Abstract
Epstein-Barr virus (EBV) is an important human dsDNA virus, which has been shown to be associated with several malignancies including about 10% of gastric carcinomas. How EBV enters an epithelial cell has been an interesting project for investigation. “Cell-in-cell” infection was recently reported an efficient way for the entry of EBV into nasopharynx epithelial cells. The present approach was to explore the feasibility of this mode for EBV infection in gastric epithelial cells and the dynamic change of host inflammatory reaction. The EBV-positive lymphoblastic cells of Akata containing a GFP tag in the viral genome were co-cultured with the gastric epithelial cells (GES-1). The infection situation was observed under fluorescence and electron microscopies. Real-time quantitative PCR and Western-blotting assay were employed to detect the expression of a few specific cytokines and inflammatory factors. The results demonstrated that EBV could get into gastric epithelial cells by “cell-in-cell” infection but not fully successful due to the host fighting. IL-1β, IL-6 and IL-8 played prominent roles in the cellular response to the infection. The activation of NF-κB and HSP70 was also required for the host antiviral response. The results imply that the gastric epithelial cells could powerfully resist the virus invader via cell-in-cell at the early stage through inflammatory and innate immune responses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Epstein-Barr virus (EBV) is the first confirmed human tumor virus, which is a member of γ herpesviruses. More than 90% of the population are infected with EBV (Henle et al. 1969). In most of the EBV-associated tumors, the virus exists in a latent infection condition (Lu et al. 2010). The genome exists in the state of free-body DNA and only a limited portion of genes are expressed without the production of virus particle (Tsurumi et al. 2005). EBV is mainly associated with lymphatic and epithelial malignancies such as Burkitt’s lymphoma (BL), Hodgkin’s disease (HD), T cell lymphoma, nasopharyngeal carcinoma (NPC) and Epstein-Barr virus-associated gastric carcinoma (EBVaGC) (Morales-Sanchez and Fuentes-Panana 2014). Gastric cancer is one of the top threats to human health among malignancies. As a unique feature subtype of gastric cancer, EBVaGC takes about 10% of gastric cancer worldwide (Shinozaki-Ushiku et al. 2015). EBV may play a role in causing abnormal changes in epithelial cells, thus ultimately leading to cell carcinogenesis in EBVaGC (Li et al. 2014).
In vitro, EBV can not effectively infect epithelial cells because this kind of cells lacks the classical receptor of CR2/CD21 as that on the surface of B lymphocytes that interacts with EBV envelope glycoprotein gp350 (Nemerow et al. 1987, 1989). This is also an important reason for the lack of suitable epithelial cell models to study the interaction between EBV and epithelial cells. In a recent report, an efficient “cell-in-cell” infection way in which EBV can infect nasopharynx or NPC epithelial cells has been discovered (Ni et al. 2015). The Akata cell line used in the approach was latently infected with recombinant EBV containing a neomycin resistance gene and an eGFP gene (Cohen et al. 2011; Takada et al. 1991). The Akata cells and the epithelial cells were co-cultured for a period of time, and then Akata cells were nearly able to fuse together with the epithelial cells through the cell membrane and formed “cell-in-cell” structures (Nemerow et al. 1987; Ni et al. 2015). EBV can be released into the recipient epithelial cells after the entry of EBV-harboring B-lymphocytes cells. The present approach aimed to evaluate the “cell-in-cell” infection method which possibly allows EBV to enter gastric mucosal epithelial cells, since the technique suggested a possible new method to establish EBV-positive epithelial cell lines independent of the CR2 receptor. While in the repeated attempts, we found it was not easy to do. Selecting with G418 failed to obtain stable EBV-positive cell lines. This implied that the cells might confer strong defense to the Akata-EBV, leading to the detection of a few pro-inflammatory cytokines.
Materials and Methods
Cell lines and Cell Culture
The GES-1 cell line is derived from normal gastric mucosal epithelial cells. The BL-derived Akata cell line is EBV-producing with a GFP-tagged viral genome (Cohen et al. 2011; Takada et al. 1991). The cell lines were routinely maintained in RPMI 1640 medium (Gibco, USA) supplemented with 10% of Fetal Bovine Serum. For the culture of Akata cells, 750 μg/mL of G418 (Sangon, China) was added to the medium.
Cell-in-Cell Infection of EBV in GES-1 Cells
When more than 80% of the EBV-positive Akata cells showed GFP expression by using G418 selection, they were co-cultured with GES-1 cells in 6-well plates at a ratio of 10:1. EBV-negative Akata cells were used for a control. The Akata cells were washed at 24 h post-infection and then used for further analyses. The GES-1 cells were observed for GFP expression under a fluorescence microscope (Olympus BX60). The GES-1 cells co-cultured with EBV-positive Akata cells were selected by using 40–400 μg/mL of G418.
