Abstract
In the current work, we investigated the concentration of Ni and Pb in different organs of Phragmites australis to evaluate its potential application as a phytoremediator to remove these two metals from contaminated water and sediment in Lake Burullus (a Ramsar site in Egypt). Above- and below-ground biomass of P. australis, water and sediment were sampled monthly for 1 year at six sites of Lake Burullus (three sites represent each of the northern and southern parts of the lake) using six randomly distributed quadrats (each of 0.5 × 0.5 m) at each sampling site. Significant variation was detected for Ni and Pb concentrations in the sediments and waters between the northern and southern sites of the lake. The biomass of P. australis in the southern sites was greater than that in the northern sites; in addition, the above-ground biomass was higher than the below-ground biomass. The above-ground organs accumulated higher concentrations of Ni and Pb than the below-ground organs. The Ni and Pb standing stocks data indicated that the organs of P. australis extracted higher amounts of Ni and Pb per its area from the southern rather than the northern sites. In the current study, the Ni and Pb above-ground standing stocks increased from the early growing season (February) and reached its peak during August and then decreased. The highest monthly Ni and Pb standing stock (18.2 and 18.4 g m− 2, respectively) was recorded in the above-ground organs of plants in the southern sites in August. The bioaccumulation factor of Ni was 157.6 and 153.4 in the northern and southern sites, respectively, whereas that of Pb was 175.3 and 158.3. The translocation factor of Ni and Pb from the below- to above-ground organs was generally > 1. Thus, this reed species is a potential candidate for Ni and Pb phytoextraction. Based on our results, P. australis could be used for the extraction of Ni and Pb to reduce the pollution in Lake Burullus, if the above-ground biomass is harvested at its maximum value in August, as was the case regarding the maximum standing stock of Ni and Pb.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Contamination of coastal lakes and wetlands with heavy metals is a common global environmental issue, due to anthropogenic activities (Bonanno and Vymazal 2017; Eid et al. 2020a). Pollution of these aquatic ecosystems with heavy metals represents a serious hazard to the life of aquatic species, as these fragile ecosystems have less self-cleaning ability compared to other kinds of ecosystems (Bonanno et al. 2017). As a result, they can become a significant sink for heavy metals (Vemic et al. 2014). Heavy metals, such as Ni and Pb, are non-degradable elements that cannot be altered, and their toxicity cannot be reduced by chemical or biological processes over time (Kabata-Pendias 2011). Ni is highly toxic to aquatic and terrestrial ecosystems (Duda-Chodak and Baszczyk 2008). It is a naturally occurring metal in water and soil (Hedfi et al. 2007) but reaches toxic levels through various anthropogenic activities, including industrialization, fertilizer application, use of chemicals, and disposal of sewage sludge (Hassan et al. 2019). At low concentrations, Ni is believed to be an important element for plant growth and development, becoming toxic to plants at high concentrations (Ragsdale 1998). In comparison, Pb is one of the most toxic heavy metals to humans globally (Li et al. 2014). It is a pollutant that is of extremely high priority in the scientific community because, as well as being toxic to ecosystems, it can bioaccumulate in biological organs, even at very low concentrations (Gilbert et al. 2011). Pb reaches natural surface water and ground water from industrial effluents, agricultural runoff, and municipal wastewater (Yurtsever and Şengil 2009).
Wetlands dominated by emergent macrophytes are among the greatest productive ecosystems globally (Mitsch and Gosselink 2015; Owers et al. 2018). Consequently, these macrophytes are used in water quality assessments and the management of wetlands (Vymazal and Březinová 2016). Harvesting emergent macrophytes, such as Phragmites australis (Cav.) Trin. ex Steudel (common reed), has been proposed as a management tool for improving and maintaining the water quality of lakes (Eid et al. 2020b). P. australis is a cosmopolitan emergent macrophyte present in a wide range of aquatic habitats and has been widely investigated as a bioaccumulator of heavy metals, acting as a biofilter to reduce water pollution (Bonanno et al. 2018). It is commonly used in constructed wetlands to improve water quality (Vymazal and Březinová 2016) because of its fast growth rate and the high potential for heavy metal accumulation in its organs (Bonanno 2013).
The use of native plants able to accumulate heavy metals for phytoremediation has an important ecological and global significance as these plants are more functional than introduced plants in terms of survival, rapid growth and reproduction under situations of environmental stress (Oyuela Leguizamo et al. 2017). Therefore, the effectiveness of a phytoremediation process depends on the selection of convenient plants for specific environments (Galal et al. 2018). Moreover, information on the accumulation possibility of aquatic plants can facilitate choosing suitable plants for phytoremediation of aquatic ecosystems (Eid et al. 2020a). To our knowledge, thus far, there no has been comprehensive study (which examined Ni and Pb in sediment, water and plant that differentiated to stems, leaves and below-ground organs) over a cycle of one year available on naturally colonizing P. australis in Lake Burullus for phytoremediation. Therefore, the present study was carried out to investigate the potential application of P. australis as a phytoremediator to remove Ni and Pb from contaminated water and sediment in Lake Burullus. In this study, we investigated the (1) concentration of Ni and Pb in the organs of P. australis and (2) extent of Ni and Pb mobility from the sediment and water to the below-ground organs and inside the plant. The current study is part of our ongoing research (Eid and Shaltout 2014; Eid et al. 2012; Eid et al. 2020c, d) to explore the capacity of some macrophytes as a bioindicator and phytoremediator of heavy metal pollution in Lake Burullus (a Ramsar site in Egypt).
