Abstract
Epstein-Barr virus (EBV) infection has a strong correlation with the development of nasopharyngeal carcinoma (NPC). Aquaporin 3 (AQP3), a member of the aquaporin family, plays an important role in tumor development, especially in epithelial-mesenchymal transition. In this study, the expression of AQP3 in EBV-positive NPC cells was significantly lower than that in EBV-negative NPC cells. Western blot and qRT-PCR analysis showed that LMP1 down-regulated the expression of AQP3 by activating the ERK pathway. Cell biology experiments have confirmed that AQP3 affects the development of tumor by promoting cell migration and proliferation in NPC cells. In addition, AQP3 can promote the lysis of EBV in EBV-positive NPC cells. The inhibition of AQP3 expression by EBV through LMP1 may be one of the mechanisms by which EBV maintains latent infection-induced tumor progression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Epstein-Barr virus (EBV), a member of the gamma-herpesvirus family, also known as human herpesvirus-4 (HHV-4), infects approximately 90% of the global population and is the first human tumor virus to be identified, with infection associated with a variety of lymphatic and epithelial cell cancers, including malignant lymphoma, nasopharyngeal carcinoma (NPC), and approximately 10% of stomach cancers [1, 2]. Among them, the most closely related to EBV infection is NPC [3]. The incidence of NPC is strongly correlated with regions, with higher prevalence in Africa and Southeast Asia and lower incidence in Western countries [4]. Among the three NPC (squamous cell carcinoma, non-keratinized carcinoma, and undifferentiated carcinoma), more than 90% of undifferentiated NPC were associated with EBV infection [5, 6]. EBV-associated tumors have latency periods of type I, II and III, respectively. EBV from NPC belongs to type II latency, and the genes expressed are EBNA1, LMP1, LMP2A, LMP2B, EBERs, BARTs [2, 7].
Latent membrane protein 1 (LMP1), a member of the tumor necrosis factor receptor superfamily, is an oncogene of EBV and is expressed in a variety of malignancies including NPC, Hodgkin lymphoma and immunosuppressant associated lymphoma [8]. It plays an important role in the occurrence and development of tumors by regulating cell proliferation, apoptosis, immune regulation, angiogenesis, metastasis and invasion [9]. LMP1 can activate many signaling pathways, including NF-κB, PI3K-AKT, ERK-MAPK, JNK, JAK-STAT, and p38/MAPK, and achieve carcinogenicity through downstream targets of these pathways [10]. Studies have shown that LMP1 induces the expression and activation of FGFR1 in NPC, promoting aerobic glycolysis of NPC cells. At the same time, LMP1 promotes cell growth, transformation, migration, and invasion by inducing FGFR1 signaling to turn over [11]. LMP1 can also induce epithelial-mesenchymal transformation (EMT) of NPC cells through TGF-β/Smad3/NRP1 signaling pathway, thus promoting the migration and invasion of NPC cells [12]. In NPC, the presence of cancer-related fibroblasts around cancer cells indicates poor prognosis. LMP1 can not only recruit and transform fibroblasts through the ERK-MAPK-dependent mechanism, but also enhance the viability and aggressiveness of fibroblasts and their ability to transform into myofibroblast-like phenotypes, thus leading to metastatic disease [13].
Aquaporins (AQPs) are widely expressed in the body and can form water channels on the cell membrane to promote the passive transport of water across the cell membrane [14]. Studies have found that AQPs is highly expressed in a variety of tumors and plays an active role in cell migration, adhesion and polarity, while regulating cell proliferation, apoptosis and invasion through intercellular signaling [15]. Among them, AQP3 is a hydroglycerol channel protein subtype of AQP family, which is mainly expressed in renal collecting ducts, epidermis, airway epithelium, conjunctiva, atmospheric ducts and bladder, and can transport water and other small molecules (such as glycerol and urea) [16, 17]. There has been a strong correlation between AQP3 and the occurrence and development of tumors [18]. Hara-Chikuma et al. [19] found that AQP3 can participate in cell migration. Kusayama et al. [20] showed that AQP3 is highly expressed in primary squamous cell carcinoma and plays an important role in its growth. In gastric cancer, AQP3 induces EMT through the PI3K/AKT/SNAIL signaling pathway, thus promoting the migration and invasion of gastric cancer cells [21]. AQP3 promotes the growth of pancreatic ductal adenocarcinoma cells by activating the mTOR pathway [22]. Our previous research found that in EBV-associated gastric cancer (EBVaGC), EBV causes loss of expression by inducing CpG island methylation of the AQP3 promoter [23]. This study investigated whether the expression of AQP3 in NPC is regulated by EBV and the biological role of AQP3 in NPC.
Materials and methods
GEO database
We downloaded sequencing data from the GEO database (GSE181906 dataset) for EBV-positive NPC cell lines (C666-1) and EBV-negative NPC cell lines (CNE-2, SUNE-1), analyzed differentially expressed genes using the limma R package, and mapped the volcano. Screening threshold: |LogFC|> 1.5, P-value < 0.05.
