon aquatic biota (as Ca is an essential nutrient), particularly for Ca-rich organisms such as the Cladocera.
Ca is a structural element in the crustacean carapace (Greenway 1985), and among the cladocerans comprises 2.5–7.7% of the dry weight of Daphnia spp., but only 0.2–0.4% for non-daphniid taxa such as Bosmina spp. (Wærvågen et al. 2002; Jeziorski and Yan 2006). Laboratory analyses have demonstrated that among the Ca-rich daphniids, Ca is primarily obtained via active transport from the surrounding water (Cowgill et al. 1986; Tan and Wang 2009), but the majority of this body Ca is lost to the surrounding medium during each shedding (moult) of the carapace, and must be replaced (Alstad et al. 1999; Tan and Wang 2009). The relationship between aqueous Ca concentration and community composition influences the distribution and competitive success of crustacean zooplankton species with varying Ca burdens, relegating taxa with relatively high Ca demands to lakes with higher ambient Ca (Wærvågen et al. 2002). The impacts of reduced calcium availability over time have been examined using paleolimnological techniques, and long-term declines in the relative abundances of daphniid species have been observed in lakes that have fallen near or below an experimentally determined fitness threshold level for the species D. pulex (1.5 mg l−1; Ashforth and Yan 2008; Jeziorski et al. 2008). Further investigations into the importance of ambient (Ca) upon cladoceran community structure are necessary to address emerging questions regarding the ecological implications of ongoing Ca declines in softwater regions such as the Canadian Shield. Long-term monitoring programs, such as those conducted by the Ontario Ministry of the Environment in south-central Ontario, have been invaluable for examining the combined effects of environmental stressors such as acid deposition, climate change, cottage development, and Ca decline for the past ~30 years (Yan et al. 2008a). However, as the onset and impacts of many environmental stressors (including Ca decline) often pre-date direct monitoring, indirect methods such as paleolimnological techniques (that utilize indicators preserved in lake sediments to track long-term changes in lake systems; Smol 2010) are required to address many long-term questions.
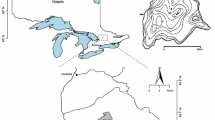
We examine the relationship between aqueous Ca levels and cladoceran community structure in a softwater Canadian Shield lake. Dickie Lake (45°09′N; 79°05′W; Fig. 1) near the town of Dorset, Ontario, is of particular interest due to the large changes in Ca concentrations it has experienced during recent decades (Fig. 2). Relatively low Ca levels (2.1–2.2 mg l−1) were documented in Dickie Lake from 1980 to 1992 (Fig. 2), prior to and during the implementation of SO2 emission reductions (Jeffries et al. 2003a), and during this time period pH levels varied from 5.7 to 6.1 (Fig. 2). Subsequently, relatively large increases in Ca concentration have occurred since the late 1990s (maximum 3.2 mg l−1), coincident with the application of calcium chloride as a dust suppressant to the surrounding roads (Yao et al. 2011), while the pH has largely remained above 6. Changes in aqueous Ca availability, such as through inadvertent liming, are of particular interest in softwater environments due to the potential impacts upon Ca-rich daphniid taxa (Jeziorski et al. 2008; 2011; DeSellas et al. 2011). Liming practices have been previously used to combat lake and stream acidification and to ameliorate toxic effects of aluminum (Henrikson et al. 1995; Eggleton et al. 1996), and may provide an approach to managing aqueous Ca levels; however the impacts on biological communities sensitive to low Ca availability are poorly understood at present.
Long-term changes in the Ca concentration of Dickie Lake due to acid deposition, followed by the recent addition of Ca-rich dust suppressant, provide a unique case study for examining the impact of aqueous Ca decline on Ca-rich cladoceran assemblages, followed by the potential restoration effects of the inadvertent liming of the watershed. Here, we investigate how the cladoceran community of Dickie Lake has changed relative to temporal changes in lakewater Ca concentration. The loss of daphniids from the Dickie Lake sediment record during the period of prolonged acid deposition and depletion of the watershed’s Ca reservoir (~1950–1990), followed by their return upon the recent addition of Ca-rich dust suppressant, adds to growing evidence of the importance of aqueous Ca availability in structuring cladoceran communities.
