Abstract
We studied nutrient removal by Phragmites australis in the Albujón rambla, the main drainage system that discharges into the Mar Menor, a Mediterranean coastal lagoon of high conservation interest, but highly threatened by point and nonpoint pollution derived from tourism and agricultural activities. We measured aerial biomass and N and P concentrations in both aboveground and belowground tissues of common reed during an annual cycle that included two cutting events and two periods of reed growth (one at the end of summer after cutting and another at the beginning of spring, following their natural cycle). The temporal variation of N and P concentrations was related to the phenology of the plant and cutting events. The maximum nutrient concentrations were recorded in young stems in the initial stages of the autumn growing season (35.86 mg N g−1 and 2.38 mg P g−1). The phosphorus dynamics showed evidence of translocation processes related with growth activity, although no evidence of N translocation was found. In November and in summer, when aerial growth ceases because of the hard conditions, the P concentration in rhizomes was higher than in stems, while in spring and in September, the period of maximal growth, the reverse relation was found. The highest total amounts of the two elements in the aboveground biomass (0.54 Tm N ha−1 and 0.25 Tm P ha−1) were reached in July, coinciding with the highest biomass (3.72 kg DW m−2), which then decreased to approximately half in August. Nutrient content in the aboveground tissues was highly dependent on the ammonium and nitrate water concentrations. In addition, the N content was inversely related to the Corg/N of sediments, while the P content was influenced positively by the phosphorous concentration of the water. Common reed of the Albujón rambla corresponds to the assimilation type, adapted to nutrient-rich habitats, which is characterized by a pronounced external N cycle and P internal reserves. Based on the results obtained, we propose a management plan for common reed to help control eutrophication of the Mar Menor lagoon. This would bring forward reed cutting to the beginning of summer, instead of August, coinciding with the time of maximum aerial biomass, greater nutrient retention, and lower risk of strong precipitation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Reed (Phragmites australis (Cav.) Trin. Former Steud) is a cosmopolitan emergent macrophyte species occurring in a wide range of aquatic habitats and has been widely investigated as bioabsorbent of macroelements and heavy metals. It functions well as a filter, reducing point and nonpoint pollution sources (Brix and Schierup 1989; Kiedrzynska et al. 2008). Common reed is one of the emergent plants most commonly used in constructed wetlands for the enhancement of water quality in water treatment systems (Wathugala et al. 1987; Biddlestone et al. 1991; Brix 1994; Gómez Cerezo et al. 2000; Borin et al. 2001; Meuleman et al. 2002; Vyamazal 2002) due to its high growth rate and great capacity for nutrient accumulation in its stems, roots, and rhizomes (Ulrich and Burton 1985; Hocking 1989; Granéli et al. 1992; Kuehl and Khol 1993; Greenway and Woolley 1999; Romero et al. 1999; Wolfgang et al. 1999; Asaeda et al. 2002; Baldatoni et al. 2003, Kiedrzynska et al. 2008). The capacity of natural wetlands to filter wastewater and nutrients from arable land also has been demonstrated (White et al. 2000; Newman and Pietro 2001; Cirujano et al. 2005; Álvarez-Rogel et al. 2006). Besides, reed beds have other important wetlands functions, including acting as a major breeding habitat for passerines (Poulin et al. 2002) and contributing to margin sediment stabilization and to habitat biodiversity, thus increasing its ecological and conservation value. However, reed tends to form monospecfic and dominant stands and may alter ecological functions by the excesive expansion, adopting an invasive behavior (Lelong et al. 2007), especially in coastal marshes, where there are an increase of inputs of freshwater and nutrient loads and major presence of no vegetation areas (Minchinton and Bertness 2003).
The Mar Menor is the largest coastal lagoon in the Western Mediterranean and, together with its associate wetlands, forms an ecosystem of high ecological, fishing, and tourist value, recognized both nationally and internationally. It is included in the Ramsar List of Wetlands of International Importance, and it is also a Special Protected Area of Mediterranean Interest, Specially Protected Area under the EU Wild Birds Directive, and Site of Community Importance to be integrated in the Nature 2000 Network (EU Habitats Directive). Despite this protection status, it is increasingly threatened by intense agricultural and tourism activities. The high anthropogenic pressure in the surrounding watershed of the Mar Menor has led to an increase in nutrients and pollutants flowing through the watercourses into the lagoon, which causes changes in the physical chemistry of water and sediments and in the biological communities of the lagoon (Terrados and Ros 1991; Pérez-Ruzafa et al. 2002; Lloret et al. 2005; Velasco et al. 2006).