Cell culture
EBV-negative (CNE-1, HONE 1, CNE2) and EBV-positive (C666-1, CNE2-EBV) NPC cells were selected for this study. CNE-1 was purchased from Xiamen Immocell Biotechnology, HONE-1 was purchased from BeNa Culture Collection, and CNE-2 was donated by the Affiliated Hospital of Qingdao University. C666-1 cells were donated by the Affiliated Cancer Hospital of Sun Yat-sen University. The EBV-negative NPC cells were cultured in DMEM (Gibco, USA) supplemented with 10% fetal bovine serum (TransGen Biotech, China), 100IU /mL penicillin, and 100 mg/mL streptomycin. C666-1 cells were cultured in 1640 supplemented with 10% fetal bovine serum (TransGen Biotech, China), 100IU /mL penicillin, and 100 mg/mL streptomycin. All cells were kept in a humidified atmosphere at 37 °C with 5% CO2.
Virus preparation and infection
Akata-EBV-GFP cells were treated with 0.8% (v/v) goat anti-human IgG for 6 h to induce EBV to shift from incubation period to lysis cycle, and then to produce EBV. After 3 days of culture in fresh medium, the supernatant was collected by centrifugation at 20,000× g for 30 min at 4 °C. The pellets were resuspended in 10% FBS DMEM and used for cell-free infection. Infected CNE-2 cells were sorted by flow cytometry and cultured in medium containing 300–700 μg/mL G418 until more than 90% of the cells showed green fluorescence under fluorescence microscopy.
RNA isolation and real-time fluorescence quantitative PCR (qRT-PCR)
Total RNA was extracted using TRIzol (Invitrogen, USA) and reverse-transcribed into cDNA using First Strand cDNA (Takara, Japan). Using the obtained cDNA as template, the mRNA transcription level was detected using the FastStart DNA Master SYBR Green Kit (Roche, Germany) in the Light Cycler 96 sequence detection system. The results were normalized by β-actin regression, and the relative gene expression was measured by 2−ΔΔCt method. PCR primers are listed below:
Forward | Reverse | |
|---|---|---|
β-actin | CACCATTGGCAATGAGCGGTT | AGGTCTTTGCGGATGTCCACGT |
AQP3 | CATCCTGGTGATGTTTGGCTG | GTGACAGCAAAGCCAAAGGC |
LMP1 | CCTTGGTCTACTCCTACTGATGATCA | CAGCACAATTCCAAGGTACAATG |
EBNA1 | CAAGGAGGTTCCAACCCGAA | TGTGGAATAGCAAGGGCAGT |
BZLF1 | AGGCCAGCTAACTGCCTATC | TGATTCTGGGTTATGTCGGA |
Western blot analysis
The total protein was obtained by cracking RIPA buffer mixture (RIPA: PMSF: phosphatase inhibitor, 100:1:1) for 20 min, and the protein was determined by the dicinchonic acid detection kit (CWBIO, China). The protein extract was isolated on a 10% or 12% acrylamide SDS-PAGE and transferred to a polyvinylidene difluoride (PVDF) membrane (Millipore, USA) by a transfer device. Subsequently, PVDF membrane was sealed with 5% skim milk for 2 h at room temperature, and incubated with primary antibody at 4 °C overnight, followed by rabbit or mouse secondary antibody coupled with horseradish peroxidase at room temperature for 2 h. Finally, an enhanced chemiluminescence detection system was used to visualize the proteins of interest. Antibodies used include: anti-β-actin (HUABIO, EM21002, 1:10,000); anti-AQP3 (Abcam, ab125219, 1:1000); anti-ERK (HUABIO, ET1601-29, 1:5000); anti-p-ERK (CST, #4370, 1:1000); anti-EBNA1 (Santa Cruz, sc-81581, 1:1000); anti-BZLF1 (Santa Cruz, sc-53904, 1:1000); anti-ZEB1 (Proteintech, 21,544–1-AP, 1:2000); anti-β-catenin (Abmart, M24002, 1:3000); anti-vimentin (CST, #5741, 1:1000); anti-N-cadherin (Abcam, ab76011, 1:1000); anti-snail (Santa Cruz, sc-271977, 1:1000); anti-LMP1 (Abcam, ab78113, 1:1000).
Transfection with siRNAs targeting LMP1, AQP3 and ERK1/2
The siRNA target sequences for LMP1, AQP3 and ERK1/2 were designed and synthesized by GenePharma (China). The sequences are as follows: AAGAGCCUUCUCUGUCCACU for siLMP1, GGCUGUAUUAUGAUGCAAUTT for siAQP3, GAAACUACCUACAGUCUCUTT for siMAPK3/ERK1, GUGCUCUGCUUAUGAUAAUTT for siMAPK1/ERK2. Then 50 nM siRNA was transfected with Lipofectamine 2000 Reagent. The cells were harvested 48 h later and the expression of related genes was detected.
Overexpression of LMP1 and AQP3
LMP1 and AQP3 were cloned into pcDNA3.1 containing GFP fluorescent protein coding sequence, respectively. Plasmid transfection was performed using Lipofectamine 2000 transfection reagent (Invitrogen, China) according to the manufacturer’s protocol, with a total of 2.5 μg plasmid DNA per six-well plate. Cells were harvested 48 h later, or cells selected with G418 were used to construct stable LMP1-expressing cells, and transfection efficiency was verified by qRT-PCR or Western blot.
Detection of EBV DNA copy number
According to the instructions of the DNA extraction kit (TianGen, China), DNA was extracted from the EBV-positive NPC cell line C666-1 transfected with AQP3 and its control plasmid. The EBV specific sequences (EBNA1 and BamHI-W) were quantitatively analyzed by absolute qRT-PCR. The number of copies is calculated according to the standard curve.