2 Materials and Methods
Sediment cores were collected from the deep basin of Dickie Lake (Fig. 1) in September 2010 using a Glew (1989) gravity corer and were then immediately sectioned on shore using a Glew (1988) vertical extruder. The cores were sectioned at a 0.25-cm resolution (a slice thickness typically representing ~1–3 years of accumulated sediment in this geographic region; Mills et al. 2009) to ensure the recent period of Ca chloride additions to the watershed were well represented. Prior to analysis, the sediment was stored in a cold room at the Paleoecological Environmental Assessment and Research Lab (PEARL) at Queen’s University in Kingston, Ontario. The sediment intervals selected for analysis were chosen to ensure a higher resolution of sediments from recent decades (the period of direct monitoring and Ca additions), while also extending the analysis prior to initial European settlement of the region (~1850). Sediment age was determined from 210Pb activity detected by gamma spectroscopy via the constant rate of supply (CRS) model (Binford 1990).
Preparation of slides for enumeration of cladoceran assemblages followed the methods detailed by Korhola and Rautio (2001). The identification of cladoceran remains primarily followed Szeroczyñska and Sarmaja-Korjonen (2007), Sweetman and Smol (2006) and Smirnov (1974). All cladoceran remains (e.g., carapaces, headshields, postabdominal claws) were tabulated separately, and the most abundant remain was used to calculate the number of individuals (Frey 1986). A minimum of 70 individuals were enumerated from each interval, a value demonstrated to be sufficient to characterize most assemblages (Kurek et al. 2010). Daphniid remains were assigned to one of two species complexes based on the postabdominal claw morphology; the Daphnia pulex species complex was identified by the presence of stout spines on the middle comb of the postabdominal claw, and the Daphnia longispina species complex was identified by the absence of these stout spines (Szeroczyñska and Sarmaja-Korjonen 2007).
For statistical analyses, only species with greater than 2% relative abundance in at least two intervals were included. Cladoceran assemblages were inspected for both general trends over time as well as analyzed more rigorously using cluster analyses (constrained incremental sum of squares [CONISS]; Grimm 1987) with the number of zones identified using the broken stick model (Bennett 1996) by means of the vegan package (Oksanen et al. 2011) for the R software environment (R Development Core Team 2010). To explore the relationships between the cladoceran species assemblages through time, a principal component analysis (PCA) was performed.
3 Results
The sedimentary cladoceran assemblages of Dickie Lake over the past ~150 years have been dominated by pelagic taxa (Fig. 3), primarily Bosmina spp., but also the Daphnia longispina species complex (D. ambigua, D. dubia, D. mendotae, D. longiremis, D. retrocurva), the D. pulex species complex (D. catawba, D. pulex), and Holopedium gibberum. Following cluster analysis, three zones in the cladoceran community were identified by the broken-stick model: ~1880–1950 (S1a), ~1950–1990 (S1b) and ~1990–present (S2), that appear to be driven principally by changes in daphniid abundances through time. The oldest cladoceran communities present in the Dickie Lake sediment core (S1a) are characterized by high relative abundances of bosminid remains, low abundances of the D. longispina complex (mean abundance 4%), the virtual absence of the D. pulex complex and low abundances of several littoral taxa (notably Alona spp. and Alonella spp.). The community changes present in the second zone (Fig. 3) identified by the cluster analysis (~1950–1990, S1b) were principally driven by decreases in the relative abundances of the D. longispina species complex and littoral taxa, concurrent with increases in the D. pulex complex (S1b). A third community zone is present from ~1990 to the present (S2, Fig. 3), and is markedly different from the prior zones due to the increases in both daphniid species complexes, while Bosmina spp. relative abundance decreased.
