Abstract
Brucellosis is the most common zoonotic bacterial disease. Prevention of human brucellosis is achieved through pasteurization of dairy products, appropriate sanitation and vaccination of domestic animals against the Brucella species. B. abortus unlipidated 19 kDa outer membrane protein (U-Omp19) is a promising candidate for a subunit vaccine against brucellosis. This study investigates immunogenicity of Omp19 alone and with Freund’s adjuvant (Omp19-IFA) and N-trimethyl chitosan (TMC/Omp19) nanoparticles, as well as the effect of Omp19 administration route on immunological responses and protection. The omp19 gene was expressed in E. coli BL21 (DE3). After purification, the recombinant Omp19 was loaded onto TMC nanoparticles by ionic gelation with tripolyphosphate. Particle size and loading efficiency of the nanoparticles were determined. Omp19-IFA was administered intraperitoneally while TMC/Omp19 nanoparticles were administered orally and intraperitoneally. The results indicated that intraperitoneal (i.p.) immunization by Omp19-IFA and TMC/Omp19 nanoparticles induced Th1 and Th2 immune responses, respectively, whereas oral immunization of TMC/Omp19 nanoparticles induced a mixed Th1/Th17 immune response. Moreover, oral immunization increased IgA levels in feces. Immunized mice were challenged with virulent B. melitensis 16 M and B. abortus 544. Oral immunization with TMC/Omp19 nanoparticles induced a remarkably high protection level against B. melitensis and B. abortus. The results showed that immunization route has a pivotal role in immune response polarization and protective efficiency of Omp19 antigen. Also, it was deduced that the higher protection level achieved through oral administration of TMC/Omp19 nanoparticles may be due to the elicited Th17 response.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brucellosis is the most common zoonotic bacterial disease that is transmitted from animals to humans. This disease is caused by a facultative intracellular pathogen belonging to the genus Brucella (B), the natural reservoir of which is domestic and wild animals (Cloeckaert et al. 2001). B. suis, B. melitensis and B. abortus mostly cause human brucellosis whereas B. canis and some Brucella species found in marine mammals cause human infection only occasionally. Of all of these, B .melitensis has severe pathogenicity for humans (Brown et al. 2009; Saraiva et al. 2009).
The possible means of brucellosis acquisition are occupational contact with infected animals, consumption of contaminated dairy products and infection from a contaminated environment (Luna-Martinez and Mejia-Teran 2002; Pappas et al. 2006). Some clinical symptoms of the disease include: periodic fever, weakness, headache, anorexia, malaise, nausea, pain and inflammation in the spleen, liver and superficial lymph nodes (Shakir 1986). Because of the intracellular residence of Brucella, a few antibiotics are impressive against this pathogen. Since there is a high probability of brucellosis relapse in single-agent therapy, the antibiotics are frequently administered in combination (Ariza et al. 1992; Montejo et al. 1993). Hence there is a considerable requirement for impressive treatments or vaccines for human brucellosis.
Subunit vaccines, such as recombinant proteins, are promising vaccine candidates because they are nonpathogenic, avirulent, well defined, nonviable and safer for manipulators, and economical. Subunit vaccines are produced at high purity and yield. They can be manipulated to improve desirable properties and minimize undesirable ones (Perkins et al. 2010). Despite their advantages, recombinant vaccines, as opposed to whole bacteria, are relatively less immunogenic and require adjuvants to stimulate the immune response (Perrie et al. 2008). Moreover, the effectiveness of a recombinant vaccine greatly depends on its components and the route of administration, which instructs the selective induction of different antigen-specific immune responses (Abkar et al. 2015; Mohanan et al. 2010; Pasquevich et al. 2011; Tabynov et al. 2014a, b).
As the oral route is the main site of entrance of Brucella into the body, the design and development of a mucosal-administered vaccine for brucellosis seems to be a logical option (Neutra and Kozlowski 2006). However, the delivery of antigens through mucosal surfaces remains a main challenge due to undesirable physiological situations (pH and enzymes) and important biological barriers, which limit the uptake of antigens (Baumann 2008; Fooks 2000; Kostrzak et al. 2009). In order to overcome this problem, multiple delivery systems such as the nanoparticle delivery system and the plant-based vaccine have been developed (Gregory et al. 2013; Kammona and Kiparissides 2012). Among various subunit vaccines, B. abortus unlipidated 19 kDa outer membrane protein (U-Omp19) has been recognized as a promising vaccine candidate for oral vaccination against brucellosis. This antigen has shown protection against three Brucella species (B. melitensis, B. abortus and B. suis) in mice (Pasquevich et al. 2011). Since recombinant vaccines have low immunogenicity, use of an impressive antigen delivery system and adjuvant is critical. The application of the nanoparticle-mediated delivery system is an impressive strategy for site-specific delivery of vaccines (Hall et al. 2007).