Transwell migration assay
A transwell chamber (Corning, USA) with 8 μm holes was placed into a 24 well plate. 200 μL serum-free DMEM cell suspension (2.5 × 104 cells) was added to the upper chamber, and 750 μl DMEM medium containing 20% fetal bovine serum was added to the lower chamber. After incubating in the cell incubator for 48 h, it was fixed with methanol for 30 min, then stained with 1% crystal violet and observed and counted under a microscope.
Wound healing test
After the treated cells in the six-well plate grow and fuse, the confluent cell layer is scraped with the autoclaved medium gun tip to form a linear wound. The cells were washed twice with PBS to remove the shed cell debris. The size of the wound at the same location is then monitored and measured at a specified time (0 h, 48 h).
CCK-8 determination
Cell growth was measured using the CCK-8 kit (TargetMol, USA) according to the instructions. The 96-well plates were inoculated with 2500 cells per well and cultured in a specified medium with 6 repeat wells per sample. According to the manufacturer's plan, 10 μl CCK-8 (the ratio of CCK-8 to medium was 1:9) was added at 0, 24, 48, 72, 96 h, and incubated at 37 °C for 1 – 4 h to detect the absorbance value of each hole at 450 nm wavelength. All experiments were performed 3 times.
Colony formation experiment
The treated cells (300 cells/well) were inoculated into a six-well plate and cultured for 2–3 weeks. Fixed with methanol for 15 min and dyed with 1% crystal violet for 15 min. Finally, the number of colonies was quantified. All experiments were performed 3 times.
Statistical analysis
Data were analyzed using independent sample Student’s t test (p < 0.05 was considered significant; ns, no significant; *p < 0.05, **p < 0.01, ***p < 0.001). Statistical analysis was performed using SPSS 19.0 statistical Software (SPSS, Chicago, IL) and GraphPad Prism 8.0 (GraphPad Software, La Jolla, CA, USA). All data are expressed as mean standard error (SEM). All experiments were repeated at least 3 times.
Results
The expression of AQP3 in EBV-positive NPC cells is much lower than that in EBV-negative NPC cells
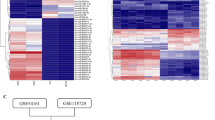
By analyzing the sequencing data of EBV-positive and EBV-negative NPC cell lines in GEO database, it was found that the expression of AQP3 in EBV-positive NPC cells was significantly lower than EBV-negative NPC cells (Fig. 1a). Therefore, to determine the expression and role of AQP3 in EBV-negative and EBV-positive nasopharyngeal carcinoma cells, we established EBV-infected nasopharyngeal carcinoma cell lines with CNE-2 cells and screened them with G418 until more than 90% of the cells showed green fluorescence under fluorescence microscopy (Fig. 1b), as described above [24]. Subsequently, western blot and qRT-PCR were used to analyze the expression levels of AQP3 in EBV-negative nasopharyngeal carcinoma cell line CNE-2 and EBV-positive nasopharyngeal carcinoma cell line CNE-2-EBV. The expression of AQP3 in EBV-positive nasopharyngeal carcinoma cells was significantly lower than that in EBV-negative nasopharyngeal carcinoma cells at both protein and mRNA levels (Fig. 1c, d).
Differential expression of AQP3 in EBV-negative and EBV-positive NPC cells a The expression of AQP3 in EBV-negative NPC cells CNE-2, SUNE-1 and EBV-positive NPC cells C666-1 was analyzed by bioinformatics. b EBV-infected CNE-2-EBV cell lines have been established, and green fluorescence is the signal that green fluorescent protein is excited under blue light when cells are successfully infected. c Results of AQP3 and LMP1 western blotting in CNE-2 and CNE-2-EBV cells. d The mRNA expression of AQP3 in CNE-2 and CNE-2-EBV cells was calculated by comparing the cycle threshold (Ct) value (2−ΔΔCt) and β-actin as the internal standard. All results represent three independent experiments. (***p < 0.001; **p < 0.01; *p < 0.05)
LMP1 down-regulates the expression of AQP3
To determine the cause of AQP3 downregulation caused by EBV infection, we overexpressed LMP1 and its control plasmid in EBV-negative NPC cells (CNE-1 and HONE-1), and established stable overexpressed cell lines (Fig. 2a). Meanwhile, Western blot and qRT-PCR were used to detect the expression of LMP1 and AQP3 at the protein level and transcription level (Fig. 2b-e). It was found that compared with the control group, the expression of AQP3 in CNE-LMP1 and HONE-LMP1 cells with stable overexpression of LMP1 was inhibited. In addition, after LMP1 knockdown in C666-1 cells, the expression of AQP3 protein level and mRNA level were increased (Fig. 2f, g). This suggests that LMP1 can inhibit AQP3 expression.