Relative frequency diagram of the most common cladoceran remains recorded in the sediments of Dickie Lake, with major daphniid community stages (S1a, S1b, S2) demarked as determined by the constrained incremental sum of squares (CONISS) cluster analysis. Only taxa that comprised >2% of at least two intervals were included, and Alonella spp. and Chydorus spp. were grouped for analysis. 210Pb dates are shown to the right
The PCA analysis explained 34% of the variation in the Dickie Lake cladoceran assemblages through time (Fig. 4). The differences among the assemblages from the three zones were principally associated with the daphniid species complexes.
Principal component analysis (PCA) biplot showing the relationship between the sample scores (points) and species scores (vectors) for Dickie Lake. The two primary axes account for a total of 34% of the explained variance. The radiometrically determined (210Pb) ages of the sediment intervals are indicated for those intervals available. Major daphniid community stages (S) are indicated and approximated here by the circles. Species arrows are coded as follows: A. curv = Acantholebris curvirostis, A. affi = Alona affinis, A. cost = Alona costata, A. gutt = Alona guttata, A. inte = Alona intermedia, A. nana = Alonella nana, A. rust = Alona rustica, Bosm. spp. = Bosmina spp., C. bico = Chydorus bicornatus, C. spha = Chydorus sphaericus, D. brac = Diaphanosoma bracyrum, D. long = Daphnia longispina, D. pule = Daphnia pulex, H. gibb = Holopedium gibberum, L. kind = Leptodora kindti, L. seti = Latona setifera, M. disp = Monospilus dispar, R. falc = Rhynchotalona falcata
4 Discussion
Changes among the sedimentary cladoceran assemblages preserved in the Dickie Lake sediment record coincide with broad changes in aqueous Ca concentration over recent decades (Fig. 3), demonstrating the influence of Ca availability on microcrustacean community structure. The timing of the three stratigraphic zones identified in the cluster analysis of cladoceran assemblages correspond approximately to the pre-industrial period (~1880–1950, S1a), the period of time when south-central Ontario was experiencing acid deposition and expedited Ca leaching (~1950–1990, S1b; Dillon et al. 1978), and most recently, the additions of Ca to the surrounding watershed via the dust suppression program (~1990–present, S2; Yao et al. 2011). The most striking changes in cladoceran community composition in response to the changes in lakewater chemistry across these time periods have been amongst the daphniid species complexes.
Prior to large-scale industrial activity and the accompanying increase in sulphate emissions and acid deposition (~1900–1950, S1a), remains from the D. longispina species complex (typically represented in south-central Ontario by D. ambigua, D. dubia, D. mendotae, D. longiremus, and D. retrocurva; Yan et al. 2008b) were present at a consistent relative abundance (~4%), in contrast with the virtual absence of the D. pulex species complex (typically represented in south-central Ontario by D. catawba, D. pulicaria and D. pulex; Yan et al. 2008b). The cladoceran assemblage changed abruptly during the 1950s (1950–1990, S1b), with decreases in the relative abundances of the D. longispina species complex and littoral chydorid taxa coincident with increases in the D. pulex species complex (Fig. 3). The timing of these changes are consistent with the rise of sulphate emissions in eastern North America and the subsequent acid deposition (Jeffries et al. 2003b; Stoddard et al. 1999), resulting in decreases in lakewater pH and, over time, reduced Ca concentration due to the depletion of the pool of exchangeable Ca in watershed soils (Watmough and Dillon 2003; Houle et al. 2006). The differential response of the daphniid species complexes is of particular interest, as the rise in the relative abundance of the D. pulex species complex is likely due to the species D. catawba (a species known to be both acid-tolerant and Ca-insensitive; Sprules 1975; Cairns 2010; Jeziorski et al. 2011). However, the decline of the D. longispina complex in Dickie Lake appears to be similar to other declines in daphniids that have been observed with falling aqueous Ca concentration (Jeziorski et al. 2008). Where the Dickie Lake sediment record differs from other paleolimnological examinations of the relationship between the Cladocera and lakewater Ca concentration is in the most recent stratigraphic zone. Beginning in the late 1990s with large additions of Ca dust suppressant to roads in the watershed (Yao et al. 2011), there has been a return of the D. longispina complex and continued persistence of the D. pulex complex (~1990–present, S2; Fig. 2) resulting in a daphniid assemblage very different from the pre-impact conditions. Thus, changes in Ca availability over the past several decades are likely the principal driver behind the fluctuations in the daphniid community of Dickie Lake.