In this study, we measured nutrient removal by P. australis in the Albujón rambla, the main drainage system that discharges into the Mar Menor, in wich it forms a dense monospecific bed along the channel. Traditionally, in this area, the aerial biomass of the common reed is harvested each year in late summer as a cleaning measurement to prevent the catastrophic effect of flash floods on urban infrastructures. Our specific objectives were: (1) to analyze the seasonal variation of aerial biomass and nutrient concentrations (N and P) in common reed in the Albujón rambla, both in the aboveground and belowground tissues; (2) to analyze the effect of cutting aerial biomass on nutrient bioaccumulation and water and sediment nutrient levels; (3) to determine environmental controlling factors of nutrient retention; and (4) to propose a management plan to increase the nutrient retention efficiency by plant and reduce the input of nutrients into the Mar Menor lagoon.
2 Materials and Methods
2.1 Study Site
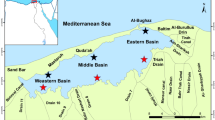
The Mar Menor is a hypersaline coastal lagoon in the SE of the Iberian Peninsula with an area of 135 km2, a volume of 580 hm3 and a mean depth of 4 m. In its drainage basin, the main land uses are intensive agriculture and residential areas. The Campo de Cartagena, one of the main horticultural producers of Europe, is a lowland plain (1,440 km2), slightly inclined to the Mar Menor, of clayed soils dedicated to intensive agriculture with irrigated horticultural and citrus crops. A large part of the nutrients contained in agricultural soils of Campo de Cartagena enters to the lagoon through superficial or underground drainage. It is drained by several ephemeral watercourses or ramblas, which flow into the lagoon after episodic storm rainfall events, usually in autumn, causing washing and erosion processes with massive exports of water, sediments, and nutrients (Velasco et al. 2006). The Albujón rambla is the principal watercourse (Fig. 1), draining a surface of 441 km2, about one third of the total surface of the Campo de Cartagena. Since the 1980s, the last 7 km of the channel has flowed permanently due to the drainage from irrigation lands and sewage discharges. The principal urban point source of pollution is the effluent of Los Alcazares wastewater treatment plant, which insufficiently treats sewage from a population over 100,000 habitants in summer. Urban wastewater discharges into the Albujón rambla 2 km upstream of its mouth in the Mar Menor, and it is in this reach of permanent water where the reed is well developed and occupies the floodplain.
The climate is semiarid Mediterranean, with a mean annual temperature of 17°C (Conesa 1990). The mean annual rainfall is 300 mm, with most precipitation concentrated into short episodic storm events in autumn and spring. At the end of the summer, traditionally, the Confederación Hidrográfica del Segura (the watershed hydrological management agency) cuts the aboveground biomass of common reed in the Albujón rambla, using heavy machinery that causes a geomorphological alteration of the channel and the floodplain. Also, the stems are left in the floodplain, forming a thick layer of coarse detritus that are lixiviated and decomposed in the stream and/or exported to the Mar Menor in the case of flood events.
2.2 Sampling and Processing of Samples
The present study was carried out between September 2003 and September 2004, in the mouth of the Albujón rambla. This period includes two cutting events and two periods of reed growth, one at the end of summer 2003 after its cut (rhizomes are not removed, and the good climatic conditions in autumn favor their growth) and another at the beginning of spring (following their natural cycle).
Nine sampling points were selected in the floodplain spatially distributed along three transects, as a function of the distance to the coastline: 50, 65, and 80 m. Inside each transect, sampling points in the floodplain were located at intervals according to distance to the channel: 0–5, 10–15, and 20–25 m (Fig. 1d). Seven samplings were carried out during the annual cycle, and in each sampling, samples were taken of the aerial (stems, leaves, and panicles) and subterranean parts (roots and rhizomes) of common reed. Also, at each date, aboveground biomass was estimated using the Thursby et al. method (2002), counting the stems included in a square plot (quadrat) of 25 cm side and the length of the five tallest stems. Nine plots per date were sampled. Previously, we calculated the length–mass relationship from 50 individuals and the total biomass in five plots counting and measuring the total individuals. A regression equation was obtained between real biomass (B) and biomass calculated using the mean length of the five tallest stems (x), and this relationship was used to estimate aerial biomass on each date.
To determine the effect of cutting on nutrient concentrations in common reed, we compared the stand at the mouth (stand 1) with other stand situated 1 km up from the mouth (stand 2, which was not affected by the harvest), before cutting in August, and after cutting in September 2004. In stand 2, Phragmites samples were taken in three points in a unique transect.
On each date, water temperature, conductivity, salinity, and dissolved oxygen were measured in situ using a multiparametric recorder (WTW, Multiline P4). Discharge was estimated from measurements of depth and current velocity along a cross-section in the channel. Also, sediments and superficial water samples were taken both in the floodplain and in the channel. The samples were hermetically closed, kept at 4°C for transportation to the laboratory for later analyses of the nutrients. Water samples were filtered through pre-ashed and pre-weighed Whatman GF/F glass-fiber filters. Ammonium was converted to ammonia by adding 10 M NaOH solution and measured with an ammonia selective electrode connected to a pH/mV meter. The rest of the nutrients were determined according to standard methods (APHA 1992): nitrate by cadmium reduction method, nitrites by sulfanylic acid colorimetry, and phosphate by ascorbic acid colorimetry.