Materials and Methods
Lake Burullus (Long. 31° 22′–31° 35′ N, Lat. 30° 31′–31° 08′ E) is a northern lake in Egypt. It is a portion of the Deltaic Mediterranean coast and is connected to the Mediterranean Sea via the Al-Bughaz natural outlet (Fig. 1). It covers an area of about 410 km2 and is bordered with agricultural lands to the south and a sand bar separating it from the Mediterranean Sea to the north. Lake depth varies between 20 cm beside the shoreline and 200 cm near the Al-Bughaz outlet (Shaltout and Khalil 2005). The lake was listed as a Ramsar site in 1998 due to its value for the wintering, foraging, refuge, and breeding of migrant birds (Kassas 2002). Lake Burullus is a major site for the drainage of agricultural water in Egypt, receiving about 4 billion m3 year− 1 of drainage water from the agricultural lands of the Nile Delta, responsible for 97 % of its water inflow (Shaltout and Khalil 2005). Major sources of pollution in the catchment area of Lake Burullus are domestic, industrial, and agricultural drainage from human settlements, factories, and reclaimed lands (Shaltout and Khalil 2005). The lake is in an arid region with warm summers (20–30°C) and mild winters (10–20°C).
Drainage water from agricultural lands of the Nile Delta and wastewater from fish farms, which join Lake Burullus from the south, generate a pronounced nutrients gradient from the south to the north (Eid et al. 2020b). Hence, sampling was conducted at six sites, with three sites on each of the northern and southern parts (Fig. 1). All six sites had pure or nearly pure P. australis flooded stands year-round. At each sampling site, the above-ground biomass of P. australis was harvested monthly for 1 year. Within six randomly distributed quadrats (0.5 × 0.5 m) at each site, all P. australis shoots were cut off at ground level, separated into leaves (including sheaths) and stems, and transferred to the laboratory in polyethylene bags. Care was taken to select quadrats randomly and to ensure that sampling was not conducted from previously sampled quadrats. Below-ground organs (roots and rhizomes) were obtained from the same quadrats at a depth of 0.5 m (the deepest point of below-ground organ penetration; Eid et al. 2010). These below-ground organs were then washed with lake water until they were free of sediment and then transferred to the laboratory in polyethylene bags. At the laboratory, all collected plant samples were closely washed with tap water and cleaned with de-ionized water over a 4-mm mesh sieve to minimize the loss of material. Plants were then oven dried at 85°C to a constant weight, weighed, and ground using a metal-free plastic mill and stored in paper bags for further analysis. All biomass values were determined as grams of dry matter per square meter (g DM m− 2). One composite sample from each quadrat from each P. australis organ at each of the six sampling sites per month, in total, 432 plant samples per organ were used to determine Ni and Pb.
At each sampling site, six water samples were collected monthly from the same sampling quadrats of plant materials. Water samples were collected as integrated composite samples from the top of the water surface down to 50 cm. Samples were collected in plastic bottles, transferred to the laboratory, and filtered using Whatman nylon membrane filters (pore size 0.45 µm, diameter 47 mm). Samples were acidified to pH 2.0 using sulfuric acid (Analar) to preserve the Ni and Pb in the samples. Then, samples were deep-frozen (− 20°C) for later analysis of Ni and Pb. At each sampling site, six sediment samples were collected monthly from the same sampling quadrats of plant materials to 50 cm depth using a hand sediment corer. Sediment cores were slowly extracted out of the corer and stored in plastic bags. Samples were transferred to the laboratory where sediments were air dried and passed through a 2 mm sieve to separate out gravel and debris. They were then stored under room temperature until further analysis.
For sediment samples, diethylenetriaminepentaacetic acid solution (DTPA) was used to extract available Ni and Pb. Heavy metals were extracted from 0.5 to 1 g of plant organs (leaf, stem, and below-ground organs) using a tri-acid mixture of HNO3:HClO4:HF (1:1:2, v:v:v) in a microwave sample preparation system (PerkinElmer Titan MPS, PerkinElmer Inc., USA) till a transparent color appeared, then plant digests were filtered and diluted to 25 mL with double de-ionized water (Allen 1989). For plant, water, and sediment samples, Ni and Pb were determined by atomic absorption spectroscopy (Shimadzu AA-6200; Shimadzu Co. Ltd., Japan). De-ionized water was used throughout the study. Cleaned glassware and analytical grade reagents were used. Blank reagents were used to correct instrument readings. The variation coefficient of replicate analysis was determined for different measurements to calculate analytical precision. To calibrate the system, standard solutions with known concentrations of Ni and Pb were used. The instrument setting and operational conditions were performed in accordance with the manufacturers’ specifications. Detection limits for Ni and Pb were 10.0 and 2.0 µg L− 1, respectively. Analyses procedures followed Allen (1989) and APHA (1998). Chemical concentrations were expressed based on dried matter.
The bioaccumulation factor (BAF) was calculated to determine the efficiency of P. australis in accumulating a given heavy metal from the sediment (Eid et al. 2020d): BAF = concentration of a heavy metal in the below-ground organs (mg kg− 1)/concentration of the same heavy metal in the sediment at the same site (mg kg− 1). The TF was calculated to determine the ability of P. australis to translocate a given heavy metal from the below-ground to the above-ground organs (Eid et al. 2020d): TF = concentration of a heavy metal in the above-ground organs (mg kg− 1)/concentration of the same heavy metal in the below-ground organs (mg kg− 1). Finally, the standing stock of Ni and Pb of the above- and below-ground organs (g m− 2) was calculated by multiplying the Ni and Pb concentrations with the biomass of the respective organs (g DM m− 2).