Recent interest in the impacts of regional Ca declines has spurred investigation into the impacts of declining Ca concentrations on the growth, reproduction and survival of individual daphniid taxa (Ashforth and Yan 2008; Cairns and Yan 2009; Tan and Wang 2010). Daphniids principally regulate Ca through adjusting efflux rates (Tan and Wang 2009); therefore, specific differences among daphniids in their Ca uptake efficiency may impact the ability to cope with reduced Ca availability (Tan and Wang 2010). Decreased efficiency of Ca uptake and retention is likely an underlying cause for the decreased relative abundance of the D. longispina complex during the S1b stratigraphic zone (~1950–1990; Fig. 3). Although Dickie Lake’s pH was slightly below 6 during the 1970s (Fig. 2), an indication of modest acidification (Hall and Smol 1996), this drop in pH alone is unlikely to be responsible for the complete loss of the D. longispina complex, as remains from this species complex are often found in the sediments of nearby lakes with comparable pH (e.g., Jeziorski et al. 2011). However, the modest acidification is an indication that buffering capacity of the surrounding watershed was depleted, and that Ca levels likely fell to concentrations detrimental to the competitiveness of members of the D. longispina complex. A recent survey of 304 softwater lakes in south-central Ontario lakes has identified lake Ca concentration to be a significant predictor of the presence of five common daphniid species (D. longiremus, D. dubia, D.mendotae, D. retrocurva, and D. pulicaria) and unrelated to the presence of two others (D. ambigua and D. catawba; Cairns 2010). Interestingly, four of the five species for which lakewater Ca is a significant predictor belong to the D. longispina species complex, and each differs in their specific optimal (2.76–4.87 mg l−1) and critical lower Ca thresholds (1.26–1.69 mg l−1; Cairns 2010). Although the Ca concentration of Dickie Lake has likely always been suboptimal for most members of the D. longispina complex (currently ~3 mg l−1 following recent Ca additions; Fig. 2), decreasing ambient Ca concentrations, and therefore greater energy expenditures on maintaining Ca balance may have reduced the fitness of this taxon and explain its disappearance from zone S1b (Fig. 3). In contrast, a lower critical threshold was not identified for either member of the D. pulex species complex (D. pulicaria and D. catawba) examined by Cairns (2010), indicating Ca insensitive species from this group may have been provided a competitive advantage during the period of acidification/Ca decline. The addition of Ca to Dickie Lake via road dust suppressant in the most recent stratigraphic zone (~1990–present, S2; Yao et al. 2011) is the most likely explanation for the reestablishment of the D. longispina species complex. However, the limited taxonomic resolution due to uncertainties in the identification of daphniids remains complicates this analysis.