In Situ Hybridization (ISH)
ISH was performed to investigate EBER expression. The paraffin-embedded gastric cancer samples were collected from Xiangya Hospital. Procedures for the EBER ISH of tissues from GC patients have been reported previously (Lu et al. 2010), followed the instructions for Sensitive Enhanced In Situ Hybridization Assay Kit I (BOSTER, China). In brief, deparaffinized sections were pretreated with proteinase K and incubated with a DIG-conjugated EBER PNA probe (Sangon Biotech, China), followed by incubation with HRP-conjugated antibody. Images were taken with an Olympus fluorescence microscope. Figures were constructed using Adobe Photoshop software.
Transmission Electronic Microscopy (TEM)
For TEM observation, GES-1 cells were fixed in 0.1 mol/L PBS (pH7.4) containing 2.5% glutaraldehyde at 4 °C for 2 h and post-fixed in 0.1% PBS with 1% osmium tetroxide. By dehydrating through gradient ethanol solutions, the specimens were embedded in Epon-812 (Fluca, Switzerland). Ultrathin sections were prepared with an ultramicrotome (Leica, Germany). Pale-gold sections were collected on 200-mesh copper grids. Ultrathin sections were stained with 3% uranyl acetate and lead citrate and examined with transmission electron microscope (Hitachi, H7700).
Immunofluorescence Assay
GES-1 cells were seeded on sterile, acid-treated 20-mm coverslips in 6-well plates and incubated with EBV-positive Akata cells. The coverslips were collected by fixation with 4% PFA for 30 min at room temperature. After washing with PBS, cells were permeabilized in PBS containing 0.1% Triton X-100 for 5 min and then washed three times with PBS. Non-specific binding was blocked by treatment with PBS containing normal goat serum (BOSTER, China) for 1 h. After the removal of blocking buffer, the cells were incubated with rabbit anti-human E-cadherin (CST, USA), mouse anti-human CD20 (Proteintech, China) primary antibodies diluted in PBS containing 1% BSA for 1 h and washed three times with PBS, and then respectively incubated with Alexa Fluor 488 Donkey anti-Rabbit IgG (H + L) and Alexa Fluor 594 goat anti-mouse IgG (H + L) secondary antibodies (Life Technologies, USA) for 1 h at room temperature. DAPI dye (Invitrogen, USA) was added before the observation to localize the nucleus. Images were taken with a confocal laser scanning microscope (Leica, TCS SP8 X&MP, Germany).
Western Blotting
Cells were collected at different time points after the co-culture, lysed in RIPA buffer (cwbiotech, China) for 1 h at 4 °C and centrifuged at 12,000 rpm at 4 °C for 20 min. Protein concentrations were determined by BCA assay (Vazyme Biotech, China). Proteins (50 μg) were separated by 10% SDS-PAGE and electrotransferred to a polyvinylidene fluoride (PVDF) membranes. Membranes were blocked with 5% milk in 0.01 mol/L Tris-buffered saline containing 0.1% Tween-20 (PBST) for 1 h at room temperature. The membrane was incubated with the primary antibody at 4 °C overnight. After a wash of three times with tris-buffered saline containing 0.1% Tween 20 (TBST) (15 min/per), the membrane was incubated with an HRP-conjugated secondary antibody (anti-mouse or anti-rabbit IgG) at 37 °C for 1 h. Protein bands were visualized by enhanced chemiluminescence reagent (Pierce Protein Biology, USA), and analyzed with a Gel Doc XR System. The primary antibodies were: anti- EBNA1 (Santa Cruz, USA), LMP1 (DAKo, The Kingdom of Denmark), HSP70 (CST, USA), IL-1β (Cusabio,China), Phospho-NF-κB (p65) (CST, USA) and GAPDH (internal control) (Proteintech Group, USA).
RT and Real-Time qPCR
Total RNA was extracted from the collected cells and reversely transcribed into cDNA (Thermo Scientific RevertAid First Strand cDNA Synthesis Kit, #K1622; Thermo Fisher Scientific, USA). The cDNA was then subject to real-time quantitative PCR (qPCR) detection at the mRNA expression levels (TransStart Tip Green qPCR SuperMix, #AQ141-02; TransGen Biotech, China). The qPCR assay was performed by using specific primers targeting IL-1β, IL-6, IL-8, TNF-α or β-actin (internal control) as listed in Supplementary Table S1. The qPCR cycling conditions were: 10 min at 95 °C for one cycle, followed by 45 cycles at 95 °C for 15 s, 60 °C for 60 s. Dissociation curves were used to confirm that the amplicon signals were unique. Expression levels were normalized to the actin mRNA level, which was obtained through parallel assays. Three independent experiments were performed for each mRNA detection, and the mean values were used for the analyses.
Statistical Analysis
Data were presented as mean ± SEM. Statistical analyses were performed using the unpaired two-tailed Student’s t test of the GraphPad Prism 5 software (GraphPad Software, USA). Values of P < 0.05 were considered as statistically significant. All experiments were repeated at least three times.