Data were collated for all six sites, generating 18 replicates for each sampling date at each location in the lake (north/south). Before performing ANOVA, data were tested for normality of distribution using Shapiro-Wilk’s W test and homogeneity of variance using Levene’s test. When necessary, data were log-transformed. Data on the biomass and metal concentrations in water and sediment were subjected to two-way analysis of variance (ANOVA-2) to test for differences between sites over time. Ni and Pb data (concentrations and standing stocks) for P. australis organs underwent three-way analysis of variance (ANOVA-3) to test for differences between organs and sites over time. Statistical analyses were carried out using SPSS 21 software (SPSS 2012).
Results and Discussion
Descriptive statistics of the two-way analysis of variance (ANOVA-2) showed significant (p < 0.01−0.001) monthly variation in the concentrations of water Ni and Pb between the northern and southern sites of the lake. The Ni and Pb concentrations (total mean) in the water at the southern sites (3.4 and 3.5 mg L− 1, respectively) of Lake Burullus were greater than those in the northern sites (3.1 and 3.3 mg L− 1) (Fig. 2). The highest concentrations of Ni in the northern and southern sites were recorded during February and January (6.2 and 4.2 mg L− 1), respectively. In comparison, the lowest concentrations of Ni in the northern and southern sites were recorded during January and February (1.7 and 2.1 mg L− 1), respectively. The highest concentrations of Pb in the northern and southern sites were recorded during June and August (4.3 and 5.5 mg L− 1), respectively. In comparison, the lowest concentrations in the northern and southern sites were recorded during April and June (1.9 and 2.1 mg L− 1), respectively.
Monthly variation in water Ni and Pb concentrations at the northern and southern sites supporting Phragmites australis in Lake Burullus, Egypt over 1 year. Vertical bars show the standard errors of the means (n = 18). F-values represent two-way analysis of variance (ANOVA-2), **p < 0.01, ***p < 0.001
Significant (p < 0.01−0.001) variation was detected for Ni and Pb concentrations in the sediments between the northern and southern sites of the lake (Fig. 3). The Ni and Pb total mean concentrations in the sediment at the southern sites (12.6 and 13.7 mg kg− 1, respectively) of Lake Burullus were greater than those in the northern sites (11.9 and 12.7 mg kg− 1) (Fig. 3). The highest sediment Ni concentrations in the northern and southern sites were recorded during February and October (13.0 and 14.4 mg kg− 1), respectively. In comparison, the lowest concentrations of Ni in the northern and southern sites were recorded during October and February (10.0 and 10.7 mg kg− 1), respectively. In comparison, the highest sediment Pb concentrations in the northern and southern sites were recorded during January and August (16.0 and 15.6 mg kg− 1), respectively. The lowest concentration of Pb in the northern sites (8.8 mg kg− 1) was recorded during October and in southern sites (12.0 mg kg− 1) was recorded during October-January.
Monthly variation in sediment Ni and Pb concentrations at the northern and southern sites supporting Phragmites australis in Lake Burullus, Egypt, over 1 year. Vertical bars show the standard errors of the means (n = 18). F-values represent two-way analysis of variance (ANOVA-2), **p < 0.01, ***p < 0.001, ns: not significant (i.e., p > 0.05)
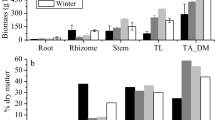
In both northern and southern areas, the above-ground biomass of P. australis reflected the growing season, expanding from February to reach a peak in August and then diminishing towards January (Fig. 4). In the northern sites, values for the above-ground biomass were 2969 g DM m− 2 in February, 7884 g DM m− 2 in August, and 3702 g DM m− 2 in January. Equivalent above-ground biomass values for samples obtained from the southern sites were 4137, 8317 and 4119 g DM m− 2, respectively. The below-ground biomass showed an opposite growth pattern, declining from February to a nadir in July, and then expanding to reach a peak in December. This trend was seen in both northern and southern sampling sites. In the northern sites, values for the below-ground biomass of P. australis, were 2335 g DM m− 2 in February, 863 g DM m− 2 in July, and 2768 g DM/m2 in December (Fig. 4). Equivalent below-ground biomass values for samples obtained from the southern sites were 3191, 942 and 3254 g DM m− 2, respectively.
Monthly variation in the above- (AGB) and below-ground biomass (BGB) of Phragmites australis in the northern and southern sites of Lake Burullus, Egypt, over 1 year. Vertical bars show the standard errors of the means (n = 18). Stem biomass: FMonth = 31.7***, FSite = 38.7***, FMonth × Site = 0.2ns; Leaf biomass: FMonth = 20.6***, FSite = 9.6**, FMonth × Site = 3.2**; Above-ground biomass: FMonth = 99.7***, FSite = 101.4***, FMonth × Site = 0.1ns; Below-ground biomass: FMonth = 41.8***, FSite = 27.9***, FMonth × Site = 0.3ns. F-values represent two-way analysis of variance (ANOVA-2), **p < 0.01, ***p < 0.001, ns not significant (i.e., p > 0.05)
Significant (p < 0.01−0.001) monthly variation in the concentrations of Ni and Pb between the northern and southern sites was detected (Fig. 5). Above-ground organs accumulated higher concentrations of Ni and Pb than below-ground organs. The highest annual average Ni concentration (2115 mg kg− 1) was recorded in the stems of the southern sites, while the lowest concentration (1865 mg kg− 1) was recorded in the below-ground organs of the northern sites. However, the highest annual average Pb concentration (2226 mg kg− 1) was recorded in the leaves of plants in the northern sites, while the lowest concentration (2122 mg kg− 1) was recorded in the stems of plants in the southern sites. The highest and lowest monthly concentration of Ni (2412 and 1590 mg kg− 1, respectively) was recorded in the leaves of plants in the northern sites in January and October, respectively. The highest monthly concentration of Pb (2582 mg kg− 1) was recorded in the leaves of plants in the northern sites in April, while the lowest concentration (1808 mg kg− 1) was recorded in the leaves of plants in the southern sites in January.