EBV encoding product LMP1 down-regulates the expression of AQP3. a Stable transfection efficiency of LMP1 plasmid by CNE-1 and HONE-1 cells. Green fluorescence is the signal that green fluorescent protein is excited under blue light when plasmid transfection is successful. b, c After overexpression of LMP1, AQP3 protein levels in CNE-1 and HONE 1 cells were detected by Western blot. d, e Compared the period threshold (Ct) value (2−ΔΔCt) and β-actin as internal standard to calculate the mRNA level of AQP3 in cells after overexpression of LMP1. The results were normalized using β-actin. f After interfering with LMP1, Western blot was used to detect the expression of AQP3 in C666-1. g The mRNA level of AQP3 in cells after interference with LMP1 was calculated using a comparative period threshold (Ct) value (2−ΔΔCt) and β-actin as the internal standard. All results represent three independent experiments. (***p < 0.001; **p < 0.01; *p < 0.05)
LMP1 regulates AQP3 expression through the ERK signaling pathway
In order to clarify the regulatory mechanism of LMP1 downregulation of AQP3, we detected the expression levels of ERK pathway related proteins ERK and p-ERK in CNE-LMP1 and HONE-LMP1. It was found that the protein expression level of p-ERK was significantly higher than that of the control group (Fig. 3a, b). In addition, after treating CNE-1 and HONE-1 with the ERK pathway inhibitor PD-0325901 at a concentration of 10 μM for 24 h, Western blot results showed that the ERK pathway was effectively inhibited and the expression of AQP3 was significantly increased (Fig. 3c, d). At the same time, the results showed that the expression of AQP3 increased with the decrease of ERK1/2 after knockdown ERK1 and ERK2 in CNE-1 and HONE-1 by siRNAs (Fig. 3e, f). After knocking down LMP1 with siRNA in C666-1, it was found that compared with the control group, the expression of AQP3 increased and the expression level of p-ERK protein decreased (Fig. 3g). After that, we also used siRNA to knock down ERK1 and ERK2 in CNE-1-LMP1 and HONE-1-LMP1 respectively in the case of LMP1 expression, and found that the expression of AQP3 was also elevated (Fig. 3h, i). These results suggest that the inhibitory effect of LMP1 on AQP3 is achieved by activating the ERK pathway.
LMP1 regulates AQP3 expression via the ERK pathway. a, b Protein levels of ERK and p-ERK in cells after overexpression of LMP1 detected by Western blot. c, d CNE-1 and HONE-1 were treated with the ERK pathway inhibitor PD-0325901 at 10 μM concentration for 24 h, and the changes of p-ERK/AQP3 protein levels were detected by Western blot. e, f The expression of AQP3 was detected by Western blot after interference of ERK1/2 with siERK1 and siERK2. g After interfering with LMP1, the expression of ERK and p-ERK in C666-1 was detected by Western blot. h, i In CNE-1-LMP1 and HONE-1-LMP1, the expression of AQP3 was detected by Western blot after interference of ERK1/2 with siERK1 and siERK2. The results were normalized using β-actin. All results represent three independent experiments. (***p < 0.001; **p < 0.01; *p < 0.05)
EBV infection affects the proliferation and migration of NPC cells
EBV infection plays an important role in the occurrence and development of NPC. Therefore, we detected the proliferation ability of CNE-2 and CNE2-EBV cells through CCK-8 proliferation experiment and colony formation experiment, and found that CNE2-EBV had stronger proliferation ability than CNE-2 (Fig. 4a,b). Meanwhile, Western blot analysis showed that the expressions of EMT-related genes vimentin and ZEB1 in CNE2-EBV were higher than those in CNE-2, while the expressions of snail and N-cadherin showed no significant differences (Fig. 4c). The transwell experiment and Wound healing experiments showed that the migration capacity of CNE-2-EBV was significantly higher than that of CNE-2 (Fig. 4d, e). Therefore, we believe that EBV infection can enhance the proliferation of NPC cells and promote the migration of NPC cells through EMT.
EBV promotes proliferation, EMT and migration of NPC cells. a, b The effect of EBV on cell proliferation was detected by CCK-8 and colony formation assay. c Western blot analysis of vimentin, ZEB1, snail and N-cadherin protein levels in CNE-2 and CNE2-EBV cells. The results were normalized using β-actin. (d, e) The effect of EBV on cell migration was evaluated by transwell migration test and wound healing test, and microscope images were obtained after 48 h (100 x). All results represent three separate experiments. (***p < 0.001; **p < 0.01; *p < 0.05)
AQP3 affects the migration of NPC cells
It has been reported that AQP3 can promote EMT in cells, so we used siRNA to interfere the expression of AQP3 in CNE-1 and HONE-1. Western blot analysis showed that inhibition of AQP3 expression resulted in decreased expression of EMT related proteins ZEB1, snail, vimentin and N-cadherin, while the expression of EMT-related proteins ZEB1, snail, vimentin, and N-cadherin was significantly increased after overexpression of AQP3 in EBV-positive NPC cells C666-1 (Fig. 5a, b, c).
AQP3 promotes EMT and migration of cells. a, b Western blot analysis of vimentin, ZEB1, N-cadherin, snail and AQP3 protein levels in CNE-1 and HONE-1 cells after interference with AQP3. The results were normalized using β-actin. c Western blot analysis of vimentin, ZEB1, N-cadherin, snail and AQP3 protein levels after AQP3 overexpression in C666-1. The results were normalized using β-actin. d, e The effect of AQP3 on cell migration was assessed using transwell migration tests and wound healing tests, and microscopic images were obtained after 48 h (100 x). All results represent three independent experiments. (***p < 0.001; **p < 0.01; *p < 0.05)
The corresponding transwell experiment showed that significantly fewer cells passed through the transwell chamber after knocking down AQP3 compared to the control group. Wound healing experiments also showed that cell migration ability was significantly reduced after interference with AQP3 (Fig. 5d, e).