Results
Detection of EBV Infection in GC Tissues and Readout in Gastric Epithelial Cells
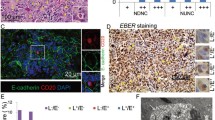
The presence of EBV in gastric carcinoma (GC) tissues was confirmed by in situ hybridization experiments to detect EBV-encoded EBER-1 (Fig. 1A). In EBV positive tissues, EBER-1 was expressed in the nucleus. The cell-in-cell structures could be observed in the tissues (Fig. 1A). We tried to simulate the in vitro infection of EBV by “cell-in-cell” way, GES-1 cells were firstly incubated with Akata cells as described. The GES-1 cells could be observed with green fluorescence around the cell membrane as in Fig. 1B after 2 days of the infection. This phenomenon could sustain for a few days till the cells grew to 100% confluence or even after several generations of culture. If G418 of low concentration was added to the media for a selection at this stage, the cells might be killed completely.
The detection of EBV infection in GC tissues and the GES-1 cell. (A) EBV genome detection in GC specimens by EBER-1 in situ hybridization (ISH) (magnification, 400 ×). Two cases of tissues showed to be EBV-positive (EBV +) and EBV-negative (EBV-) respectively. The cell-in-cell structures are indicated by yellow arrows. A magnified image is showed at the upper left corner. (B) The GFP expression in GES-1 cells post-infection of “cell-in-cell”. The fluorescence was observed at 48 h post-infection under a fluorescence microscope.
Detection of EBV Infection by “cell-in-cell”
In order to observe whether EBV-harboring Akata cells entered the GES-1 and released the virus, the GES-1 cells with “green membranes” were detected under an electron microscope. As showed in Fig. 2A, the Akata cells are gathered beneath the cell membrane with the emergence of large amounts of vacuole structures. Some Akata cells seemed to have broken membranes with a trend of releasing virus particles. These cell-in-cell structures were characterized by the appearance of CD20+ B cells (EBV-positive Akata cells) co-localizing within GES-1 cells based on immunofluorescence staining being observed under a confocal microscope (Fig. 2B).
EBV observation and detection in gastric epithelial cell co-culture with EBV positive Akata cell. (A) The observation of Akata-EBV infection in GES-1 cells by electronic microscopy. (a) EBV-bearing Akata cells penetrated into GES-1. (b) Viruses were released from Akata into the cytoplasm of GES-1. N represents the nucleus, C represents the cytoplasm and red arrows indicate EBV-containing Akata cells. (B) Detection of EBV-positive Akata cells in GES-1 cells by using immunofluorecence assay. CD20 antibody was used for the detection indicating the membrane of Akata cells (red). E-cadherin staining (green) indicates the cell outline, and DAPI staining (blue) indicates the nucleus. A confocal microscope was used for the observation and image-taking. Scale bar, 10 μm.
The Expression of EBV-Encoded Proteins in GES-1 with “cell-in-cell” Infection
In order to further ensure the entry of EBV-positive Akata, the EBV-encoded EBNA1 and LMP1 proteins were detected in GES-1 with cell-in-cell infection. The result showed that both EBNA1 and LMP1 were expressed in the GES-1 cells after co-cultured with EBV-positive Akata cells (Fig. 3).
The Dynamic Expression of Pro-Inflammatory Cytokines at mRNA Levels
The observations in the infection suggested that EBV-harboring Akata cells could not finish the infection process. This reminded a strong defense from host cells existed. Since innate immunity is the first line of host defense, real-time qPCR was performed to detect cytokines’ responses in the GES-1 cells. The inflammatory factors of IL-1β, IL-6 and IL-8 in the infected cells were changed greatly and rapidly as shown in Fig. 4. In comparison, the control with co-culture of EBV-negative Akata cells could give rise to a much milder increase or no change of these inflammatory factors. While they decreased to a normal level after 3–4 days post-infection. TNF-α did not have a significant change during the infection.
The dynamic expression of inflammatory factors in co-cultured GES-1 cells detected by qPCR. The expression of IL-1β, IL-6, IL-8 and TNF-α were detected, showing by the mean ± SD for three independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001; ns, no significance (P > 0.05). All the unlabelled comparisions also showed ns (P > 0.05).
The Dynamic Expression of Inflammatory Factors at Protein Levels
Based on the changes of inflammatory factors at mRNA levels, we further determined the expression of IL-1β, as well as HSP70 and NF-κB (p65) using Western blotting assay. HSP70 is a stress-associated protein, and NF-κB is an immune-associated transcriptional factor. The phosphorylation of p65 represents the activation of NF-κB. As shown in Fig. 5A, the protein expression of IL-1β initially increased at 24 h post-infection for EBV-positive Akata and mantained at high levels. Both HSP70 and p-p65 showed increased expressions at 1–3 days post-infection (Fig. 5B). Regarding the protein expression of IL-1β, HSP70 and p-p65, the infected cells had a milder response to EBV-negative Akata than that of EBV-positive Akata infection.