Sediments and common reed samples were dried previously in the oven at 60°C and next were ground with a mortar (sediments) and a mechanical grinder (P. australis) until a fine phase was obtained that facilitated nutrient analysis. The nitrogen and carbon concentrations of sediments and nitrogen concentration of reed samples were determined using an elementary analyzer (Carlo Erba CNHS-0 EA-1108). However, for phosphorus analysis, both types of samples (1 g of sediments and 0.25 g of reed) were subjected to sulfuric acid and potassium persulfate digestion in an autoclave at 115°C for 1 h and then left to cool. The diluted digested solution was analyzed following the standard method for phosphate analysis by ascorbic acid colorimetry (APHA 1992).
2.3 Data Analyses
Analysis of the variance (ANOVA, one factor) was carried out to test differences in the concentrations of nutrients between the different treatments: in the distance gradients to the channel and the mouth, aboveground, and belowground tissues of the reed, water, and sediments in the floodplain and channel, and between reed stands. Previously, data were log10-transformed to meet normality assumptions. Pearson correlation coefficients were calculated between nutrient concentrations in common reed and environmental variables measured together with the mean monthly air temperature and Corg/N and N/P ratios. Multiple lineal regression models (Stepwise Forward) were carried out, the dependent variables being nutrient concentrations of the reed and the independent variables the environmental parameters analyzed. Statistical analyses were made with the Statistica 6 program.
3 Results
3.1 Physico-chemical Water Characteristics of the Albujón Rambla
The mean discharge of the Albujón rambla into the Mar Menor lagoon during the study period was 322.76 l s−1, the highest value (949.20 l s−1) being reached in April after strong spring rains. In general, superficial water in the mouth of the rambla was hyposaline (5.5 g l−1 mean salinity, 12.31 mS cm−1 mean conductivity), of basic pH (7.59 mean pH), poorly oxygenated (mean values of 5.44 mg O2 l−1 and 61.41% O2 saturation). The highest values for phosphates and ammonium in channel water were measured in August (0.74 and 9.20 mg l−1, respectively) reflecting the high urban wastewater discharges. The highest concentrations of nitrites (348.63 μg l−1) and nitrates (29.44 mg l−1) occurred in April, probably due to the washing of surrounding cultivated soils by the spring rains.
Significant differences were found in nitrite and nitrate concentrations between channel and floodplain, being higher in the former. However, the floodplain presented higher ammonium and phosphate concentrations than the channel, although these differences were not significant. Temporal variations in the water nutrient concentrations were greater in the floodplain than in the channel, as observed from the larger standard errors, particularly for phosphates (Table 1).
When water nutrient levels between reaches were compared, a significant increase in concentration of phosphate (583.3 μg l−1), nitrate (15.41 mg l−1), and nitrite (112.6 μg l−1) was observed in September 2003 after reed cutting in stand 1 in relation to stand 2, while in September 2004, only the nitrate level increased (9.51 mg l−1), and the nitrite decreased (104 μg l−1; Table 3).
3.2 Nutrient Sediment Content
The nitrogen, phosphorus, and organic carbon contents in sediment were significantly greater in the floodplain that in the channel (Table 2) through the accumulation of dead shoots of common reed. The lowest nitrogen concentration in the floodplain occurred in September 2004 (0.60 mg N g−1), while the maximum values were observed in November 2003 (2.43 mg N g−1). Total phosphorus concentration peaked in August 2004 in the floodplain (0.59 mg P g−1) and in July 2004 in the channel sediments (0.44 mg P g−1). The Corg values were highest in summer, in July 2004 (230.43 mg Corg g−1 in the floodplain and 39.87 mg Corg g−1 in the channel sediments). No effect of cutting was observed on the nutrient content of sediment, when comparing reaches after the harvest of common reed, in both September 2003 and 2004 (Table 3).
3.3 Phenology and Aboveground Biomass of P. australis
P. australis is a perenial helophyte with strong winter dormancy requirements (Tylová et al. 2008). Its shoots are sensitive to frost, and senescence starts in autumn and is accompanied with the translocation of N- and P-rich compounds to the belowground organs (rhizomes) as reserves for over-wintering (Kohl et al. 1998). During this period, rhizomes grow horizontally before terminating in an upward apex and becoming dormant until spring. During the spring growing season, the aerial shoots arise from the rhizomes. Large buds formed the previous fall are the first to emerge, with smaller spring-formed buds emerging later. Following the emergence, stems grow rapidly, and floration occurs in summer (Gucker 2008).
Phenology of P. australis in the Albujón rambla during the studied period follows the general pattern, although with some variations in relation to weather conditions and cutting events (Fig. 2). Stems in the mouth of the rambla were cut during the last week of August in both years (2003 and 2004). After cutting, a new fast growth period started, the new stems reaching a mean length of 46 and 57 cm after 10−15 days, respectively. At the beginning of November 2003, the stems reached 136 cm mean height. In December and January, the senescence of the aboveground biomass began, and by the middle of February, practically all the old stems were dry, althought new stems also began to emerge at this time. By the end of April, the new stems had reached a mean heigh of 145 cm. Stems attained their maximum height and biomass by the middle of July (Fig. 3), while the biomass decresed in August. Flowering started in August.