AQP3 affects the growth of NPC cells
In addition, after the expression of AQP3 was knocked down, the proliferation capacities of the cells at 24 h, 48 h, 72 h and 96 h were analyzed by CCK-8 method and cell growth curves were plotted. Cells treated with AQP3 siRNA were found to have lower OD values at 72 h and 96 h compared to controls (Fig. 6a, b). Similarly, colony formation experiments were performed after knockdown of AQP3 to evaluate its effect on EBV-negative NPC cell growth. Fewer colonies were formed after knockdown of AQP3 compared to the control group. These results suggest that AQP3 can promote the proliferation of NPC cells (Fig. 6c, d).
AQP3 promotes lysis of EBV
Based on the biological characteristics of AQP3 and its expression in EBV-negative and EBV-positive NPC cells, we further investigated its influence on the latent and lytic state of EBV. After overexpression of AQP3 in C666-1, western blot results showed that the expression of lytic protein BZLF1 increased in C666-1-AQP3, while the expression of latent protein EBNA1 decreased (Fig. 7a). At the same time, the effects of AQP3 on EBNA1 and BZLF1 were detected by qRT-PCR at the transcription level, and the similar results were obtained (Fig. 7b). In addition, we also detected the DNA copy number of EBV encoding gene in C666-1-AQP3, and found that the DNA copy number of EBV increased significantly after overexpression of AQP3, indicating that AQP3 can promote the lytic replication of EBV (Fig. 7c).
AQP3 promotes lysis of EBV. a Western blot analysis of BZLF1 and EBNA1 protein levels after AQP3 overexpression. b The relative expressions of BZLF1 and EBNA1 after overexpression of AQP3 were calculated using the comparative period threshold (Ct) value (2−ΔΔCt) and β-actin as the internal standard. c Differences in DNA copy number of EBV after AQP3 overexpression of C666-1. All results represent three independent experiments. (***p < 0.001; **p < 0.01; *p < 0.05)
Discussion
Cancer poses a serious threat to human health and has become one of the leading causes of death worldwide [25]. As a multi-factorial disease, cancer has a complex pathogenesis, and its possible pathogenic factors include smoking, drinking, unhealthy eating habits, genome mutations, genetic factors and infectious agents [26]. Among them, EBV, as the first tumorigenic virus discovered, has been widely concerned about its carcinogenic effect on related tumors, but the detailed mechanism of EBV on tumorigenesis and development remains to be further studied. Our previous studies found that AQP3 expression was much lower in EBV-associated gastric cancer than in non-EBV-associated gastric cancer [27]. In this study, through bioinformatics analysis, we found that AQP3 expression in NPC was also significantly correlated with EBV infection. The expression of AQP3 in EBV-positive NPC cells is significantly lower than that in EBV-negative NPC, but the mechanism of regulation by EBV remains unclear. Wang et al. [23] reported that the low expression of AQP3 in EBVaGC was caused by hypermethylation of its promoter. However, in NPCs, we found that the low expression of AQP3 was not related to methylation (data not shown), but was regulated by the EBV-encoded product LMP1.
EBV, as the earliest tumor-associated virus, plays a very important role in the occurrence and development of tumors. Our study also proves that latent infection of EBV can promote the proliferation and migration of tumor cells. As a major oncogene encoded by EBV, LMP1 can activate a variety of signaling pathways including NF-κB, PI3K-AKT, ERK-MAPK, etc., and play a role in cell growth, invasion and EMT [28]. Kung et al. [29] proposed that LMP1 activates EGFR, STAT3 and ERK pathways under the action of PKCδ through its carboxy-terminal activation domain 1 (LMP1-CTAR1). LMP1 can promote epithelial cell migration and invasion through the ERK-MAPK pathway to realize its carcinogenic properties [30]. Extracellular vesicles containing LMP1 secreted by cells can activate the ERK, AKT and NF-κB signaling pathways and enhance the adhesion, proliferation, migration, and invasion of recipient cells [31].
In addition, studies have also shown that LMP1 can affect the occurrence and development of tumors by inhibiting the expression of certain oncogenes. In NPC, Du et al. [32] found that LMP1 and LMP2A could up-regulate miR-155, resulting in decreased expression of its target gene JMJD1A, and the decrease of JMJD1A was significantly correlated with lower 5 years overall survival and 5 years disease-free survival in NPC patients. These findings suggest that miR-155 and JMJD1A have potential as therapeutic targets for NPC. In EBVaGC, LMP1 up-regulates miR-146a to target and inhibit the expression of chemokine receptor 4 (CXCR4), thereby inhibiting the proliferation and migration of tumor cells and promoting the apoptosis of tumor cells [33]. In Burkitt lymphoma, LMP1 down-regulates the oncogene TCL1 via miR-29b, which may be the basis of its B-cell lymphoma growth antagonistic properties [34]. After infection with EBV in primary B cells, LMP1 mediates the activation of NF-κB and exerts an inhibitory effect on the TLR9 promoter, thereby inhibiting the expression of TLR9 in B cells to suppress the host immune response [35]. In conclusion, EBV, as an important tumorigenic virus, can promote the occurrence and development of related tumors, but its coding genes, such as LMP1, can target certain host oncogenes to achieve immune escape and maintain latent infection.