Discussion
Epstein-Barr virus (EBV) is a γ herpesvirus with a genome size of 172 kb. EBV infects more than 90% of human populations and establishes a lifelong latent infection in the host (Murata and Tsurumi 2014; Yu et al. 2011). Studies have revealed that EBV is associated with a variety of human malignancies of lymphatic and epithelial origins (Liu et al. 2017; Young and Rickinson 2004; Yu et al. 2012; Zuo et al. 2017a; Tu et al. 2017), and in recent years it has been found to be related with about 10% of gastric cancer. EBVaGC, as a subtype of gastric cancer, has its unique molecular and clinicopathological features (Ding et al. 2013; Hu et al. 2015). In vitro, EBV virions are able to effectively infect B lymphocytes but are difficult to infect epithelial cells (ECs) due to the lack of EBV envelope glycoprotein gp350 receptor CR2 on the surface of epithelial cells (Nemerow et al. 1989; Zheng et al. 2014). On the other hand, epithelial cells usually form the stratified structure in vivo. Thus it is difficult to simulate a similar environment for EBV infection in vitro (Temple et al. 2014). Recently, a novel factor, EphA2 (Tyrosine-Protein Kinase Receptor ECK) has been reported as a critical player for EBV epithelial cells entry (Chen et al. 2018; Zhang et al. 2018). This should be helpful in the establishment of EBV-infected epithelial cell models.
To establish suitable EC models for the study of mechanisms related to EBV infection, we tried several methods in CR2-independent manners according to reports. “Cell-to-cell” has been a well-documented way for EBV infection through B lymphocyte-mediated transferring in vivo and in vitro (Bornkamm et al. 2006; Shannon-Lowe et al. 2006). Whereas, it seemed not efficient to be used for the establishment of a stable EBV-infected nasopharyngeal carcinoma (NPC) cell line as in our trials (data not shown). Nevertheless, the cell-to-cell infection has given one reasonable explanation of EBV infection into non-susceptible ECs. The in-cell infection by forming “cell-in-cell” structures is a newly reported method, which was used for EBV infection into the NPC cell line, CNE2 (Ni et al. 2015). According to the report, EBV-bearing Akata cells occur to be non-cytotoxic cells and are activated rapidly after entering into ECs without any treatment. Compared with cell-to-cell infection, the EBV tropism in “cell-in-cell” infection rarely relies on the conjugate formation between host cells and the virus, and the transmission occurs more fleetly happened after 6–24 h as reported (Ni et al. 2015). To accomplish the transmission of viruses, the internalized EBV-loaded Akata cells always died with lysosome-dependent pathway (entosis) (Ni et al. 2015; Overholtzer et al. 2007). There are also a lot of vesicles in host cells caused by invading Akata cells in our approach (Fig. 2A). Our original intention was to establish an EBV-infected gastric epithelial cell line (GES-1) for future study. However, a stable cell line was not easy to obtain successfully in our trial because the cells could not undergo a selection with G418. During the early cell-in-cell infection, GFP-EBV or GFP-Akata were observed to be maintained around the membranes (Fig. 1). The same phenomenon was also observed in the NPC cell line of 6-10B (data not shown). If G418 was used for a selection at this stage, the cells hardly survived. The detection of CD20, an indicator of the membrane of Akata cells (Ni et al. 2015), validated the formation of cell-in-cell structure observed by using a confocal microscope (Fig. 2B). EBNA1 and LMP1 are the latent proteins of EBV (Ding et al. 2007; Lu et al. 2017; Tao et al. 2015). In our result, EBNA1 and LMP1 were detected to be expressed in co-cultured GES-1 cells with EBV-positive Akata, indicating the existence of EBV into the cells mediated by the “cell-in-cell” infection (Fig. 3). The result is consistent with that of cell-in-cell infection in NPC cells (Ni et al. 2015). Since the EBV particles harboring in Akata cells could be released to the epithelial cells, the expression pattern of EBV genes seemed similar to that in the infection of direct virus particle.