Monthly variation of the Ni and Pb concentrations in the organs of Phragmites australis in the northern and southern sites of Lake Burullus, Egypt, over 1 year. Vertical bars show the standard errors of the means (n = 18). Ni: FMonth = 5.9***, FSite = 33.6***, FOrgan = 29.0***, FMonth × Site = 6.5***, FMonth × Organ = 2.7***, FSite × Organ = 5.3**, FMonth × Site × Organ = 3.7***; Pb: FMonth = 11.9***, FSite = 7.8**, FOrgan = 2.3ns, FMonth × Site = 5.6***, FMonth × Organ = 3.6***, FSite × Organ = 1.4ns, FMonth × Site × Organ = 1.9*. F-values represent three-way analysis of variance (ANOVA-3), *p < 0.05, **p < 0.01, ***p < 0.001, ns not significant (i.e., p > 0.05)
Below-ground organs had the greatest potential to uptake Ni and Pb from the sediments at both the northern and southern sites (Table 1). The BAF of Ni was 157.6 and 153.4 in the northern and southern sites, respectively, whereas that of Pb was 175.3 and 158.3. The TF of Ni and Pb from the below- to above-ground organs (stems and leaves) were generally > 1, with the highest value (1.11) being recorded for Ni of the stems in the southern plants and the lowest value (1.00) for Pb of the stems in the southern plants. Descriptive statistics of the ANOVA-3 showed significant (p < 0.001) monthly variation in the standing stocks (g m− 2) of Ni and Pb between the northern and southern sites of the lake and between above- and below-ground organs (Fig. 6). Standing stocks data indicated that organs of P. australis extracted higher amounts of Ni and Pb per area from the southern rather than the northern sites (Fig. 6). In the current study, the Ni and Pb above-ground standing stocks increased from the early growing season (February) and reached its peak during August and then decreased. The highest monthly Ni and Pb standing stock (18.2 and 18.4 g m− 2, respectively) was recorded in the above-ground organs of plants in the southern sites in August.
Monthly variation of the Ni and Pb standing stock in the above- and below-ground organs of Phragmites australis in the northern and southern sites of Lake Burullus, Egypt, over 1 year. Vertical bars show the standard errors of the means (n = 18). Ni: FMonth = 12.9***, FSite = 113.3***, FOrgan = 2198.8***, FMonth × Site = 1.2ns, FMonth × Organ = 57.0***, FSite × Organ = 30.2***, FMonth × Site × Organ = 1.4ns; Pb: FMonth = 4.0***, FSite = 19.7***, FOrgan = 1119.4***, FMonth × Site = 0.5ns, FMonth × Organ = 35.9***, FSite × Organ = 3.7ns, FMonth × Site × Organ = 0.3ns. F-values represent three-way analysis of variance (ANOVA-3), ***p < 0.001, ns not significant (i.e., p > 0.05)
Heavy metal pollutants in Lake Burullus originate from various anthropogenic activities, including industrial facilities and agricultural drainage (Eid and Shaltout 2014). Many studies address the relationships between land-use and water pollution (El-Zeiny and El-Kafrawy 2017). The current study demonstrated that the concentrations of Ni (3.1–3.4 mg L− 1) in the water samples from six locations in Lake Burullus exceeded the maximum level of Ni (0.2 mg L− 1) detected in irrigation water by Rowe and Abdel-Magid (1995). In contrast, the concentrations of Pb (3.3–3.5 mg L− 1) in the same water samples were below the maximum level of Pb previously documented in irrigation water (5.0 mg L− 1) (Rowe and Abdel-Magid 1995). The Pb (12.7–13.7 mg kg− 1) and Ni (11.9–12.6 mg kg− 1) concentrations in the sediment at these six locations were within ranges that are considered safe (2.0–200.0 mg kg− 1 for Pb and 0.5–100.0 mg kg− 1 for Ni) (Kabata-Pendias 2011; Nagajyoti et al. 2010). Moreover, Pb concentration in sediment and water was higher during summer, which was attributed to the excessive waste discharges into the lake during this period which brought high load of nutrients into the lake (ElTohamy et al. 2014), in addition to the high evaporation rate in the lake. The monthly average volume discharged into the lake through the drain system varies from about 240 × 106 m3 during winter to about 420 × 106 m3 during summer (Ali 2011). Most of the waste discharged into the lake originated from anthropogenic activity at the southern part of the lake; consequently, Ni and Pb concentrations in the sediment and water were greater at the southern sites compared to the northern sites of the lake. Our results agree with El-Zeiny and El-Kafrawy (2017) and Elsayed et al. (2019), who determined that the maximum concentrations of water pollutants were in the southern part of the lake, especially at sites polluted by fertilizer, run-off animal wastes and domestic sewage.