Various synthetic and natural polymers have been tested as delivery systems for mucosal vaccines (Mahapatro and Singh 2011). These polymers can prolong the residence time of the antigen, thereby increasing the antigen uptake by professional antigen-presenting cells (APCs); they may, in addition, have intrinsic adjuvant properties (Gebert et al. 2004). Among various delivery systems for mucosal vaccines, N-trimethyl chitosan (TMC), a partially quaternized derivative of chitosan, nanoparticles has received particular attention (Amidi et al. 2007; Bal et al. 2011; Subbiah et al. 2012; Verheul et al. 2011). Previous studies have shown that these nanoparticles can enhance the immunogenicity of the antigen in oral, nasal and parenteral immunization. Other interesting features of TMC nanoparticles as a vaccine delivery system include low cytotoxicity, high uptake by intestinal M-cells, high loading capacity and high interaction of the antigen with dendritic cells (Garg et al. 2010; Jabbal-Gill et al. 2012; Slutter et al. 2009).
The aim of this study is to conduct a survey of the immunogenicity property of U-Omp19 alone or with TMC nanoparticles, to investigate the influence of administration route on type of immune response.
Materials and methods
Bacterial strains and plasmid
B. melitensis 16 M and B. abortus 544 were used in the protection assay. B. melitensis Rev.1 and B. abortus S19 were applied as vaccine controls. All the strains were obtained from Razi Vaccine and Serum Research Institute, Karaj, Iran. E. coli BL21 (DE3) and pET28a vector (Novagen, Madison, WI) were applied for expression of recombinant protein.
Mice and ethics statement
The 4 to 6 week old female BALB/c mice were obtained from Pasteur Institute (Tehran, Iran) and housed in standard polypropylene cages maintained at 20–22 °C while undergoing 12-h light/dark cycles. All experimental procedures on animals were approved by the ethical committee of Razi Vaccine and Serum Research Institute (No. 515.92 GD, 26.1.2010).
Cloning, expression and purification of recombinant protein
B. abortus omp19 gene was amplified using forward primer (5-ATAAGGATCCGCCACCATGCAATCTTCAAG-3) and reverse primer (5-TACCCTCGAGTTATCTGCTTAAAGTAACAGCCTG-3) from a synthetic gene (GenBank Accession Number: JQ965699). The underlined parts of the primer sequences above represent the restriction sites for BamHI and XhoI, respectively. The PCR product was inserted in pET28a vector. The recombinant plasmid was transformed into competent E. coli BL21 (DE3) cells. A single colony was cultured overnight in LB medium (Merck Frankfurte, Germany) containing 50 μg/ml of kanamycin at 37 °C and subsequently diluted 10-fold with fresh LB medium containing kanamycin. IPTG at a final concentration of 1 mM was used for induction of the recombinant protein expression. The bacterial cells were incubated overnight at 37 °C with shaking at 150 rpm. The cells were harvested by centrifugation (15,300 g for 15 min). The pellet was suspended in lysis buffer (Tris 50 mM, EDTA 5.0 mM, pH 8.0). After sonication, cell lysate was subjected to centrifugation at 15,300 g and 4 °C for 30 min. The supernatant containing Omp19 was analyzed by SDS-PAGE. The recombinant protein was identified by western blot with mouse anti-His antibodies (1:5000, Sigma). The recombinant protein was purified by chromatography through Ni-NTA Agarose (Qiagen) in accordance with the manufacturer’s protocol. The concentration of purified protein was determined by Bradford method (Giambartolomei et al. 2004).
Nanoparticle preparation
TMC was provided by Dr. Sahebghadam Lotfi (Department of Clinical Biochemistry, Faculty of Medicine, Tarbiat Modares University, Tehran, Iran). TMC nanoparticles were prepared by ionic complexation with pentasodium tripolyphosphate (TPP) (Merck Frankfurte, Germany) as a cross-linking agent (Calvo et al. 1997). Omp19 and TMC were dissolved in a 5 mM HEPES (Sigma-Aldrich) buffer (pH 7.4) to a final concentration of 0.1 mg/ml and 1 mg/ml, respectively. TPP was added under continuous stirring to an Omp19: TPP: TMC weight ratio of 1:3:10. Nanoparticles were harvested by centrifugation (30 min, 16,000 g) on a glycerol bed to avoid aggregation. The supernatant was discarded and the nanoparticles were resuspended in phosphate buffered saline (PBS).