AQP3 is a aquaporin responsible for transporting water and other small molecules including glycerol and urea, and is also involved in a variety of signal transduction pathways in cancer cells [36]. Our study found that ERK can inhibit the expression of AQP3 in NPC cells, while previous studies in our lab have found that AQP3 can activate the ERK pathway in gastric cancer cells [27]. We hypothesize that there is a homeostasis between them, and it is this homeostasis that keeps the expression levels of AQP3 and ERK in NPC cells at reasonable levels. However, at the moment this is only a hypothesis and needs to be further proved by subsequent relevant studies. A large number of studies have reported that AQP3 plays a very important role in the occurrence and development of various cancers. In human urothelial carcinoma, the expression of AQP3 is related to the grade and stage of the tumor [37]. In pancreatic ductal adenocarcinoma, after silencing AQP3 and AQP5, cell membrane fluidity increases and cell migration ability decreases [38]. AQP3 can also promote cisplatin resistance in gastric cancer cells through autophagy [39]. In gastric cancer cells, c-Met promotes the expression of AQP3 through the ERK signaling pathway, and the migration and proliferation of gastric cancer cells are significantly inhibited after the knockdown of AQP3 expression [40]. At the same time, there are other studies of the link between other members of the aquaporin family and viruses. Sakurai et al. [41] found that HCV infection down-regulates the expression of miR-27b-targeted AQP11, which leads to a decrease in HCV genome copy number in cells. In addition, in human sperm, HPV infection can inhibit the expression and function of AQP8 and may promote the sensitivity of sperm cells to oxidative stress [42]. In this paper, we found that the migration ability and proliferation ability of cells were down-regulated after interference with AQP3.
EBV has a bidirectional life cycle of incubation and lysis [43], and after infecting B cells and epithelial cells of the host oropharynx, persistent infection is established in memory B cells. In epithelial cells, EBV often undergoes lytic replication, while in B cells, it can establish lifelong latent infection through occasional reactivation [44]. The presence of viruses and occasional reactivation can increase the risk of malignant transformation of cells [45]. It has been proved that all EBV-related malignancies are composed of latent cells, but due to the lack of suitable animal models, the role of lytic viral infection in EBV-positive tumors remains unclear [46]. If effective artificially induced lytic replication can be achieved, oncolytic therapy of EBV-associated tumors may be achieved, and the reactivation of EBV from latency depends on the expression of BZLF [47]. Some studies suggest that LMP1-mediated antiviral effects may contribute to the establishment or maintenance of EBV latency [48].
Our study found that after AQP3 was overexpressed in C666-1, the expression of BZLF1 was increased and the expression of EBNA1 was decreased, which promoted the NPC cells to enter the lytic phase. However, how AQP3 regulates EBV to enter the cleavage state still needs to be further elucidated. Our previous studies have shown that MUS81 expression in EBVaGC is much lower than in EBV-negative GC, and that overexpression of MUS81 in EBVaGC cells inhibits EBNA1 expression [49]. In lymphoma, KSHV infection upregulates the expression of PLK1, and lytic infection of KSHV is thus inhibited. Similar effects are also seen in EBV with similar latency and lytic replication [50]. Therefore, we suggest that this inhibition of viral lysis may be a potential tumorigenic mechanism of EBV.
Conclusion
This study revealed that AQP3 expression was significantly reduced in EBV-positive NPC cells compared with EBV-negative NPC cells, and LMP1 inhibited AQP3 expression by activating ERK signaling. At the same time, AQP3 can also promote the proliferation and migration of NPC cells. In addition, AQP3 can promote the expression of BZLF1 and enable EBV to enter the lysis phase. Therefore, EBV in NPC cell C666-1 with low expression of AQP3 maintains its latent state, which may be the possible cause of carcinogenesis of EBV in NPC.
Data availability
The data used to support the findings of this study are available from the corresponding author upon request.