Wetland plants is essential for photosynthesis and the flow of energy through the ecosystem. Thus, a quantitative measure of wetland plant biomass yields valuable information relating to both the size and productivity of that community (Owers et al. 2018). It also provides a measure of the overall well-being, composition and function of the wetland community (Pacini et al. 2018). In the present study, a greater biomass was seen in the southern sites compared with those in the north. Our previous investigation (Eid et al. 2020b) indicated that above-ground biomass of P. australis increased with increasing nutrients along north-south direction in Lake Burullus, where lake eutrophication influenced P. australis growth positively. In the northern and southern sites of the lake, the annual average above-ground biomass of P. australis was 5.2 and 6.0 kg DM m− 2. These figures were greater than previously documented for temperate climes (0.6–3.5 kg DM m− 2, Eid et al. 2020b), but somewhat less than that seen in Australia (9.9 kg DM m− 2, Hocking 1989) and Iraq (13.5 kg DM m− 2, Rezk and Al-Edany 1981). Whilst acknowledging potential genetic variation among the populations (Lambertini et al. 2008), this observed increase in above-ground biomass of P. australis in Lake Burullus may also reflect the high solar irradiance, augmented length of growing season and a positive influence of the ambient temperature on growth (Eid et al. 2010), Karunaratne et al. (2003) have theorized that the P. australis biomass is significantly affected by temperature and photoperiod, especially in the presence of elevated nutrient bioavailability (Ho 1979). Atkin et al. (2006) have reported that biochemical and physiological pathways, and thus macrophyte growth, can be enhanced by a rise in temperature even within the normal physiological spectrum. Lake Burullus is recognized as having eutrophic water properties (Eid et al. 2020b) and this eutrophication is likely to underpin the profuse productivity of P. australis seen in the region.
In the present work, above-ground biomass increased from February (2969–4137 g DM m− 2) until it reached its maximum in August (7884–8317 g DM m− 2), after which it decreased again until January (3702–4119 g DM m− 2). A similar growth trend in P. australis was reported by Quan et al. (2007). In addition, Karunaratne et al. (2003) reported that the shoot growth of aquatic macrophytes starts in March, with above-ground biomass peaking during summer, and then decreasing from the onset of senescence. Consequently, summer is the ideal season for sequestering high heavy metal concentrations by P. australis. In comparison, below-ground biomass was the lowest during summer and peaked during winter. The decrease in rhizome biomass during spring and summer might be attributed to the partial translocation of rhizome carbohydrates to the new shoots produced during this period, since rapid shoot growth occurs before foliar structures develop (Granéli et al. 1992).
The uptake of heavy metals depends on the concentration and solubility of a given heavy metal and the plant species involved (Hassan et al. 2019). In this study, we demonstrated the potential of P. australis to mitigate Ni and Pb contamination in the water and sediments of Lake Burullus using an ex-situ phytoremediation approach, in accordance with the study of Cicero-Fernández et al. (2017). Above-ground organs of P. australis accumulated greater Ni and Pb concentrations than the below-ground organs, constituting a significant pool of absorbed Ni and Pb. This finding was comparable to that obtained by Eid and Shaltout (2016), who reported that P. australis accumulated higher concentrations of Pb in the stems compared to the below-ground organs. In addition, Galal and Shehata (2016) reported that Arundo donax (an emergent macrophyte with a growth form similar to that of P. australis) accumulated higher concentrations of Pb in the leaves compared to the below-ground organs. However, other studies reported that heavy metals are largely retained in below-ground organs (Bonanno 2013; Eid and Shaltout 2014; Bonanno et al. 2017). The distribution of heavy metals in different plant organs depends on their form, water transport, and plant species (Ouzounidou et al. 1995). Variation in heavy metal concentrations across plant organs has been attributed to compartmentalization and translocation through the vascular system (Kim et al. 2003). Plant physiological factors, variations in the solubility and availability of each heavy metal, and plant organization mechanisms to control above-ground concentrations could be other causes for the different concentrations of heavy metals between above- and below-ground organs (Eid and Shaltout 2014).
The usefulness of P. australis in the elimination of aquatic pollutants is well known (Eid et al. 2020b). Moreover, their application is also both cost-effective and environmentally sympathetic factor (Bonanno et al. 2017). It is generally used in constructing wetlands for enhancement of water quality in water treatment systems (Vymazal and Březinová 2016) because of its adaptation to a wide range of environmental conditions (Bonanno et al. 2018) and great capacity for accumulating heavy metals in its organs (Bonanno 2013; Eid et al. 2020a). The current study showed that organs of P. australis accumulated high concentrations of Ni and Pb, exceeding the safe range for normal plants (Nagajyoti et al. 2010). These results indicated that P. australis can grow on sediments polluted with Ni and Pb; therefore, this plant appears to tolerate these heavy metals to the given levels. In addition, P. australis accumulated Ni and Pb concentrations > 1000 mg kg− 1; thus, it is deemed as a hyperaccumulator of these two heavy metals (Singh and Ma 2007). Moreover, Ni and Pb concentrations in the organs of P. australis in Lake Burullus were higher than the range of concentrations registered for the same species in other natural and constructed wetlands (Table 2). Results of the present study might differ from those of previous studies due to differences in the sampling time, pollution levels, physico-chemical characteristics of water and sediment at the sampling sites, and the analytical methods used in the digestion of plant materials (Du Laing et al. 2003, 2009; Kabata-Pendias 2011).
The estimation of the BAF represents a simple method to characterize quantitatively the transport of available heavy metals from sediment or water to plant, while the efficacy of the internal transport of heavy metals from the roots of plants to their shoots is illustrated by the TF (Branzini et al. 2012). According to Bello et al. (2018), high BAF coupled with low TF is found in heavy metal-phytostabilization plants, whereas BAF and TF > 1.0 is found in heavy metal-phytoextraction plants (Sellal et al. 2019). The current research reveals that P. australis was recognized by a BAF and TF > 1.0 in respect of the two heavy metals considered in this investigation. This suggests that P. australis can amass heavy metals in its organs and is more applicable for phytoextraction use. These results agreed, to a great extent, with Galal et al. (2017a) on Vossia cuspidate, Galal et al. (2017b) on Cyperus articulates, and Galal and Shehata (2016) on A. donax. The earlier studies by Sellal et al. (2019) and Ullah et al. (2015) indicate that P. australis sequester substantial concentrations of Ni and Pb considered in this study and this plant has a capacity to accumulate them in cellular vacuoles (as organic complexes) in the shoot organs.