3.4 Nutrient Content of P. australis
Preliminary tests pointed to no differences in nutrient content tissues along transects in stand 1. Mean N concentration was higher in the aboveground tissues (stems and leaves) than in belowground parts (roots and rhizomes) of the plant, while no significant differences were found for P (Table 4).
The pattern of temporal variation of N and P concentrations was related to plant phenology (Fig. 4). The maximum concentrations for the two nutrients were observed in young stems in the initial stages of the autum growing season (35.86 mg N g−1and 2.38 mg P g−1, in September 2003). The dynamics of the nitrogen content followed a similar pattern in aboveground and belowground biomass, although variations were bigger in the aerial part (Fig. 4 a). During the cold period, N levels decreased in both parts, and no clear evidence of N translocation was observed. Later, during the spring growing season, a small increase in the N content ocurred in the new stems and then fell in summer to reach the minimum values in both parts of the plant. However, P dynamics showed evidence of translocation processes related to growth activity (Fig. 4 b). In November and in summer, the periods when aerial growth stops because of the harsh conditions, the P concentration in rhizomes was higher than in stems, while in spring and in September, the periods of maximum growth, the reverse relation was found. In February, both concentrations were very similar.
The highest total amounts of the two elements in the aboveground biomass were reached in July (0.54 TmN ha−1 and 0.025 TmP ha−1) but decreased to approximately half in August (Table 5).
Nonsignificant differences in the concentrations of P and N between the two studied stands of P. australis were found in August 2004, just before cutting (Table 6). However, in September 2004, after reed cutting, the levels of these elements in the young stems and of P in rhizomes were significanfly greater in stand 1 than in the mature stand 2.
3.5 Nutrient Bioaccumulation in Relation to Environmental Variables
The N concentration in stems showed a positive correlation with the ammonium of the water of floodplain and channel and the N/P of sediments, while it showed the inverse relation with the Corg/N of sediments (Table 7). Phosphorus retention in the aboveground tissues was highly correlated with phosphate concentration of channel water. Ammonium of the channel water was also positively correlated with the P concentration both in the aboveground and belowground tissues. The nitrate content of water in the channel and the air temperature also showed a positive correlation with P concentrations in the stems, while P retention in the rhizomes was negatively correlated with nitrates in the floodplain water (Table 7).
Predictive models of nutrient concentration in the aboveground biomass obtained are shown in Table 8. The N content in the aboveground tissues was highly dependent on amonium and nitrate content of water from the channel (with positive sign) and the Corg/N ratio of sediments (with negative sign). The P model accounts for 70% of its variability and was defined by the ammonium, nitrate, and phosphate concentrations of water of the rambla besides the salinity as independent variables, all with positive sign. As regards the nutrient levels in the underground part, no significant model was found.
4 Discussion
4.1 Nutrient Dynamics
Numerous studies have provided data of nutrient levels in aquatic plant tissues and growth rates in relation to nutrient concentrations in the environment, particularly with regard to the use of macrophyte species in the control of eutrophication (e.g. Carignan and Kalff 1980, Fernández-Alaez et al. 1999). Plants displayed a significantly higher P content in nutrient-rich sites than in nutrient-poor ones (Garbey et al. 2004), growing deficiently in conditions of nutrient shortage. Romero et al. (1999), in hydroponic culture in the laboratory, showed that nitrogen and phosphorus concentration in the P. australis tissues generally increased with N level in the root solution and significantly affected the relative growth rate of the plants. Similar results have been obtained by other authors (Ulrich and Burton 1985; Rickey and Anderson 2004). Tylová et al. (2008) experimentally demonstrated that nutrient enrichment affects plant growth, phenology, and the accumulation of N storage compounds in belowground organs.
In streams under heavy influence of agriculture, such as the Albujón rambla, nitrate is the predominant N form. Nevertheless, during summer months, it is common to find high phosphate and ammonium concentrations due to the increase in the discharge of wastewater. The mean content of nitrogen and phosphorus in water during the studied period corresponded to hypereutrophic conditions (Vollenweider 1968). Nutrient levels in P. australis were high in relation to the high nutrient concentrations in the environment and similar to those obtained by other authors in eutrophic systems: Lake Averno (Naples, Italy; Baldatoni et al. 2003), constructed wetlands in Queensland, Australia (Greenway and Woolley 1999), el Hondo wetland (Elche, Spain; Cirujano et al. 2005), and Pilica River (Poland; Kiedrzynska et al. 2008).