The sustaining of GFP-EBV at the EC membrane suggested that a strong resistance existed in the host cells in response to the infection, leading to our further detection of host cytokines or inflammatory factors. The result demonstrated that interleukin 1β (IL-1β) was greatly increased at both mRNA and protein levels. The result was consistent with that of EBV infection in gastric carcinoma (Chong et al. 2002). IL-1β also closely related to Helicobacter pylori (H. pylori) infection, and thus increasing the risk of gastric mucosal injury and gastric cancer (Chakravorty et al. 2004; Queiroz et al. 2013). More importantly, IL-1β is an effector at the downstream of IFN-dependent anti-virus innate immune pathways (Ludigs et al. 2012; Yao et al. 2016). IL-1β was reported to increase the cell growth of the GC cell line, TMK1. EBV-associated gastric carcinoma specifically produces IL-1β in vivo. Thus, IL-1β may act as an autocrine growth factor in EBVaGC (Chong et al. 2002). It is reported that overexpressed IL-1β inhibit gastric acid secretion during H. pylori and EBV infection (Matsusaka et al. 2014). Therefore, our result implied the strong host immune response to the virus invasion. Interleukin 6 (IL-6) may act as a pro-inflammatory or anti-inflammatory cytokine. It plays a role in immune response, hematopoiesis, platelet production, acute phase reaction and tumor development (Chen et al. 2015; Zhu et al. 2012). In GC, IL-6 can affect tumor progression via STAT3/NF-κB pathway (Yin et al. 2013; Zhao et al. 2016). In NPC, activated IL-6/STAT3 signaling may promote EBV-infected premalignant nasopharyngeal epithelial cells into cancer cells, and strengthen the malignant performances of NPC cells (Zhang et al. 2013). Interleukin 8 (IL-8) belongs to the CXC chemokine family, also a neutrophil chemotactic factor (Chakravorty et al. 2004). In EBV associated disease, EBV may increase the recruitment of lymphocytes to sites of infection by inducing IL-8 release (McColl et al. 1997). The plasma of EBV load is positively related to IL-8 levels, which may be promising markers for the presence and progression of EBV associated disease (Savitri and Haryana 2015). IL-8 can be also stimulated by IL-1β in GC cells (Hwang et al. 2004; Lian et al. 2016). In our results, the expression pattern of IL-8 was substantially consistent with that of IL-1β, suggesting a relationship of them in EBV infection (Dehne et al. 2013; Pereira et al. 2014). TNF-α is able to induce inflammation and to inhibit tumorigenesis and viral replication by regulated immune cells. Imbalance of TNF-α is associated with the development of hepatocellular carcinoma, breast cancer and gastric cancer (McIlwain et al. 2013). EBV can evade the antiviral inflammatory response by inhibiting TNF-α (Li et al. 2016). Overall, the induction of these inflammatory cytokines could be a response of host cells to the EBV infection and might subsequently help cancer progression, as well as the viral infection under some circumtances. Apart from these genes, in our future work, we would examine other genes such as interferon (IFN) and IFN-stimulated genes (ISGs), which are also associated with host anti-virus response.
HSP70 is an influential member of the heat shock protein family, which functions to resist various stressors and regulate cell growth and apoptosis. The increase of HSP70 can keep cancer cells from apoptosis (Arora et al. 2017). There is reported that high HSP70 levels in gastric tumors is associated with poor overall survival in intestinal type of gastric cancer patients (Lee et al. 2013). The expression level of HSP70 is low in normal cells and can be significantly increased in stress states such as hyperthermia, hypoxia and infection (Choi et al. 2009). NF-κB is known to play a key role in regulating the immune response to infection and cancer. Moreover, NF-κB can regulate the release of IL-1β, IL-6, IL-8 and TNF-α. Abnormal activation of NF-κB promotes gastric carcinogenesis (Sokolova and Naumann 2017). And EBV is able to regulate NF-κB signaling to establish latent infection in NPC (Tsao and Tsang 2017; Zuo et al. 2017b). The activation of NF-κB needs the phosphorylation and nucleus entry. Activated NF-κB can regulate gene transcription, promoting tumorigenesis (Gambhir et al. 2015). In this approach, HSP70 and phosphorylated NF-κB were up-regulated as other cytokines did at the early stage of infection, indicating a sustained host inflammatory responses. The results implied that the activation of NF-κB and HSP70 pathways were required for the host responses to the viral invasion.
Taken together, the results revealed that host cells could make a rapid response at the early stages of EBV infection by “cell-in-cell”. The activation of NF-κB pathway and HSP70 pathways was also required for the host immune response. These observations demonstrated that a coordinated mobilization of several cellular inflammation and immune processes participated the first line of defense. This is also a reason for the problem in the establishment of EBV cell lines by this method. To establish a latent infection, EBV would have to overcome the defense of these host factors.