Stoltz and Greger (2002) stated that plants with a higher metal concentration than the sediment are accumulators, with the storage capacity of a given heavy metal varying across organs and species. In this study, we recorded the highest storage capacity of Ni and Pb in the above-ground organs of P. australis during summer, whereas that of the below-ground parts was recorded during winter, reflecting the documented trend in biomass. Therefore, harvesting P. australis during summer could be used to control the flow of these heavy metals in Lake Burullus, when considering the dynamic cycling of and seasonal changes in the accumulation potential of this reed (Ruiz and Velasco 2010). In addition, periodic harvesting stimulates P. australis growth and increases its capacity to accumulate heavy metals in new shoots (Greenway and Woolley 1999). Based on our results, P. australis could be used for removal of Ni and Pb to reduce the pollution in Lake Burullus, if the above-ground biomass is mowed at its maximum value in August, as was the case regarding the maximum standing stock of Ni and Pb. Considering values for peak total above-ground biomass and that P. australis dominates an area of approximately 8200 ha in Lake Burullus (Eid et al. 2010), as much as 1205.4–1492.4 t Ni and 1303.8–1508.8 t Pb could be theoretically extracted yearly from the lake by mowing above-ground biomass of P. australis in August. Regrettably, we do not have quantitative data about Ni and Pb load of the water releasing to Lake Burullus. Harvested materials could be used as roof or fencing materials or could be used as substrate for biogas production and carbonization to make charcoal.
References
Abdel-Shafy H, Hegemann W, Teiner A (1994) Accumulation of metals by vascular plants. Environ Manage Health 5:21–24
Ahmad SS, Reshi ZA, Shah MA, Rashid I, Ara R, Andrabi SMA (2014) Phytoremediation potential of Phragmites australis in Hokersar Wetland: a Ramsar site of Kashmir Himalaya. Int J Phytoremediat 16:1183–1191
Ali EM (2011) Impact of drain water on water quality and eutrophication status of Lake Burullus, Egypt, a southern Mediterranean lagoon. Afr J Aquat Sci 36:267–277
Allen S (1989) Chemical analysis of ecological materials. Blackwell Scientific Publications, London
APHA (American Public Health Association) (1998) Standard methods for the examination of water and wastewater. American Public Health Association, Washington DC
Atkin OK, Loveys BR, Atkinson LJ, Pons TL (2006) Phenotypic plasticity and growth temperature: understanding interspecific variability. J Exp Bot 57:267–281
Bello AO, Tawabini BS, Khalil AB, Boland CR, Saleh TA (2018) Phytoremediation of cadmium-, lead- and nickel-contaminated water by Phragmites australis in hydroponic systems. Ecol Eng 120:126–133
Bonanno G (2013) Comparative performance of trace element bioaccumulation and bio- monitoring in the plant species Typha domingensis, Phragmites australis and Arundo donax. Ecotoxicol Environ Saf 97:124–130
Bonanno G, Lo Giudice R (2010) Heavy metal bioaccumulation by the organs of Phragmites australis (common reed) and their potential use as contamination indicators. Ecol Indic 10:639–645
Bonanno G, Vymazal J (2017) Compartmentalization of potentially hazardous elements in macrophytes: insights into capacity and efficiency of accumulation. J Geochem Explor 181:22–30
Bonanno G, Borg JA, Di Martino V (2017) Levels of heavy metals in wetland and marine vascular plants and their biomonitoring potential: a comparative assessment. Sci Total Environ 576:796–806
Bonanno G, Vymazal J, Cirelli GL (2018) Translocation, accumulation and bioindication of trace elements in wetland plants. Sci Total Environ 631–632:252–261
Bragato C, Brix H, Malagoli M (2006) Accumulation of nutrients and heavy metals in Phragmites australis (Cav.) Trin ex. Steudel and Bolboschoenus maritimus (L.) Palla in a constructed wetland of the Venice lagoon watershed. Environ Pollut 144:967–975
Branzini A, González RS, Zubillaga M (2012) Absorption and translocation of copper, zinc and chromium by Sesbania virgata. J Environ Manage 102:50–54
Cicero-Fernández D, Peña-Fernández M, Expósito-Camargo JA, Antizar-Ladislao B (2017) Long-term (two annual cycles) phytoremediation of heavy metal-contaminated estuarine sediments by Phragmites australis. New Biotechnol 38:56–64
Du Laing G, Tack FMG, Verloo MG (2003) Performance of selected destruction methods for the determination of heavy metals in reed plants (Phragmites australis). Anal Chim Acta 497:191–198
Du Laing G, Van de Moortel AMK, Moors W, De Grauwe P, Meers E, Tack FMG, Verloo MG (2009) Factors affecting metal concentrations in reed plants (Phragmites australis) of intertidal marshes in the Scheldt estuary. Ecol Eng 35:310–318
Duda-Chodak A, Baszczyk U (2008) The impact of nickel on human health. J Elementol 13:685–696