Differences in Ca optima and thresholds within daphniid species complexes add ambiguities to the interpretation of changes in the daphniid sedimentary assemblages as typically only the postabdominal claw is preserved in lake sediments. Postabdominal claws are then assigned to either the D. pulex or D. longispina species complex according to the presence/absence of stout spines on the middle comb (Szeroczyñska and Sarmaja-Korjonen 2007). To date, the identification of additional morphological characters to better separate taxa within the species complexes have proven unsuccessful (Korosi et al. 2011). Although aqueous Ca concentration appears to be a significant explanatory variable for most members of the D. longispina species complex present in south-central Ontario (Cairns 2010), this is not the case for D. ambigua (Cairns 2010). Similarly D. longispina and D. galeata are commonly found in low Ca (<2.0 mg l−1) European lakes (Hessen et al. 1995; Wærvågen et al. 2002). Despite these potential complications and the inherent difficulties associated with comparisons of cladoceran paleolimnological and modern-day sampling data due to the incomplete species preservation in sediments, differences in the regions of the lake represented (whole-basin vs. water column), and differing time scales (integrated year-round samples vs. snapshots in time), the monitoring data for Dickie Lake show good agreement with the sediment record. During the late 1970s (S1b) the daphniids of Dickie Lake were dominated by D. catawba (~10% of total crustacean zooplankton density during the ice-free period), with several members of the D. longispina complex (D. mendotae, D. retrocurva and D. ambigua) present in only trace amounts (<1%; N. Yan, York University, Toronto, ON, Canada, unpublished data). Similarly, the monitoring data also show a return following a prolonged absence of several members of the D. longispina complex (D. mendotae and D. retrocurva) upon the additions of Ca to the watershed (N. Yan unpublished data), closely matching the changes observed post 1990 (S2) in the sediment record. Discrepancies between the onset of the S2 stratigraphic zone (in 1990) and the actual addition of Ca (in 1998) are likely due to the presence of the Ca-insensitive taxa D. ambigua responding to environmental cues other than Ca limitation.
Although the cladoceran response to the closely related changes in lake pH and Ca concentration was the motivation for this analysis, in recent decades Dickie Lake has also experienced many of the other environmental stressors influencing lakes on the Canadian Shield. Therefore, the response of sedimentary cladoceran assemblages to changes in Ca availability have accompanied broad changes in climate, nutrient availability, land use and their interactive and often counteractive effects (Yan et al. 2008b; Smol 2010). For example, accompanying the general increases in temperature, drought conditions in south-central Ontario have caused periods of re-acidification (Dillon et al. 2007), detrimental to acid-sensitive daphniid taxa, while decreasing total phosphorus may be simultaneously favouring daphniids over smaller, planktonic competitors such as Bosmina spp. (Gliwicz 1990). Specifically within Dickie Lake, chloride concentrations have risen substantially with the addition of the road dust suppressant (from 0.8–5.8 mg l−1; Molot and Dillon 2008); however, they remain several orders of magnitude below concentrations acutely toxic to daphniids (D. pulex; Gardner and Royer 2010). Despite the variety of competing influences of recent environmental changes in Dickie Lake, changes in ambient Ca concentration remain the best explanatory factor for the observed stratigraphic shifts among daphniid taxa.
The impacts of the declining Ca concentrations in softwater boreal lakes are expected to be most pronounced for Ca-rich organisms such as Daphnia spp. (Jeziorski et al. 2008; Cairns and Yan 2009). However, differences in tolerance of low Ca conditions among the daphniids warrants further investigation. This paleolimnological analysis adds to the growing evidence of the importance of aqueous Ca concentration on cladoceran community composition, as long-term declines in the D. longispina complex were reversed following the addition of large amounts of Ca to the Dickie Lake watershed. The persistence of the D. pulex complex following the inadvertent liming of Dickie Lake, despite its lack of a preindustrial presence, raises questions regarding the use of Ca additions as a lake management practice to promote or enhance recovery from aqueous Ca decline. It appears that human-mediated aqueous Ca increases have promoted a transition to a novel third state, rather than a recovery of the Dickie Lake cladoceran community.
References
Alstad, N., Skardal, L., & Hessen, D. (1999). The effect of calcium concentration on the calcification of Daphnia magna. Limnology and Oceanography, 44, 2011–2017.
Ashforth, D., & Yan, N.D. (2008). The interactive effects of falling calcium concentrations and rising temperatures on Daphnia pulex life table parameters at low and high food concentrations. Limnology and Oceanography, 53, 420–432.
Bennett, K. (1996). Determination of the number of zones in a biostratigraphical sequence. New Phytolologist, 132, 155–170.