Seasonal changes in N and P concentrations in the aboveground and belowground tissues of common reed were observed in relation with plant phenology and cutting. The greatest nutrient concentrations were reached in the newly emerging stems after cutting. N concentrations in the aboveground reed tissues were always greater than in the belowground tissues, with maximum levels in growth periods (autumn and spring) and minimum levels in winter and summer. The initial decline in concentrations of N and P in the aboveground tissues coincided with senescence of the stems in winter because of low temperatures, and the second decline was in July due to drought stress.
The phosphorous dynamics was different, the decrease in P in the shoots and the increase in rhizomes at the end of the vegetation period showing downward translocation. At the end of autumn and winter, P is translocated of stems and leaves toward the rhizomes and roots, which can recycle and withdraw nutrients from senescing plant parts for reuse (Vitousek 1982). The belowground storage pools of P compounds provide essential amounts of nutrients that are used for the growth of young stems in early spring. In early spring, at the start of its natural growth cycle, a translocation occurred again but in the opposite direction: from rhizomes to aerial part. The nutrient translocation capacity (in this case of P) from some organs to others enables plants to face nutrient fluctuations and therefore be highly competitive in habitats with fluctuating resources (Garbey et al. 2004). The formation of N reserves in rhizomes was, however, neghigible. This behavior is typical of relatively open nutrient cycles where, over the longer-term, net storage of incoming nutrients does not occur (Board 1996, Kuehl et al. 1997). Under high nitrogen levels, P. australis can allocate more resources to aboveground growth than to rhizome growth because there is little or no competition for nitrogen (Minchinton and Bertness 2003). Common reed of the Albujón rambla corresponds to the assimilation type described by Lippert et al. (1999) adapted to nutrient-rich habitats, which is characterized by a pronounced external N cycle. Internal reserves play a less important role. The reed stand is well supplied with external N pools, both from sediments and water, and is highly productive (great aerial biomass, with stems of great length and diameter, and with a high nutrient content).
4.2 Factors Controlling Nutrient Bioaccumulation
Nutrient accumulation in the aboveground reed tissue responded positively to N availability in the Albujón rambla. Nitrate and ammonium concentrations of channel water contribute to the storage of P and N in stems. Salinity and phosphates from the water channel also positively affect P retention. However, high Corg/N values in sediments can limit microbial activity and, therefore, the mineralization processes of the organic matter, negatively affecting N accumulation in the stems. On the other hand, interactions between the N and P cycles in sediments may explain the negative relationships found between the P content in the rhizomes and the nitrate concentration of the floodplain water. High nitrate concentrations in the floodplain increase the denitrification process. Denitrification with FeS as the reducing agent produces FeOOH, which will strongly enhance P binding onto sediment. The overall result is a loss of N compounds and a stronger P fixation, which limits the P release of sediments and therefore its assimilation by the rhizomes (Golterman 2004). Andersen (1982) obtained similar results in lakes, finding a positive correlation between high nitrate concentration and low P release from sediment.
4.3 Proposed Management Plan
Several authors have proposed to control the flow of nutrients and heavy metals in lakes or reservoires through reed harvesting, considering the dynamic cycling of nutrients and the seasonal changes in accumulation (Nikolaidis et al. 1996; Asaeda and Karunaratne 2000; Kiedrzynska et al. 2008). Besides, the periodic cutting of common reed stimulates its growth and increases nutrient content of new shoots (Greenway and Woolley 1999).
The high nutrient content of P. australis in the Albujón rambla means it can be used as a “green filter” to reduce the pollution load reaching the Mar Menor lagoon, if the aboveground biomass is harvested when it contains the maximum amounts of nutrients. In the Albujón rambla, the aboveground biomass reached the maximum value in July as did as the maximum standing stocks of N and P. However, in August, it decreased probably due to drought stress and high temperature conditions in the study area. Based on the obtained results, we propose that reed cutting be brought forward to the beginning of summer, instead of August, coinciding with the time of maximum aerial biomass, greatest nutrient retention, and less risk of high precipitation. A second harvest in November, before the autumn shoots began to die and before P translocation to rhizomes, could be recommended to maximize the nutrient reduction in nutrient-rich habitats because the following spring growth will not be limited. However, the effects of harvesting frequently on the plants need to be evaluated. We recommend removing the stems immediately after cutting to avoid nutrient leaching from shoots to sediments and water and that the use of heavy machinery for cutting be avoided because this alters the floodplain sediments and favors the P release (Golterman 2004). Traditional reed cutting in the Albujón rambla at the end of summer and litter deposition on the floodplain lead to the rapid release of soluble compounds from leaves and stems, facilitated by the fragmentation to which they are subjected (Larsen 2003).
The correct management of common reed in coastal ramblas and wetlands (cutting and removing the biomass from the floodplain) represents an economic and simple measure to reduce the contamination of the Mar Menor lagoon in conjunction with other watershed strategies (wastewater treatment plants, good agricultural practices, geomorphic stream restoration, etc). The stream restoration designed to reconnect stream channel with floodplains that promote flooding and dissipation of erosive force, increasing the residence time during floods, could increase the removal of nutrients by common red and denitrification rates (Kaushal et al. 2008).