Duman F, Cicek M, Sezen G (2007) Seasonal changes of metal accumulation and distribution in common club rush (Schoenoplectus lacustris) and common reed (Phragmites australis). Ecotoxicology 16:457–463
Eid EM, Shaltout KH (2014) Monthly variations of trace elements accumulation and distribution in above- and below-ground biomass of Phragmites australis (Cav.) Trin ex. Steudel in Lake Burullus (Egypt): a biomonitoring application. Ecol Eng 73:17–25
Eid EM, Shaltout KH (2016) Bioaccumulation and translocation of heavy metals by nine native plant species grown at a sewage sludge dump site. Int J Phytoremediat 18:1075–1085
Eid EM, Shaltout KH, Al-Sodany YM, Soetaert K, Jensen K (2010) Modeling growth, carbon allocation and nutrient budget of Phragmites australis in Lake Burullus, Egypt. Wetlands 30:240–251
Eid EM, Shaltout KH, El-Sheikh MA, Asaeda T (2012) Seasonal courses of nutrients and heavy metals in water, sediment and above- and below-ground Typha domingensis biomass in Lake Burullus (Egypt): perspective for phytoremediation. Flora 207:783–794
Eid EM, Galal TM, Sewelam NA, Talha NI, Abdallah SM (2020a) Phytoremediation of heavy metals by four aquatic macrophytes and their potential use as contamination indicators: a comparative assessment. Environ Sci Pollut Res 27:12138–12151
Eid EM, Shaltout KH, Al-Sodany YM, Haroun SA, Galal TM, Ayed H, Khedher KM, Jensen K (2020b) Seasonal potential of Phragmites australis in nutrient removal to eliminate the eutrophication in Lake Burullus, Egypt. J Freshwater Ecol 35:135–155
Eid EM, Galal TM, Shaltout KH, El-Sheikh MA, Asaeda T, Alatar AA, Alfarhan AH, Alharthi A, Alshehri AMA, Picó Y, Barcelo D (2020c) Biomonitoring potential of the native aquatic plant Typha domingensis by predicting trace metals accumulation in the Egyptian Lake Burullus. Sci Total Environ 714:136603
Eid EM, Shaltout KH, Al-Sodany YM, Haroun SA, Galal TM, Ayed H, Khedher KM, Jensen K (2020d) Common reed (Phragmites australis (Cav.) Trin ex. Steudel) as a candidate for predicting heavy metal contamination in Lake Burullus, Egypt: a biomonitoring approach. Ecol Eng 148:105787
El-Zeiny A, El-Kafrawy S (2017) Assessment of water pollution induced by human activities in Burullus Lake using Landsat 8 operational land imager and GIS. Egypt J Remote Sens Space Sci 20:S49–S56
Elsayed FA, Okbah MA, El-Syed SM, Eissa MA, Goher ME (2019) Nutrient salts and eutrophication assessment in Northern Delta lakes: case study Burullus Lake, Egypt. Egypt J Aquat Biol Fish 23:145–163
ElTohamy WS, Abdel Aziz NEM, El Ghobashy AE, Alzeny A (2014) Water quality assessment of Burullus Lake using multivariate analysis. World J Fish Mar Sci 6:564–572
Galal TM, Shehata HS (2016) Growth and nutrients accumulation potentials of giant reed (Arundo donax L.) in different habitats in Egypt. Int J Phytoremediat 18:1221–1230
Galal TM, Gharib FA, Ghazi SM, Mansour KH (2017a) Phytostabilization of heavy metals by the emergent macrophyte Vossia cuspidata (Roxb.) Griff.: a phytoremediation approach. Int J Phytoremediat 19:992–999
Galal TM, Gharib FA, Ghazi SM, Mansour KH (2017b) Metal uptake capability of Cyperus articulatus L. and its role in mitigating heavy metals from contaminated wetlands. Environ Sci Pollut Res 24:21636–21648
Galal TM, Eid EM, Dakhil MA, Hassan LM (2018) Bioaccumulation and rhizofiltration potential of Pistia stratiotes L. for mitigating water pollution in the Egyptian wetlands. Int J Phytoremediat 20:440–447
Gilbert UA, Emmanuel IU, Adebanjo AA, Olalere GA (2011) Biosorptive removal of Pb2+ and Cd2+ onto novel biosorbent: defatted Carica papaya seeds. Biomass Bioenergy 35:2517–2525
Granéli W, Weisner SE, Sytsma MD (1992) Rhizome dynamics and resource storage in Phragmites australis. Wetlands Ecol Manage 1:239–247
Greenway M, Woolley A (1999) Constructed wetlands in Queensland: performance efficiency and nutrient bioaccumulation. Ecol Eng 12:39–55
Hassan MU, Chattha MU, Khan I, Chattha MB, Aamer M, Nawaz M, Ali A, Khan MAU, Khan TA (2019) Nickel toxicity in plants: reasons, toxic effects, tolerance mechanisms, and remediation possibilities - a review. Environ Sci Pollut Res 26:12673–12688
Hedfi A, Mahmoudi E, Boufahja F, Beyrem H, Aissa P (2007) Effects of increasing levels of nickel contamination on structure of offshore nematode communities in experimental microcosms. Bull Environ Contam Toxicol 79:345–349
Ho YB (1979) Shoot development and production studies of Phragmites australis (Cav.) Trin. ex Steudel in Scottish lochs. Hydrobiologia 64:215–222
Hocking PJ (1989) Seasonal dynamics of production, and nutrient accumulation and cycling by Phragmites australis (Cav.) Trin. ex Steudel in a nutrient-enriched swamp in inland Australia. I. Whole plants. Aust J Mar Freshwater Res 40:421–444