References
Arora N, Alsaied O, Dauer P, Majumder K, Modi S, Giri B, Dudeja V, Banerjee S, Von Hoff D, Saluja A (2017) Downregulation of sp1 by minnelide leads to decrease in hsp70 and decrease in tumor burden of gastric cancer. PLoS ONE 12:e0171827
Bornkamm GW, Behrends U, Mautner J (2006) The infectious kiss: newly infected b cells deliver epstein-barr virus to epithelial cells. Proc Natl Acad Sci USA 103:7201–7202
Chakravorty M, Ghosh A, Choudhury A, Santra A, Hembrum J, Roychoudhury S (2004) Ethnic differences in allele distribution for the il8 and il1b genes in populations from eastern india. Hum Biol 76:153–159
Chen L, Yuan W, Chen Z, Wu S, Ge J, Chen J, Chen Z (2015) Vasoactive intestinal peptide represses activation of tumor-associated macrophages in gastric cancer via regulation of tnfalpha, il-6, il-12 and inos. Int J Oncol 47:1361–1370
Chen J, Sathiyamoorthy K, Zhang X, Schaller S, Perez White BE, Jardetzky TS, Longnecker R (2018) Ephrin receptor a2 is a functional entry receptor for epstein-barr virus. Nat Microbiol 3:172–180
Choi SR, Lee SA, Kim YJ, Ok CY, Lee HJ, Hahm KB (2009) Role of heat shock proteins in gastric inflammation and ulcer healing. J Physiol Pharmacol 60:5–17
Chong JM, Sakuma K, Sudo M, Osawa T, Ohara E, Uozaki H, Shibahara J, Kuroiwa K, Tominaga S, Hippo Y, Aburatani H, Funata N, Fukayama M (2002) Interleukin-1beta expression in human gastric carcinoma with epstein-barr virus infection. J Virol 76:6825–6831
Cohen JI, Fauci AS, Varmus H, Nabel GJ (2011) Epstein-barr virus: an important vaccine target for cancer prevention. Sci Transl Med 3: 107fs107
Dehne N, Fuhrmann D, Brune B (2013) Hypoxia-inducible factor (hif) in hormone signaling during health and disease. Cardiovasc Hematol Agents Med Chem 11:125–135
Ding L, Li L, Yang J, Zhou S, Li W, Tang M, Shi Y, Yi W, Cao Y (2007) Latent membrane protein 1 encoded by epstein-barr virus induces telomerase activity via p16ink4a/rb/e2f1 and jnk signaling pathways. J Med Virol 79:1153–1163
Ding Y, Li XR, Yang KY, Huang LH, Hu G, Gao K (2013) Proteomics analysis of gastric epithelial ags cells infected with epstein-barr virus. Asian Pac J Cancer Prev 14:367–372
Gambhir S, Vyas D, Hollis M, Aekka A, Vyas A (2015) Nuclear factor kappa b role in inflammation associated gastrointestinal malignancies. World J Gastroenterol 21:3174–3183
Henle G, Henle W, Clifford P, Diehl V, Kafuko GW, Kirya BG, Klein G, Morrow RH, Munube GM, Pike P, Tukei PM, Ziegler JL (1969) Antibodies to epstein-barr virus in burkitt’s lymphoma and control groups. J Natl Cancer Inst 43:1147–1157
Hu TZ, Huang LH, Xu CX, Liu XM, Wang Y, Xiao J, Zhou L, Luo L, Jiang XX (2015) Expressional profiles of transcription factors in the progression of helicobacter pylori‐associated gastric carcinoma based on protein/DNA array analysis. Med Oncol 32:265
Hwang YS, Jeong M, Park JS, Kim MH, Lee DB, Shin BA, Mukaida N, Ellis LM, Kim HR, Ahn BW, Jung YD (2004) Interleukin-1beta stimulates il-8 expression through map kinase and ros signaling in human gastric carcinoma cells. Oncogene 23:6603–6611
Lee HW, Lee EH, Kim SH, Roh MS, Jung SB, Choi YC (2013) Heat shock protein 70 (hsp70) expression is associated with poor prognosis in intestinal type gastric cancer. Virchows Arch 463:489–495
Li L, Zhang Y, Guo BB, Chan FK, Tao Q (2014) Oncogenic induction of cellular high cpg methylation by epstein-barr virus in malignant epithelial cells. Chin J Cancer 33:604–608
Li Y, Long X, Huang L, Yang M, Yuan Y, Wang Y, Delecluse HJ, Kuang E (2016) Epstein-barr virus bzlf1-mediated downregulation of proinflammatory factors is essential for optimal lytic viral replication. J Virol 90:887–903
Lian S, Xia Y, Ung TT, Khoi PN, Yoon HJ, Kim NH, Kim KK, Jung YD (2016) Carbon monoxide releasing molecule-2 ameliorates il-1beta-induced il-8 in human gastric cancer cells. Toxicology 361–362:24–38
Liu L, Zhou Q, Xie Y, Zuo L, Zhu F, Lu J (2017) Extracellular vesicles: novel vehicles in herpesvirus infection. Virol Sin 32:349–356