References
Álvarez-Rogel, J., Jiménez-Cárceles, F. J., & Egea, C. (2006). Phosphorus and Nitrogen Content in the Water of a Coastal Wetland in the Mar Menor Lagoon (SE Spain): Relationships with Effluents from Urban and Agricultural Areas. Water, Air, and Soil Pollution, 173, 21–38. doi:10.1007/s11270-005-9020-y.
American Public Health Association (APHA). (1992). Standard Methods for the Examination of Water and Wastewater. Washington, DC: American Public Health Association.
Andersen, J. M. (1982). Effect of Nitrate Concentration in Lake Water on Phospate Release from Sediment. Water Research, 16, 1119–1126. doi:10.1016/0043-1354(82)90128-2.
Asaeda, T., & Karunaratne, S. (2000). Dynamic Modeling of the Growth of Phragmites australis: Model Description. Aquatic Botany, 67, 301–318. doi:10.1016/S0304-3770(00)00095-4.
Asaeda, T., Nam, L., Hietz, P., Tanaka, N., & Karunaratne, S. (2002). Seasonal Fluctuations in Live and Dead Biomasa of Phragmites australis as Described by a Growth and Decomposition Model: Implications of Duration of Aerobic Conditions for Litter Mineralization and Sedimentation. Aquatic Botany, 73, 223–239. doi:10.1016/S0304-3770(02)00027-X.
Baldatoni, D., Altoni, A., Di Tomamasi, P., Giovanni, B., & Virzo De Santo, A. (2003). Assessment of Macro and Microelement Accumulation Capability of two Aquatic Plants. Environmental Pollution, 130, 149–156. doi:10.1016/j.envpol.2003.12.015.
Biddlestone, A. K., Gray, K. R., & Thurairajan, K. (1991). A Botanical Approach to the Treatment of Wastewaters. Journal of Biotechnology, 17, 209–220. doi:10.1016/0168-1656(91)90012-K.
Board, R. R. (1996). Temporal Variations in the Nitrogen Content of Phragmites australis (Cav.) Trin ex. Steud. From a Shallow Fertile lake. Aquatic Botany, 55, 171–181. doi:10.1016/S0304-3770(96)01070-4.
Borin, M., Bonaniti, G., Santamaria, G., & Giardini, L. (2001). A Constructed Surface Flow Wetland for Treating Agricultural Waste Waters. Water Science and Technology, 44, 523–530.
Brix, H., & Schierup, H. H. (1989). The Use of Macrophytes in Water-Pollution Control. Ambio, 18, 100–107.
Brix, H. (1994). Functions of Macrophytes in Constructed Wetlands. Water Science and Technology, 29, 71–78.
Carignan, R., & Kalff, J. (1980). Phosphorus Sources for Aquatic Weeds: Water or Sediments. Science, 207, 987–989. doi:10.1126/science.207.4434.987.
Cirujano, S., Moreno, M., Rubio, A. & Echeverrías, J. (2005). Capacidad depuradora del carrizo en el Parque Natural El Hondo (Alicante). Biodiversidad y Gestión de los carrizales. In Actas de las I Jornadas Científicas Parque Natural de El Hondo, Biodiversidad y Gestión de los carrizales, Crevillente.
Conesa, C. (1990). El Campo de Cartagena—Clima e hidrología de un medio semiárido. Ayuntamiento de Cartagena y Comunidad de regantes del Campo de Cartagena, Murcia: Universidad de Murcia.
Fernández-Alaez, M., Fernández-Alaez, C., & Becares, E. (1999). Nutrient Content in Macrophytes in Spanish Shallow Lakes. Hydrobiologia, 408/409, 317–326. doi:10.1023/A:1017030429717.
Garbey, C., Murphy, K. J., Thiébaut, G., & Muller, S. (2004). Variation in P-content in Aquatic Plant Tissues Offers an Efficient Tool for Determining Plant Growth Strategies Along a Resource Gradient. Freshwater Biology, 49, 346–356. doi:10.1111/j.1365-2427.2004.01188.x.
Gómez Cerezo, R., Suárez, M. L., & Vidal-Abarca, M. R. (2000). The Performance of Multi-stage System of Constructed Wetlands for Urban Wastewater treatment in a Semiarid Region of SE Spain. Ecological Engineering, 16, 501–517. doi:10.1016/S0925-8574(00)00114-2.
Golterman, H. L. (2004). The Chemistry of Phosphate and Nitrogen Compounds in Sediments. Dordrecht, The Netherlands: Kluber Academic Plubishers.
Granéli, W., Sytsma, M., & Weisner, S. (1992). Rhizome Dynamics and Resource Storage in Phragmites australis. Wetlands Ecology and Management, 1, 239–247. doi:10.1007/BF00244929.