Binford, M. (1990). Calculation and uncertainty analysis of 210Pb dates for PIRLA project lake sediment cores. Journal of Paleolimnology, 3, 253–267.
Cairns, A. (2010). Field assessments and evidence of impact of calcium decline on Daphnia (Crustacea, Anomopoda) in Canadian Shield lakes. MSc thesis, York University, Canada.
Cairns, A., & Yan, N.D. (2009). A review of the influence of low ambient calcium concentrations on freshwater daphniids, gammarids and crayfish. Environmental Reviews, 17, 67–79.
Cowgill, U., Emmel, H., Hopkins, D., Applegath, S., & Takahashi, I. (1986). Influence of water on reproductive success and chemical composition of laboratory reared populations of Daphnia magna. Water Research, 20, 317–323.
DeSellas, A., Paterson, A., Sweetman, J., & Smol, J. (2011). Assessing the effects of multiple environmental stressors on zooplankton assemblages in Boreal Shield lakes since pre-industrial times. Journal of Limnology, 70, 41–56.
Dillon, P.J., Jeffries, D., Snyder, W., Reid, R., Yan, N.D., Evans, D., Moss, J., & Scheider, W. (1978). Acidic precipitation n south-central Ontario: recent observations. Journal of the Fisheries Research Board of Canada, 35, 809–815.
Dillon, P. J., Watmough, S. A., Eimers, M. C., & Aherne, J. (2007). Long-term changes in boreal lake and stream chemistry: recovery from acid deposition and the role of climate. In G. R. Visgilio & D. M. Whitelow (Eds.), Acid in the environment: lessons learned and future prospects (pp. 59–75). New York: Springer.
Eggleton, M., Morgan, E., & Pennington, W. (1996). Effects of liming on an acid-sensitive southern Appalachian stream. Restoration Ecology, 4, 247–263.
Frey, D. (1986). Cladoceran Analysis. In B. E. Berglund (Ed.), Handbook of Holocene palaeoecology and palaeohydrology (pp. 667–692). New York: Wiley.
Gardner, K., & Royer, T. (2010). Effect of road application on seasonal chloride concentrations and toxicity in south-central Indiana streams. Journal of Environmental Quality, 39, 1036–1042.
Glew, J. R. (1988). A portable extruding device for close interval sectioning of unconsolidated core samples. Journal of Paleolimnology, 1, 235–239.
Glew, J. R. (1989). A new trigger mechanism for sediment samplers. Journal of Paleolimnology, 2, 241–243.
Gliwicz, Z. M. (1990). Food thresholds and body size in cladocerans. Nature, 343, 638–640.
Greenway, P. (1985). Calcium balance and moulting in the Crustacea. Biological Reviews, 60, 425–454.
Grimm, E. C. (1987). CONISS: a Fortran 77 program for stratigraphically constrained cluster analysis by the method of incremental sum of squares. Computers and Geosciences, 13, 13–35.
Hall, R., & Smol, J. P. (1996). Paleolimnological assessment of long-term water-quality changes in south-central Ontario lakes. Canadian Journal of Fisheries and Aquatic Sciences, 53, 1–17.
Henrikson, L., Hindar, A., & Thörnelöf, E. (1995). Freshwater liming. Water, Air, and Soil Pollution, 85, 131–142.
Hessen, D. O., Faafeng, B. A., & Andersen, T. (1995). Competition or niche segregation between Holopedium and Daphnia; empirical light on abiotic key parameters. Hydrobiologia, 307, 253–261.
Houle, D., Ouimet, R., Couture, S., & Gagnon, C. (2006). Base cation reservoirs in soil control the buffering capacity of lakes in forested catchments. Canadian Journal of Fisheries and Aquatic Sciences, 63, 471–474.
Jeffries, D., Brydges, T., Dillon, P.J., & Keller, W. (2003a). Monitoring the results of Canada/U.S.A acid rain control programs: some lake responses. Environmental Monitoring and Assessment, 88, 3–19.