Lu JH, Tang YL, Yu HB, Zhou JH, Fu CY, Zeng X, Yu ZY, Yin HL, Wu MH, Zhang JY, Li XL, Li GY (2010) Epstein-barr virus facilitates the malignant potential of immortalized epithelial cells: from latent genome to viral production and maintenance. Lab Invest 90:196–209
Lu Y, Qin Z, Wang J, Zheng X, Lu J, Zhang X, Wei L, Peng Q, Zheng Y, Ou C, Ye Q, Xiong W, Li G, Fu Y, Yan Q, Ma J (2017) Epstein-barr virus mir-bart6-3p inhibits the rig-i pathway. J Innate Immun 9:574–586
Ludigs K, Parfenov V, Du Pasquier RA, Guarda G (2012) Type i ifn-mediated regulation of il-1 production in inflammatory disorders. Cell Mol Life Sci 69:3395–3418
Matsusaka K, Funata S, Fukayama M, Kaneda A (2014) DNA methylation in gastric cancer, related to helicobacter pylori and epstein-barr virus. World J Gastroenterol 20:3916–3926
McColl SR, Roberge CJ, Larochelle B, Gosselin J (1997) EBV induces the production and release of il-8 and macrophage inflammatory protein-1 alpha in human neutrophils. J Immunol 159:6164–6168
McIlwain DR, Berger T, Mak TW (2013) Caspase functions in cell death and disease. Cold Spring Harb Perspect Biol 5:a008656
Morales-Sanchez A, Fuentes-Panana EM (2014) Human viruses and cancer. Viruses 6:4047–4079
Murata T, Tsurumi T (2014) Switching of EBV cycles between latent and lytic states. Rev Med Virol 24:142–153
Nemerow GR, Mold C, Schwend VK, Tollefson V, Cooper NR (1987) Identification of gp350 as the viral glycoprotein mediating attachment of epstein-barr virus (ebv) to the ebv/c3d receptor of b cells: sequence homology of gp350 and c3 complement fragment c3d. J Virol 61:1416–1420
Nemerow GR, Houghten RA, Moore MD, Cooper NR (1989) Identification of an epitope in the major envelope protein of epstein-barr virus that mediates viral binding to the b lymphocyte ebv receptor (cr2). Cell 56:369–377
Ni C, Chen Y, Zeng M, Pei R, Du Y, Tang L, Wang M, Hu Y, Zhu H, He M, Wei X, Wang S, Ning X, Wang M, Wang J, Ma L, Chen X, Sun Q, Tang H, Wang Y, Wang X (2015) In-cell infection: a novel pathway for epstein-barr virus infection mediated by cell-in-cell structures. Cell Res 25:785–800
Overholtzer M, Mailleux AA, Mouneimne G, Normand G, Schnitt SJ, King RW, Cibas ES, Brugge JS (2007) A nonapoptotic cell death process, entosis, that occurs by cell-in-cell invasion. Cell 131:966–979
Pereira ER, Frudd K, Awad W, Hendershot LM (2014) Endoplasmic reticulum (er) stress and hypoxia response pathways interact to potentiate hypoxia-inducible factor 1 (hif-1) transcriptional activity on targets like vascular endothelial growth factor (vegf). J Biol Chem 289:3352–3364
Queiroz DM, Rocha AM, Melo FF, Rocha GA, Teixeira KN, Carvalho SD, Bittencourt PF, Castro LP, Crabtree JE (2013) Increased gastric il-1beta concentration and iron deficiency parameters in h. Pylori infected children. PLoS One 8:e57420
Savitri E, Haryana MS (2015) Expression of interleukin-8, interleukin-10 and epstein-barr viral-load as prognostic indicator in nasopharyngeal carcinoma. Glob J Health Sci 7:364–372
Shannon-Lowe CD, Neuhierl B, Baldwin G, Rickinson AB, Delecluse HJ (2006) Resting b cells as a transfer vehicle for epstein-barr virus infection of epithelial cells. Proc Natl Acad Sci USA 103:7065–7070
Shinozaki-Ushiku A, Kunita A, Fukayama M (2015) Update on epstein-barr virus and gastric cancer (review). Int J Oncol 46:1421–1434
Sokolova O, Naumann M (2017) NF-kappaB signaling in gastric cancer. Toxins (Basel) 9:119
Takada K, Horinouchi K, Ono Y, Aya T, Osato T, Takahashi M, Hayasaka S (1991) An epstein-barr virus-producer line akata: establishment of the cell line and analysis of viral DNA. Virus Genes 5:147–156
Tao Y, Shi Y, Jia J, Jiang Y, Yang L, Cao Y (2015) Novel roles and therapeutic targets of epstein-barr virus-encoded latent membrane protein 1-induced oncogenesis in nasopharyngeal carcinoma. Expert Rev Mol Med 17:e15
Temple RM, Zhu J, Budgeon L, Christensen ND, Meyers C, Sample CE (2014) Efficient replication of epstein-barr virus in stratified epithelium in vitro. Proc Natl Acad Sci USA 111:16544–16549
Tsao SW, Tsang CM (2017) Epstein-barr virus infection and nasopharyngeal carcinoma. Philos Trans R Soc Lond B Biol Sci 372:20160270
Tsurumi T, Fujita M, Kudoh A (2005) Latent and lytic epstein-barr virus replication strategies. Rev Med Virol 15:3–15