Greenway, M., & Woolley, A. (1999). Constructed Wetlands in Queensland: Performance Efficiency and Nutrient Bioaccumulation. Ecological Engineering, 12, 39–55. doi:10.1016/S0925-8574(98)00053-6.
Gucker, C.L. (2008). Phragmites australis. In: Fire Effects Information System (Online). US. Department of Agriculture, Forest Services, Rocky Mountain Research Station, Frie Scienc Laboratory. http://www.fs.fed.us/database/feis/plants/graminoid/phraus/all.hyml (accessed 21 May, 2008).
Hocking, P. J. (1989). Seasonal Dynamics of Production, Nutrient Accumulation, and Cycling by Phragmites australis in Nutrient-enriched Swamps in Inland Australia I: Whole Plants. Australian Journal of Marine and Freshwater Research, 40, 421–444. doi:10.1071/MF9890421.
Kaushal, S. S., Groffman, P. M., Mayer, P. M., Striz, E., & Gold, A. J. (2008). Effects of Stream Restoration on Denitrification in an Urbanizing Watershed. Ecological Applications, 18, 789–804. doi:10.1890/07-1159.1.
Kiedrzynska, E., Wagner, I., & Zalewski, M. (2008). Quantification of Phosphorus Retention Efficiency by Floodplain Vegetation and a Management Strategy for a Eutrophic Reservoir Restoration. Ecological Engineering, 33, 15–25. doi:10.1016/j.ecoleng.2007.10.010.
Kohl, J. G., Woitke, P., Kühl, H., Dewender, M., & König, G. (1998). Seasonal Changes in Dissolved Amino Acids and Sugars in Basal Culm Internodes as Physiological Indicators of the C/N-Balance of Phragmites australis at Littoral Sites of Different Trophic Status. Aquatic Botany, 60, 221–240. doi:10.1016/S0304-3770(97)00096-X.
Kuehl, H., & Khol, J. G. (1993). Seasonal Nitrogen Dynamics in Reed Beds Stands (Phragmites australis (Cav.) Trin. ex. Steudel) in Relation to Productivity. Hidrobiología, 251, 1–12. doi:10.1007/BF00007158.
Kuehl, H., Woitke, P., & Kohl, J. G. (1997). Stategies of Nitrogen Cycling of Phragmites australis at Two Sites Differing in Nutrient Availability. Revue der Gesamten Hydrobiologie, 82, 57–66. doi:10.1002/iroh.19970820108.
Larsen, V. J. (2003). The Effects of Pre-drying and Fragmentation on the Leaching on Nutrient Elements and Organic Matter from Phragmites australis (Cav.) Trin. Aquatic Botany, 14, 29–39. doi:10.1016/0304-3770(82)90084-5.
Lelong, B., Lavoie, C., Jodoin, Y., & Belzile, F. (2007). Expansion Pathways of the Exotic Common reed (Phragmites australis) : A Historical and Genetic Analysis. Diversity & Distributions, 13, 430–437. doi:10.1111/j.1472-4642.2007.00351.x.
Lippert, I., Rolletschek, H., Kühl, H., & Khol, J. G. (1999). Internal and External Nutrient Cycles in Stands of Phragmites australis- A Model for Two Ecotypes. Hydrobiologia, 408/409, 343–348. doi:10.1023/A:1017008629659.
Lloret, J., Marin, A., Marin-Guirao, L., & Velasco, J. (2005). Changes in Macrophytes Distribution in a Hypersaline Coastal Lagoon Associated with the Development of Intensively Irrigated Agriculture. Ocean and Coastal Management, 48, 828–842. doi:10.1016/j.ocecoaman.2005.07.002.
Meuleman, A., Beekman, J., & Verhoeven, J. (2002). Nutrient Retention and Nutrient-use Efficiency in Phragmites australis Stands After Wasterwater Application. Wetlands, 22, 712–721. doi:10.1672/0277-5212(2002)022[0712:NRANUE]2.0.CO;2.
Minchinton, T. E., & Bertness, M. D. (2003). Disturbance-mediated Competition and the Spread of Phragmites australis in a Coastal Marsh. Ecological Applications, 13, 1400–1416. doi:10.1890/02-5136.
Newman, S., & Pietro, K. (2001). Phosphorus Storage and Release in Response to Flooding: Implications for Everglades Stormwater Treatment Areas. Ecological Engineering, 18, 23–28. doi:10.1016/S0925-8574(01)00063-5.
Nikolaidis, N. P., Koussouris, T., Murria, T. E., Bertahas, I., Diapoulus, A., & Konstantinos, G. (1996). Seasonal Variation of Nutrients and Heavy Metals in Phragmites australis of Lake Triclonis, Greece. Lake Reserve Management, 12, 364–370.
Pérez-Ruzafa, A., Gilabert, J., Gutiérrez, J. M., Fernández, A. I., Marcos, C., & Sabah, S. (2002). Evidence of a Planktonic Foof Web Response to Changes in Nutrient Input Dynamics in the Mar Menor Coastal Lagoon, Spain. Hydrobiologia, 475/476, 350–369. doi:10.1023/A:1020343510060.