Jeffries, D., Clair, T., Couture, S., Dillon, P.J., Dupont, J., Keller, W., McNicol, D., Turner, M., Vet, R., & Weeber, R. (2003b). Assessing the recovery of lakes in southeastern Canada from the effects of acidic deposition. Ambio, 32, 176–182.
Jeziorski, A., & Yan, N.D. (2006). Species identity and aqueous calcium concentrations as determinants of calcium concentrations of freshwater crustacean zooplankton. Canadian Journal of Fisheries and Aquatic Sciences, 63, 1007–1013.
Jeziorski, A., Yan, N.D., Paterson, A.M., DeSellas, A., Turner, M., Jeffries, D., Keller, B., Weeber, R., McNicol, D., Palmer, M., McIver, K., Arseneau, K., Ginn, B., Cumming, B., & Smol, J.P. (2008). The widespread threat of calcium decline in fresh waters. Science, 322, 1374–1377.
Jeziorski, A., Paterson, A. M., & Smol, J. P. (2011). Crustacean zooplankton sedimentary remains from calcium-poor lakes: complex responses to threshold concentrations. Aquatic Sciences. doi:10.1007/s00027-011-0202-y.
Keller, W., Dixit, S., & Heneberry, J. (2001). Calcium declines in northeastern Ontario lakes. Canadian Journal of Fisheries and Aquatic Sciences, 58, 2011–2020.
Korhola, A., & Rautio, M. (2001). Cladocera and other brachiopod crustaceans. In J. P. Smol, H. J. B. Birks, & W. M. Last (Eds.), Developments in paleoenvironmental research Vol 4: Tracking environmental change using lake sediments: zoological indicators (pp. 4–51). Dordrecht: Kluwer Academic Publishing.
Korosi, J. B., Jeziorski, A., & Smol, J. P. (2011). Using morphological characters of subfossil daphniid postabdominal claws to improve taxonomic resolution within species complexes. Hydrobiologia. 676, 117–128.
Kurek, J., Korosi, J., Jeziorski, A., & Smol, J. P. (2010). Establishing reliable minimum count sizes for cladoceran microfossils sampled from lake sediments. Journal of Paleolimnology, 44, 603–612.
Lawrence, G., David, M., Lovett, G., Murdoch, P., Burns, D., Stoddard, J., Baldigo, B., Porter, J., & Thompson, A. (1999). Soil calcium status and the response of stream chemistry to changing acidic deposition rates. Ecological Applications, 9, 1059–1072.
Likens, G., Driscoll, C., & Buso, D. (1996). Long-term effects of acid rain: response and recovery of a forest ecosystem. Science, 272, 244–246.
Mills, R., Paterson, A., Blais, J., David, R., Smol, J., & Mierle, G. (2009). Factors influencing the achievement of steady state in mercury contamination among lakes and catchments of south-central Ontario. Canadian Journal of Fisheries and Aquatic Sciences, 66, 187–200.
Molot, L., & Dillon, P. (2008). Long-term trends in catchment export and lake concentrations of base cations in the Dorset study area, central Ontario. Canadian Journal of Fisheries and Aquatic Sciences, 65, 809–820.
Oksanen, J., Guillaume Blanchet, F., Roeland, K., Legendre, O’Hara, P., Simpson, G., Solymos, P., Henry M., Stevens, H., Wagner, H. (2011). Vegan: community ecology package. R package version 1.17-8. http://CRAN.R-project.org/package=vegan
R Development Core Team (2010). R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, URL http://www.R-project.org/.
Smirnov, N. (1974). Fauna of the USSR: Crustacea. Jerusalem: Keter Press.
Smol, J. P. (2010). The power of the past: Using sediments to track the effects of multiple stressors on lake ecosystems. Journal of Freshwater Biology, 55(Suppl. 1), 43–59.
Sprules, W. G. (1975). Mid summer crustacean zooplankton communities in acid stressed lakes. Journal of the Fisheries Research Board of Canada, 32, 389–396.