Characterization of TMC/Omp19 nanoparticles
The size of the nanoparticles was determined by dynamic light scattering (DLS) using a NanoSizer ZS (Malvern Instruments, Malvern, UK) in 5 mM HEPES (pH 7.4) at 25 °C. The morphology of the nanoparticles was determined using FE-SEM (JEOL 7500 F). The samples were coated with gold using a sputter coater device K650X (Emitech, Hailsham, UK) before examination by FE-SEM.
The amount of protein entrapped in the nanoparticles was calculated as the difference between the total protein added to the loading solution and the amount of the concentration of non-entrapped protein remaining in the supernatant. The concentration of non-entrapped protein remaining in the supernatant was determined by Bradford method. Loading efficiency (LE) for Omp19 was computed by the following equation: LE (%) = (Total amount of Omp19-Free Omp19/ Totai amount of Omp19) × 100.
Estimation of protein integrity
The effect of the preparation process on protein integrity was examined by SDS-PAGE analysis. The entrapped proteins in TMC nanoparticles were destabilized by the addition of 1 ml of 10 % (w/v) NaCl to the nanoparticles solution. Subsequently the protein sample was prepared and electrophoresed.
Mice immunization
Mice were immunized by the i.p. and oral routes. Nine groups of mice either receiving vaccine or as negative control groups are listed in Table 1. The positive control groups were immunized intraperitoneally on day 15 with 1 × 105 CFU of B. abortus S19 and B. melitensis Rev.1.
Antibody detection
Specific indirect ELISA was done to determine total IgG, IgG1 and IgG2a titers. Briefly, Omp19 specific ELISA was performed with serum samples using purified recombinant Omp19. Each well of MaxiSorp plates (Nunc, Denmark) was coated with 1 μg (100 μl) of Omp19 in carbonate-bicarbonate buffer (pH 9.6) at 37 °C for 1 h. The plates were washed 3 times with PBST at each step. After 1 h of blocking at 37 °C with 3 % (w/v) skim milk in PBST to prevent nonspecific binding, the plates were incubated with serially diluted sera (1:250 to 1:8000) at 37 °C for 2 h. Anti-mouse IgG, IgG1 and IgG2a HRP conjugates (100 μl/well) were added to wells and incubated at 37 °C for 1 h. Following the addition of 100 μl of o-phenylenediamine dichloride (OPD; Sigma, USA) in phosphate-citrate buffer (pH 5.5) and H2O2 as a substrate, the plates were again incubated at 37 °C for 15 min. Finally, color development was stopped by the addition of 50 μl of 1 N H2SO4 and each well was measured for optical density at 450 nm by using a microtiter plate reader (Eurogenetics, Torino, Italy).
For determining anti-Omp19 IgA level, feces of mice were collected and mixed with extraction buffer (10 mg/ml bovine serum albumin (Sigma), 100 μg/ml soybean trypsin inhibitor (Sigma) and 30 mM disodium EDTA in PBS, pH 7.6) and shaken overnight at 4 ° C. After centrifugation (16,000 g, 20 min, 4 ° C), serially diluted supernatant (1:2 to 1:16) was subjected to Omp19 coated plate followed by anti-mouse IgA HRP conjugated antibody and the procedure continued as mentioned above. All antibody assays were performed in triplicate.
Cytokine assay
One month after the last immunization, 5 mice from each group were sacrificed and their spleens were dissected aseptically. The splenocytes were homogenized and suspended in RPMI 1640 medium (NUNC. Thermo Fisher Scientific Inc., Roskilde, Denmark) supplemented by 10 % heat-inactivated fetal calf serum (HyClone, UT, USA), 2 mM L-glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin. The cells were cultured at a concentration of 4 × 106 /ml in duplicate wells in the absence of additives (non-stimulated control) or in the presence of either 2 μg of CBP’s, 10 μg of purified rOmp19, or 0.25 μg of ConcanavalinA (ConA). Cultures were incubated at 37 °C with 5 % CO2. The IFN-γ, IL-4, IL-12 and IL-17 in the culture supernatants were determined 48 h after antigen stimulation using mouse ELISA kits by following the manufacturer’s instructions (R & D Systems, Minneapolis, MN, USA). All assays were performed in triplicate.