References
Ayee R, Ofori MEO, Wright E, Quaye O (2020) Epstein Barr virus associated lymphomas and epithelia cancers in humans. J Cancer 11:1737–1750. https://doi.org/10.7150/jca.37282
Yin H, Qu J, Peng Q, Gan R (2019) Molecular mechanisms of EBV-driven cell cycle progression and oncogenesis. Med Microbiol Immunol 208:573–583. https://doi.org/10.1007/s00430-018-0570-1
Tsao SW, Tsang CM, Lo KW (2017) Epstein-Barr virus infection and nasopharyngeal carcinoma. Philos Trans R Soc B Biol Sci. https://doi.org/10.1098/rstb.2016.0270
Torre LA et al (2015) Global cancer statistics, 2012. CA Cancer J Clin 65:87–108. https://doi.org/10.3322/caac.21262
Brennan B (2006) Nasopharyngeal carcinoma. Orphanet J Rare Dis 1:23. https://doi.org/10.1186/1750-1172-1-23
Li W et al (2022) Immunotherapeutic approaches in EBV-associated nasopharyngeal carcinoma. Front Immunol. https://doi.org/10.3389/fimmu.2022.1079515
Choi SJ, Jung SW, Huh S, Cho H, Kang H (2018) Phylogenetic comparison of Epstein-Barr virus genomes. J Microbiol 56:525–533. https://doi.org/10.1007/s12275-018-8039-x
Wang LW, Jiang S, Gewurz BE (2017) Epstein-Barr virus LMP1-mediated oncogenicity. J Virol. https://doi.org/10.1128/JVI.01718-16
Wang L, Ning S (2021) New look of EBV LMP1 signaling landscape. Cancers (Basel). https://doi.org/10.3390/cancers13215451
Lo AK, Dawson CW, Lung HL, Wong KL, Young LS (2021) The role of EBV-encoded LMP1 in the NPC tumor microenvironment: from function to therapy. Front Oncol 11:640207. https://doi.org/10.3389/fonc.2021.640207
Lo AK et al (2015) Activation of the FGFR1 signalling pathway by the Epstein-Barr virus-encoded LMP1 promotes aerobic glycolysis and transformation of human nasopharyngeal epithelial cells. J Pathol 237:238–248. https://doi.org/10.1002/path.4575
Ye D et al (2020) LMP1 Up-regulates calreticulin to induce epithelial-mesenchymal transition via TGF-beta/Smad3/NRP1 pathway in nasopharyngeal carcinoma cells. J Cancer 11:1257–1269. https://doi.org/10.7150/jca.37415
Davis AM, Rapley A, Dawson CW, Young LS, Morris MA (2021) The EBV-encoded oncoprotein, LMP1, recruits and transforms fibroblasts via an ERK-MAPK-dependent mechanism. Pathogens. https://doi.org/10.3390/pathogens10080982
Verkman AS, Anderson MO, Papadopoulos MC (2014) Aquaporins: important but elusive drug targets. Nat Rev Drug Discov 13:259–277. https://doi.org/10.1038/nrd4226
Login FH, Nejsum LN (2023) Aquaporin water channels: roles beyond renal water handling. Nat Rev Nephrol 19:604–618. https://doi.org/10.1038/s41581-023-00734-9
Liu YL et al (2007) Expression of aquaporin 3 (AQP3) in normal and neoplastic lung tissues. Hum Pathol 38:171–178. https://doi.org/10.1016/j.humpath.2006.07.015
Verkman AS (2005) More than just water channels: unexpected cellular roles of aquaporins. J Cell Sci 118:3225–3232. https://doi.org/10.1242/jcs.02519
Verkman AS (2012) Aquaporins in clinical medicine. Annu Rev Med 63:303–316. https://doi.org/10.1146/annurev-med-043010-193843
Hara-Chikuma M, Verkman AS (2008) Aquaporin-3 facilitates epidermal cell migration and proliferation during wound healing. J Mol Med (Berl) 86:221–231. https://doi.org/10.1007/s00109-007-0272-4
Kusayama M et al (2011) Critical role of aquaporin 3 on growth of human esophageal and oral squamous cell carcinoma. Cancer Sci 102:1128–1136. https://doi.org/10.1111/j.1349-7006.2011.01927.x
Chen J et al (2014) Aquaporin 3 promotes epithelial-mesenchymal transition in gastric cancer. J Exp Clin Cancer Res 33:38. https://doi.org/10.1186/1756-9966-33-38
Huang X, Huang L, Shao M (2017) Aquaporin 3 facilitates tumor growth in pancreatic cancer by modulating mTOR signaling. Biochem Biophys Res Commun 486:1097–1102. https://doi.org/10.1016/j.bbrc.2017.03.168
Wang J et al (2019) LMP2A induces DNA methylation and expression repression of AQP3 in EBV-associated gastric carcinoma. Virology 534:87–95. https://doi.org/10.1016/j.virol.2019.06.006
Yue W et al (2019) Early pattern of Epstein-Barr virus infection in gastric epithelial cells by “cell-in-cell.” Virol Sin 34:253–261. https://doi.org/10.1007/s12250-019-00097-1
Siegel RL, Miller KD, Fuchs HE, Jemal A (2021) Cancer Statistics, 2021. CA Cancer J Clin 71:7–33. https://doi.org/10.3322/caac.21654
Maomao C et al (2022) Current cancer burden in China: epidemiology, etiology, and prevention. Cancer Biol Med 19:1121–1138. https://doi.org/10.20892/j.issn.2095-3941.2022.0231
Yu C et al (2022) Molecular mechanism of aquaporin 3 (AQP3) regulating by LMP2A and its crosstalk with 4E-BP1 via ERK signaling pathway in EBV-associated gastric cancer. Virus Res 322:198947. https://doi.org/10.1016/j.virusres.2022.198947
Lo AK, Lo KW, Ko CW, Young LS, Dawson CW (2013) Inhibition of the LKB1-AMPK pathway by the Epstein-Barr virus-encoded LMP1 promotes proliferation and transformation of human nasopharyngeal epithelial cells. J Pathol 230:336–346. https://doi.org/10.1002/path.4201
Kung CP, Meckes DG Jr, Raab-Traub N (2011) Epstein-Barr virus LMP1 activates EGFR, STAT3, and ERK through effects on PKCdelta. J Virol 85:4399–4408. https://doi.org/10.1128/JVI.01703-10