Stoddard, J., Jeffries, D., Lukewille, A., Clair, T., Dillon, P.J., Driscoll, C., Forsius, M., Johannessen, M., Kahl, J., Kellogg, J., Kemp, A., Mannio, J., Monteith, D., Murdoch, P., Patrick, S., Rebsdorf, A., Skjelkvale, B., Stainton, M., Traaen, T., van Dam, H., Webster, K., Wieting, J., & Wilander, A. (1999). Regional trends in aquatic recovery from acidification in North America and Europe. Nature, 401, 575–578.
Sweetman, J., & Smol, J. P. (2006). A guide to the identification of cladoceran remains (Crustacea: Branchiopoda) in Alaskan lake sediments. Archiv für Hydrobiologie, Supplement, 151, 353–394.
Szeroczyñska, K., & Sarmaja-Korjonen, K. (2007). Atlas of subfossil Cladocera from Central and Northern Europe. PL: Friends of the Lower Vistula Society.
Tan, Q., & Wang, W. (2009). The regulation of calcium in Daphnia magna reared in different calcium environments. Limnology and Oceanography, 54, 746–756.
Tan, Q., & Wang, W. (2010). Interspecies differences in calcium content and requirement in four freshwater cladocerans explained by biokinetic parameters. Limnology and Oceanography, 55, 1426–1434.
Wærvågen, S., Rukke, N., & Hessen, D. (2002). Calcium content of crustacean zooplankton and its potential role in species distribution. Journal of Freshwater Biology, 47, 1866–1878.
Watmough, S., & Dillon, P.J. (2003). Base cation and nitrogen budgets for seven forested catchments in central Ontario, 1983–1999. Forest Ecology and Management, 177, 155–177.
Watmough, S., Aherne, J., Alewell, C., Arp, S., Bailey, P., Clair, T., Dillon, P., Duchensne, L., Eimers, C., Fernandez, I., Foster, N., Larssen, T., Miller, E., Mitchell, M., & Page, S. (2005). Sulphate, nitrogen and base cation budgets at 21 forested catchments in Canada, the United States and Europe. Environmental Monitoring and Assessment, 109, 1–36.
Yan, N.D., Paterson, A., Somers, K., & Scheider, W. (2008a). An introduction to the Dorset special issue: transforming understanding of factors that regulate aquatic ecosystems on the southern Canadian Shield. Canadian Journal of Fisheries and Aquatic Sciences, 65, 781–785.
Yan, N.D., Somers, K., Girard, R., Paterson, A., Keller, W., Ramcharan, C., Rusak, J., Ingram, R., Morgan, G., & Gunn, J. (2008b). Long-term trends in zooplankton of Dorset, Ontario, lakes: the probable interactive effects of changes in pH, total phosphorus, dissolved organic carbon, and predators. Canadian Journal of Fisheries and Aquatic Sciences, 65, 862–877.
Yao, H., McConnell, C., Somers, K., Yan, N.D., Watmough, S., & Scheider, W. (2011). Nearshore human interventions reverse patterns of decline in lake calcium budgets in central Ontario as demonstrated by mass-balance analyses. Water Resources Research, 47, 1–13. doi:10.1029/2010WR010159.
Acknowledgements
We thank Angelo Sorce for assistance in core collection, and the Dorset Environmental Science Centre, Ontario Ministry of Environment for providing monitoring data. We also thank an anonymous journal reviewer for their insightful comments on an earlier version of this paper. This research was funded by a Natural Sciences and Engineering Research Council grant and an Ontario Premier’s Discovery Award to John P. Smol.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shapiera, M., Jeziorski, A., Paterson, A.M. et al. Cladoceran Response to Calcium Decline and the Subsequent Inadvertent Liming of a Softwater Canadian Lake. Water Air Soil Pollut 223, 2437–2446 (2012). https://doi.org/10.1007/s11270-011-1035-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11270-011-1035-y