Lymphocyte proliferation assay
Lymphocyte proliferation was determined by MTT assay. Three days after the cell culture, 25 μl MTT dye (5 mg/ml in PBS) (Sigma-Aldrich) was added to each well and the preparation incubated at 37 °C with 5 % CO2 for 2 h. The entire culture supernatant was removed from each well. To dissolve the formazan crystals, 75 μl of 5 % dimethyl sulfoxide (DMSO) was added to each well. Finally, the absorbance (OD) of color density was measured at 540 nm.
Protection assay
One month after the final immunization, the mice were challenged with 2 × 107 CFU of B. abortus 544 and B. melitensis 16 M through i.p. injection. Four weeks later, the infected mice were sacrificed by cervical dislocation, their spleens were extracted aseptically and homogenized, and the dilutions were plated on Brucella agar to determine the number of Brucella colonies. The results were represented as the mean log CFU ± SD per group. Units of protection were calculated by subtracting the mean log10 CFU for the experimental groups from the mean log10 CFU of the negative control group.
Statistical analysis
Data obtained from antibody detection, cytokine assay and protection assay were analyzed using the two-way analysis of variance (ANOVA). p values < 0.01 were considered as statistically significant.
Results
Cloning, expression and purification of recombinant protein
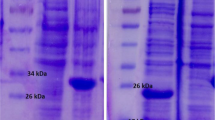
Amplification of omp19 gene produced a 500 bp DNA fragment (Fig. 1a). The PCR product was cloned successfully in the pET28a (+) expression vector. The integrity of cloning process was confirmed by double digesting and sequencing of produced plasmids. The omp19 gene was expressed in E. coli BL21 (DE3), with the N-terminal 6X-His-tag. The SDS-PAGE analysis showed the presence of recombinant Omp19 protein as a major band, as shown in Fig. 1b. Purification of the protein was performed under native conditions and SDS-PAGE analysis showed the presence of recombinant protein in the eluted fraction. The average yield of this protein was 0.458 mg/ml of culture. As shown in Fig. 1c, Omp19 protein was determined by Western blot.
a PCR product of omp19 gene (Lanes 1–3) followed by agarose gel electrophoresis. An expected rOmp19 protein was determined by SDS-PAGE (b) and Western blot (c) using an anti-His antibody dilution ratio of 1:5000. Lane 1 shows the elution of purified Omp19 at 250 mM Imidazole concentration. Lanes 2 and 3 show the induced and uninduced cell lysate of Omp19 expressing E. coli cells, respectively. d SEM image of TMC/Omp19 nanoparticles shows that the particles have an average size between 200 and 300 nm and are spherical
Characterization of TMC/Omp19 nanoparticles
DLS showed that most of TMC/Omp19 nanoparticles had a mean size distribution between 300 and 400 nm (Data not shown). SEM images showed the size of the particles to be smaller than measured with the DLS, probably due to dehydration of the sample (between 200 and 300 nm). Additionally, the TMC/Omp19 nanoparticles had a spherical appearance and a smooth surface (Fig. 1d). LE of Omp19 was calculated as 67.1 ± 5.9 %.
Estimation of protein integrity
The protein integrity of Omp19 before and after encapsulation was evaluated by SDS-PAGE analysis. The antigen integrity of the Omp19 in the nanoparticles was determined to be intact before and after antigen loading. SDS-PAGE analysis results validated the structural integrity of the Omp19 within TMC nanoparticles showing that the integrity of Omp19 has been maintained during preparation process (Data not shown).
Antibody detection
I.p. immunization with Omp19-IFA, TMC/Omp19 (multi-dose) and oral immunization (multi-dose) could induce specific IgG production. I.p. immunization with Omp19-IFA showed higher Omp19-specific IgG titers in comparison with other groups (p < 0.01) (Fig. 2a). The IgG1 and IgG2a antibody titers determined the type of immune response induced by the different formulations and routes. The main subtype produced after i.p. immunization with Omp19-IFA and oral immunization with TMC/Omp19 nanoparticles was IgG2a whereas the main subtype in i.p. immunization with TMC/Omp19 nanoparticles was IgG1 (Fig. 2b and c).