Dawson CW, Laverick L, Morris MA, Tramoutanis G, Young LS (2008) Epstein-Barr virus-encoded LMP1 regulates epithelial cell motility and invasion via the ERK-MAPK pathway. J Virol 82:3654–3664. https://doi.org/10.1128/JVI.01888-07
Nkosi D, Sun L, Duke LC, Meckes DG Jr (2020) Epstein-Barr virus LMP1 manipulates the content and functions of extracellular vesicles to enhance metastatic potential of recipient cells. PLoS Pathog 16:e1009023. https://doi.org/10.1371/journal.ppat.1009023
Du ZM et al (2011) Upregulation of MiR-155 in nasopharyngeal carcinoma is partly driven by LMP1 and LMP2A and downregulates a negative prognostic marker JMJD1A. PLoS ONE 6:e19137. https://doi.org/10.1371/journal.pone.0019137
Wang W et al (2019) LMP1-miR-146a-CXCR4 axis regulates cell proliferation, apoptosis and metastasis. Virus Res 270:197654. https://doi.org/10.1016/j.virusres.2019.197654
Anastasiadou E et al (2010) Epstein-Barr virus encoded LMP1 downregulates TCL1 oncogene through miR-29b. Oncogene 29:1316–1328. https://doi.org/10.1038/onc.2009.439
Fathallah I et al (2010) EBV latent membrane protein 1 is a negative regulator of TLR9. J Immunol 185:6439–6447. https://doi.org/10.4049/jimmunol.0903459
Chen L et al (2017) Silencing of AQP3 induces apoptosis of gastric cancer cells via downregulation of glycerol intake and downstream inhibition of lipogenesis and autophagy. Onco Targets Ther 10:2791–2804. https://doi.org/10.2147/OTT.S134016
Rubenwolf PC et al (2014) Expression of aquaporin water channels in human urothelial carcinoma: correlation of AQP3 expression with tumour grade and stage. World J Urol 32:991–997. https://doi.org/10.1007/s00345-013-1153-9
Silva PM et al (2022) Aquaporin-3 and aquaporin-5 facilitate migration and cell–cell adhesion in pancreatic cancer by modulating cell biomechanical properties. Cells. https://doi.org/10.3390/cells11081308
Dong X et al (2016) Aquaporin 3 facilitates chemoresistance in gastric cancer cells to cisplatin via autophagy. Cell Death Discov 2:16087. https://doi.org/10.1038/cddiscovery.2016.87
Wang J et al (2012) c-Met upregulates aquaporin 3 expression in human gastric carcinoma cells via the ERK signalling pathway. Cancer Lett 319:109–117. https://doi.org/10.1016/j.canlet.2011.12.040
Sakurai F et al (2019) miR-27b-mediated suppression of aquaporin-11 expression in hepatocytes reduces HCV genomic RNA levels but not viral titers. Virol J 16:58. https://doi.org/10.1186/s12985-019-1160-6
Pellavio G et al (2020) HPV infection affects human sperm functionality by inhibition of aquaporin-8. Cells. https://doi.org/10.3390/cells9051241
Houen G, Trier NH (2020) Epstein-Barr Virus and systemic autoimmune diseases. Front Immunol 11:587380. https://doi.org/10.3389/fimmu.2020.587380
Zhang Q, Xu M (2023) EBV-induced T-cell responses in EBV-specific and nonspecific cancers. Front Immunol 14:1250946. https://doi.org/10.3389/fimmu.2023.1250946
Chakravorty S, Afzali B, Kazemian M (2022) EBV-associated diseases: current therapeutics and emerging technologies. Front Immunol 13:1059133. https://doi.org/10.3389/fimmu.2022.1059133
Kenney SC, Mertz JE (2014) Regulation of the latent-lytic switch in Epstein-Barr virus. Semin Cancer Biol 26:60–68. https://doi.org/10.1016/j.semcancer.2014.01.002
Murata T, Tsurumi T (2014) Switching of EBV cycles between latent and lytic states. Rev Med Virol 24:142–153. https://doi.org/10.1002/rmv.1780
Zhang J, Das SC, Kotalik C, Pattnaik AK, Zhang L (2004) The latent membrane protein 1 of Epstein-Barr virus establishes an antiviral state via induction of interferon-stimulated genes. J Biol Chem 279:46335–46342. https://doi.org/10.1074/jbc.M403966200
Zhang Y et al (2023) Downregulation of MUS81 expression inhibits cell migration and maintains EBV latent infection through miR-BART9-5p in EBV-associated gastric cancer. J Med Virol 95:e28725. https://doi.org/10.1002/jmv.28725
Biswas A et al (2021) Inhibition of polo-like kinase 1 (PLK1) facilitates reactivation of gamma-herpesviruses and their elimination. PLoS Pathog 17:e1009764. https://doi.org/10.1371/journal.ppat.1009764
Acknowledgements
This work is supported by the National Natural Science Foundation of China (32370177); Natural Science Foundation of Shandong Province (ZR2020MH302 and ZR2020MC020).
Funding
Natural Science Foundation of Shandong Province, ZR2020MC020, ZR2020MH302, National Natural Science Foundation of China, 32370177.
Author information
Authors and Affiliations
Contributions
JL, DS, ZG, WL, YZ, and BL designed research; JL performed research; JL and YZ analyzed data; JL prepared original draft; YZ and BL reviewed and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Edited by Erik Karlsson.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, J., Shi, D., Gong, Z. et al. Aquaporin-3 is down-regulated by LMP1 in nasopharyngeal carcinoma cells to regulate cell migration and affect EBV latent infection. Virus Genes 60, 488–500 (2024). https://doi.org/10.1007/s11262-024-02096-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11262-024-02096-1