Tu C, Zeng Z, Qi P, Li X, Yu Z, Guo C, Xiong F, Xiang B, Zhou M, Gong Z, Liao Q, Yu J, He Y, Zhang W, Li X, Li Y, Li G, Xiong W (2017) Genome-wide analysis of 18 epstein-barr viruses isolated from primary nasopharyngeal carcinoma biopsy specimens. J Virol 91
Yao HL, Song J, Sun P, Song QQ, Sheng LJ, Chi MM, Han J (2016) Gene expression analysis during recovery process indicates the mechanism for innate immune injury and repair from coxsackievirus b3-induced myocarditis. Virus Res 213:314–321
Yin Y, Si X, Gao Y, Gao L, Wang J (2013) The nuclear factor-kappab correlates with increased expression of interleukin-6 and promotes progression of gastric carcinoma. Oncol Rep 29:34–38
Young LS, Rickinson AB (2004) Epstein-barr virus: 40 years on. Nat Rev Cancer 4:757–768
Yu Z, Lu J, Yu H, Yan Q, Zuo L, Li G (2011) A precise excision of the complete epstein-barr virus genome in a plasmid based on a bacterial artificial chromosome. J Virol Methods 176:103–107
Yu H, Lu J, Zuo L, Yan Q, Yu Z, Li X, Huang J, Zhao L, Tang H, Luo Z, Liao Q, Zeng Z, Zhang J, Li G (2012) Epstein-barr virus downregulates microrna 203 through the oncoprotein latent membrane protein 1: a contribution to increased tumor incidence in epithelial cells. J Virol 86:3088–3099
Zhang G, Tsang CM, Deng W, Yip YL, Lui VW, Wong SC, Cheung AL, Hau PM, Zeng M, Lung ML, Chen H, Lo KW, Takada K, Tsao SW (2013) Enhanced il-6/il-6r signaling promotes growth and malignant properties in EBV-infected premalignant and cancerous nasopharyngeal epithelial cells. PLoS ONE 8:e62284
Zhang H, Li Y, Wang HB, Zhang A, Chen ML, Fang ZX, Dong XD, Li SB, Du Y, Xiong D, He JY, Li MZ, Liu YM, Zhou AJ, Zhong Q, Zeng YX, Kieff E, Zhang Z, Gewurz BE, Zhao B, Zeng MS (2018) Ephrin receptor a2 is an epithelial cell receptor for epstein-barr virus entry. Nat Microbiol 3:1–8
Zhao G, Zhu G, Huang Y, Zheng W, Hua J, Yang S, Zhuang J, Ye J (2016) Il-6 mediates the signal pathway of jak-stat3-vegf-c promoting growth, invasion and lymphangiogenesis in gastric cancer. Oncol Rep 35:1787–1795
Zheng Y, Qin Z, Ye Q, Chen P, Wang Z, Yan Q, Luo Z, Liu X, Zhou Y, Xiong W, Ma J, Li G (2014) Lactoferrin suppresses the epstein-barr virus-induced inflammatory response by interfering with pattern recognition of tlr2 and tlr9. Lab Invest 94:1188–1199
Zhu C, Wu Y, Chen S, Yu M, Zeng Y, You X, Xiao J, Wang S (2012) Protective immune responses in mice induced by intramuscular and intranasal immunization with a mycoplasma pneumoniae p1c DNA vaccine. Can J Microbiol 58:644–652
Zuo L, Yue W, Du S, Xin S, Zhang J, Liu L, Li G, Lu J (2017a) An update: epstein-barr virus and immune evasion via microrna regulation. Virol Sin 32:175–187
Zuo LL, Zhang J, Liu LZ, Zhou Q, Du SJ, Xin SY, Ning ZP, Yang J, Yu HB, Yue WX, Wang J, Zhu FX, Li GY, Lu JH (2017b) Cadherin 6 is activated by epstein-barr virus lmp1 to mediate emt and metastasis as an interplay node of multiple pathways in nasopharyngeal carcinoma. Oncogenesis 6:402
Acknowledgements
This work was supported by the National Key Research & Development Program and National Natural Science Foundations of China (2017YFC1200204, 31670171, 81728011) and Innovation Foundations for Postgraduates of Central South University (2018zzts817).
Author information
Authors and Affiliations
Contributions
Author Contributions
JL and FZ designed and guided the study. WY and MZ performed most of the experiments; LZ, SX, JZ, LL, SL, SZ and YX participated in the experiments; WY, MZ and JL analyzed the data. JL and WY wrote and finalized the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Animal and Human Rights Statement
The study protocol was approved by the Medical Ethics Review Committees of Xiangya Hospital, Central South University.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yue, W., Zhu, M., Zuo, L. et al. Early Pattern of Epstein-Barr Virus Infection in Gastric Epithelial Cells by “Cell-in-cell”. Virol. Sin. 34, 253–261 (2019). https://doi.org/10.1007/s12250-019-00097-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12250-019-00097-1