Anti-Omp19 antibody levels: The sera were analyzed in triplicates for Omp19 specific IgG antibodies by ELISA with comparison to the control group. Sera obtained from mice belonging to different experimental groups were collected at regular upto day 45 post-primary immunization. a Antibody level of intraperitoneally and orally immunized mice. b and c Antibody isotyping: The isotype profile of Omp19 specific antibodies in serum of orally and intraperitoneally immunized mice were analyzed by ELISA using HRP conjugated anti-mouse IgG1 and IgG2a (dilution 1:5000) antibodies. Immunization groups are based on Table 1. Charts display only those with high antibody titer. The difference between groups was assessed by the ANOVA and comparisons were considered significant at p < 0.01. Different letters (a, b, c, d, e and f) represent significant difference between groups
Secretory IgA is a predominant antibody in mucosal immunity. I.p. vaccination did not elicit any detectable IgA levels in the fecal extracts whereas oral immunization with Omp19 showed increased levels of IgA (Fig. 3).
Omp19 specific mucosal IgA antibody levels in fecal samples from immunized mice. Feces of mice belonging to different experimental groups were collected at regular upto day 45 post-primary vaccination. Immunization groups based on Table 1. *p value <0.05
Cytokine assay
The supernatants of splenocyte cultures from all immunized mice were evaluated for cytokines by ELISA. The results showed IL-4 production was significantly higher in mice immunized with TMC/Omp19 in i.p. route, but not in the other groups (p < 0.01) (Fig. 4a). The significant production of IFN-γ and IL-12 was seen in cells from Omp19-IFA in intraperitoneally and TMC/Omp19 nanoparticles in orally immunized mice (Fig. 4b and c). Furthermore, the significantly high production of IL-17 was determined in orally immunized mice (Fig. 4d).
IL-4 (a), IFN-γ (b), IL-12 (c) and IL-17 (d) levels in cell supernatants were determined by ELISA. Spleen Cells (4 × 106 mL−1 in duplicate wells) were stimulated with Omp19 for 48 h. Immunization groups based on Table 1. The difference between groups was assessed by the ANOVA and comparisons were considered significant at p < 0.01. Different letters (a, b, c, d and e) represent significant difference between groups
Protection assay
The ability of Omp19 to protect against infection with virulent B. abortus 544 and B. melitensis 16 M challenge was evaluated in immunized BALB/c mice. The level of protection was analyzed by determining the numbers of CFU in spleens at 4 weeks post-challenge. Compared with the control group, the mice that were administered Omp19 orally demonstrated a higher degree of protection when challenged with B. abortus and B. melitensis, the log units of protection obtained being 2.28 and 2.15, respectively (p < 0.01). However, i.p. immunization with TMC/Omp19 nanoparticles and Omp19-IFA produced 1.39 and 1.87 log protection units against B. abortus, respectively (Table 2). Furthermore, i.p. immunization with TMC/Omp19 nanoparticles and Omp19-IFA showed 1.58 and 1.69 log protection units against B. melitensis, respectively (Table 3).
Lymphocyte proliferation assay
The results of MTT proliferation assay was shown as stimulation index (S.I.). The S.I. corresponds to the count per minute of induced splenocytes divided by the count per minute of uninduced splenocytes. As shown in Fig. 5, the S.I. for intraperitoneally vaccinated mice with Omp19-IFA and TMC/Omp19 were determined to be 1.46 and 1.16 respectively, whereas for that of orally vaccinated mice, it was found to be 1.94 when stimulated with rOmp19 (Fig. 5). Thus, this high S.I. value indicates cell stimulatory activity of rOmp19 and thus, may be one of the reasons behind a strong immune response (p < 0.01).
Lymphocyte proliferation assay of splenocytes from mice immunized with rOmp19. Mice immunized with PBS and nanoparticles were used as controls. Splenocytes from vaccinated mice (2 × 105 cells/well) were stimulated with rOmp19 (0.1 μg/well) for 72 h and the proliferative response was determined by in vitro-MTT assay. The data are the mean S.I. ± SD of five individual mice from each group with three repeats. Immunization groups based on Table 1. Different letters (a, b, c, d, e and f) represent significant difference between groups. p value < 0.01
Discussion
Several intracellular and cell surface components have been assessed as subunit vaccines against brucellosis in mouse models, and some of these have shown protective efficacy (Fu et al. 2012; Ghasemi et al. 2014a, b; Goel and Bhatnagar 2012). In previous studies, U-Omp19 elicited a T helper 1 response and oral protection with cholerae toxin (CT) as mucosal adjuvant against B. abortus infection. Additionally, i.p. immunization with U-Omp19 in Freund’s adjuvant showed a higher degree of protection against B. abortus than the corresponding lipidated form (Pasquevich et al. 2009, 2011). The expression of Omp19 is crucial for stimulation of protection by the vaccine strain B. abortus S19, since the abrogation of its gene in this strain has resulted in the loss of its protective ability in heifers, showing that the protein is a main component of a recombinant vaccine against brucellosis (Fiorentino et al. 2008).