Jastrzębska M, Cwynar P, Polechoński R, Skwara T (2010) The content of heavy metals (Cu, Ni, Cd, Pb, Zn) in common reed (Phragmites australis) and floating pondweed (Potamogeton natans). Pol J Environ Stud 19:243–246
Kabata-Pendias A (2011) Trace elements in soils and plants. CRC Press, Boca Raton, FL
Karunaratne S, Asaeda T, Yutani K (2003) Growth performance of Phragmites australis in Japan: Influence of geographic gradient. Environ Exp Bot 50:51–66
Kassas M (2002) Management plan for burullus protectorate area. MedWetCoast, Global Environmental Facility (GEF) and Egyptian Environmental Affairs Agency (EEAA), Cairo
Kim IS, Kang HK, Johnson-Green P, Lee EJ (2003) Investigation of heavy metal accumulation in Polygonum thunbergii for phytoextraction. Environ Pollut 126:235–243
Lambertini C, Gustafsson MHG, Frydenberg J, Speranza M, Brix H (2008) Genetic diversity patterns in Phragmites australis at the population, regional and continental scales. Aquat Bot 88:160–170
Li HB, Cui XY, Li K, Li J, Juhasz AL, Ma LQ (2014) Assessment of in vitro lead bioaccessibility in house dust and its relationship to in vivo lead relative bioavailability. Environ Sci Technol 48:8548–8555
Mitsch WJ, Gosselink JG (2015) Wetlands, 5th edn. Wiley, Hoboken
Nagajyoti PC, Lee KD, Sreekanth TVM (2010) Heavy metals, occurrence and toxicity for plants: a review. Environ Chem Lett 8:199–216
Ouzounidou G, Ciamporova M, Moustakas M, Karataglis S (1995) Responses of maize (Zea mays L.) plants to copper stress I. Growth, mineral content and ultrastructure of roots. Environ Exp Bot 35:167–176
Owers CJ, Rogers K, Woodroffe CD (2018) Terrestrial laser scanning to quantify above-ground biomass of structurally complex coastal wetland vegetation. Estuar Coast Shelf Sci 204:164–176
Oyuela Leguizamo MA, Fernández Gómez WD, Gutíerrez Sarmiento MC (2017) Native herbaceous plant species with potential use in phytoremediation of heavy metals, spotlight on wetlands - a review. Chemosphere 168:1230–1247
Pacini N, Hesslerová P, Pokorný J, Mwinami T, Morrison EHJ, Cook AA, Zhang S, Harper DM (2018) Papyrus as an ecohydrological tool for restoring ecosystem services in afro-tropical wetlands. Ecohydrol Hydrobiol 18:142–154
Quan WM, Han JD, Shen AL, Ping XY, Qian PL, Li CJ, Shi LY, Chen YQ (2007) Uptake and distribution of N, P and heavy metals in three dominant salt marsh macrophytes from Yangtze River estuary, China. Mar Environ Res 64:21–37
Ragsdale SW (1998) Nickel biochemistry. Curr Opin Chem Biol 2:208–215
Rezk MR, Al-Edany TY (1981) Ecology of Phragmites australis (Cav.) Trin. ex Steud. in Shat Al-Arab, Iraq. II. Reed growth as affected by the chemical composition of its beds. Pol Arch Hydrobiol 28:19–31
Rowe DR, Abdel-Magid IM (1995) Handbook of wastewater reclamation and reuse. CRC Press, Boca Raton, FL
Ruiz M, Velasco J (2010) Nutrient bioaccumulation in Phragmites australis: Management tool for reduction of pollution in the Mar Menor. Water Air Soil Pollut 205:173–185
Sellal A, Belattar R, Bouzidi A (2019) Trace elements removal ability and antioxidant activity of Phragmites australis (from Algeria). Int J Phytoremediat 21:456–460
Shaltout KH, Khalil MT (2005) Lake burullus (burullus protected area). Egyptian Environmental Affairs Agency (EEAA), Cairo
Singh N, Ma LQ (2007) Assessing plants for phytoremediation of arsenic-contaminated soils. In: Willey N (ed) Phytoremediation: methods and reviews. Humana Press Inc., Totowa, NJ, pp 319–347
SPSS (2012) SPSS base 21.0 user’s guide. SPSS Inc, Chicago
Stoltz E, Greger M (2002) Accumulation properties of As, Cd, Cu, Pb and Zn by four wetland plant species growing on submerged mine tailings. Environ Exp Bot 47:271–280
Ullah A, Heng S, Munis MF, Fahad S, Yang X (2015) Phytoremediation of heavy metals assisted by plant growth promoting (PGP) bacteria: a review. Environ Exp Bot 117:28–40
Vemic M, Rousseau D, Du Laıng G, Lens P (2014) Distribution and fate of metals in the Montenegrin part of Lake Skadar. Int J Sed Res 29:357–367
Vymazal J, Březinová T (2016) Accumulation of heavy metals in aboveground biomass of Phragmites australis in horizontal flow constructed wetlands for wastewater treatment: a review. Chem Eng J 290:232–242
Yurtsever M, Şengil IA (2009) Biosorption of Pb (II) ions by modified quebracho tannin resin. J Hazard Mater 163:58–64
Acknowledgements
This work was supported by the Deanship of Scientific Research at King Khalid University under Grant number (R.G.P. 2/56/40).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Eid, E.M., Shaltout, K.H., Al-Sodany, Y.M. et al. Temporal Potential of Phragmites australis as a Phytoremediator to Remove Ni and Pb from Water and Sediment in Lake Burullus, Egypt. Bull Environ Contam Toxicol 106, 516–527 (2021). https://doi.org/10.1007/s00128-021-03120-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-021-03120-y