Poulin, B., Lefebvre, G., & Mauchamp, A. (2002). Habitat Requirements of Passerines and Reedbed Management in Southern France. Biological Conservation, 107, 315–325. doi:10.1016/S0006-3207(02)00070-8.
Rickey, M. A., & Anderson, R. C. (2004). Effects of Nitrogen Addition on the Invasive Grass Phragmites australis and a Native Competitor Spartina pectinata. Journal of Applied Ecology, 41, 888–896. doi:10.1111/j.0021-8901.2004.00948.x.
Romero, J. A., Brix, H., & Comín, F. A. (1999). Interative Effects of N and P on Growth, Nutrient Allocation and NH -4 Uptake Kinetics by Phragmites australis. Aquatic Botany, 64, 369–380. doi:10.1016/S0304-3770(99)00064-9.
Terrados, J., & Ros, J. D. (1991). Production dynamics in a macrophyte-dominated ecosystem: the Mar Menor coastal lagoon (SE Sapin). In J. D. Ros & N. Prat (Eds.), Homenage to Ramon Margalef—Why there is such Pleasure in Studing Nature, (pp. 255–270), p. 10. Oecologia Aquatica: Barcelona.
Thursby, G., Chintala, M., Stetson, D., Wigand, C., & Champlin, D. (2002). A Rapid, Non Destructive Method for Estimating Aboveground Biomass of Salt Marsh Grasses. Wetlands, 22, 626–630. doi:10.1672/0277-5212(2002)022[0626:ARNDMF]2.0.CO;2.
Tylová, E., Steinbachová, L., Votrubová, O., Lorenzen, B., & Brix, H. (2008). Different Sensitivity of Phragmites australis and Glyceria maxima to High Availability of Ammonium-N. Aquatic Botany, 88, 93–98. doi:10.1016/j.aquabot.2007.08.008.
Ulrich, K. E., & Burton, T. M. (1985). The Effects of Nitrate, Phosphate, and Potassium Fertilization on Growth and Nutrient Uptake Patterns of Phragmites australis. Aquatic Botany, 21, 53–62. doi:10.1016/0304-3770(85)90095-6.
Wathugala, A. G., Suzuki, T., & Kurihara, Y. (1987). Removal of Nitrogen, Phosphorus and COD from Waste Water Using Sand Filtration System with Phragmites australis. Water Research, 21, 1217–1224. doi:10.1016/0043-1354(87)90173-4.
Velasco, J., Lloret, J., Millán, A., Marín, A., Barahona, J., Abellan, P., et al. (2006). Nutrient and Particulate Inputs into the Mar Menor Lagoon from an Intensive Agricultural Watershed. Water, Air, and Soil Pollution, 176, 37–56. doi:10.1007/s11270-006-2859-8.
Vitousek, P. M. (1982). Nutrient Cycling and Nutrient Use Efficiency. American Naturalist, 119, 553–572. doi:10.1086/283931.
Vollenweider, R. A. (1968). Scientific fundamentals of the eutrofication of lakes and flowing waters, with particular reference to nitrogen and phosphorus as factors in eutrofication. Paris: Organisation for Economic Cooperation and Development, DAS/CSI/68.27.
Vyamazal, J. (2002). The use of Sub-surface Constructed Wetlands for Wastewater Treatment in the Czech Republic: 10 years Experience. Ecological Engineering, 18, 663–646. doi:10.1016/S0925-8574(02)00055-1.
White, J. S., Bayley, S. E., & Curtis, P. J. (2000). Sediment Storage of Phosphorus in a Northern Prairie Wetland Receiving Municipal and Agro-industrial Wastewater. Ecological Engineering, 14, 127–138. doi:10.1016/S0925-8574(99)00024-5.
Wolfgang, P., Grosser, S., & Melzer, A. (1999). Nitrogen and Carbohydrate Storage in Rhizomes of Phragmites australis (Cav.) Trin. ex Steud., at Different Aquatic Sites of Lakes in Upper Bavaria. Limnologica, 29, 36–46. doi:10.1016/S0075-9511(99)80037-1.
Acknowledgments
This work was partially funded by the Consejería de Agricultura, Agua y Medio Ambiente of the Murcia Region, Programa Séneca, 2001 (Project AGR/24/FS/02). We thank R. Alcántara, J. Lloret, C. Gutierrez, and D. Bruno for assistance in the field work, J. Lloret for design Fig. 1, and O. Belmar for assistance in processing samples in the laboratory, also A. Millán for his helpful comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ruiz, M., Velasco, J. Nutrient Bioaccumulation in Phragmites australis: Management Tool for Reduction of Pollution in the Mar Menor. Water Air Soil Pollut 205, 173–185 (2010). https://doi.org/10.1007/s11270-009-0064-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11270-009-0064-2