Previous data consider that control of an intracellular microorganism such as Brucella requires Th1 response, i.e., IFN-γ, IL-12 and TNFα, to provoke cell-mediated immunity, whereas Th2 response has a minor role in this kind of infection. Th1 cytokines (especially IFN-γ) stimulate macrophages for more effective killing and replication inhibition of intracellular pathogens such as Brucella (Murphy et al. 2001; Vitry et al. 2012). Th17 response is an important immune cell that manages mucosal host defense against many extracellular and intracellular microorganisms. In this line, Pasquevich et al. showed that IL-17 plays a critical role in vaccine mediated anti-Brucella immunity. A possible role for Th17 cell involvement in protective responses against Brucella is that pathogen-specific Th17 cells may boost or work synergistically with Th1 cells for high protection (Kumar et al. 2013; Pasquevich et al. 2011). Our results showed that the significant production of IFN-γ and IL-12 was achieved in Omp19-IFA in i.p. route and TMC/Omp19 nanoparticles in orally immunized mice (Fig. 5). Additionally, the high titer of IL-17 was determined in orally administered mice. By contrast, IL-4 production was significantly higher only in the group immunized with TMC/Omp19 in i.p. route, but not in the other groups. These results are in accordance with observations made by other authors suggesting that i.p. (Omp19-IFA) and oral administration (plant-expressed Omp19) of Omp19 induce production of Th1 and mixed Th1-Th17 responses, respectively (Pasquevich et al. 2011).
As the Brucella bacterium often enters the body via contaminated food and water, mucosal immunity can serve as a first line of defense to prevent the infection before it reaches the bloodstream (Golding et al. 2001). There is not known role for IgA in protection against brucellosis, but IgA can reflect stimulation of the common mucosal immune system. So, one of the goals of this study was the induction of anti-Brucella IgA in mucosal sites. Our results showed that when TMC/Omp19 nanoparticles were orally administered, the specific anti-Omp19 IgA was detected in feces of the mice (Fig. 4). Omp19 in oral immunization (multi-dose) and i.p. immunization with Freund’s adjuvant and TMC nanoparticles (multi-dose) induced high IgG titers (Fig. 3a). Our results are in accordance with observations by Chen et al., showing that subcutaneous (s.c.) injection of Urease-loaded TMC nanoparticles into mice generated high levels of IgG titers but low IgA titers. By contrast, orally administered Urease-loaded TMC nanoparticles elicited high titers of both IgA and IgG antibodies (Chen et al. 2008). However, our results are in contrast with the Boontha et al. study which suggests that oral administration of TMC/Ovalbumin induces low IgA titers (Mobley et al. 1995). Additionally, the isotype antibody responses (IgG1 and IgG2a) suggest that oral immunization with TMC nanoparticles and i.p. immunization with Freund’s adjuvant may direct the antigen-specific immune response towards Th1, whereas i.p. immunization with TMC nanoparticles may direct the antigen specific immune response towards Th2 (Fig. 3b). The same results were observed by Amidi et al. after nasal immunization with influenza antigen-loaded TMC nanoparticles (Amidi et al. 2007). Altogether, our results indicate that i.p. immunization by Omp19-IFA and TMC/Omp19 nanoparticles induces Th1 and Th2 immune responses, respectively, whereas oral immunization induces a mixed Th1/Th17 immune response.
The OMPs of Brucellas spp. have often shown good immunogenicity and protective antigenicity. Vemulapalli et al. indicated that inoculation with B. abortus Omp18 elicits production of Omp18-specific antibodies and a Th1 response in BALB/c mice. However, Omp18 preparations did not result in protection against challenge with the virulent strain B. abortus 2308 (Vemulapalli et al. 2000). Another study showed that plant-expressed U-Omp19 can induce significant protection when administered to BALB/c mice by the oral route as purified proteins and within the crude leaf material of transgenic tobacco plants. The protection level gained was equivalent to those elicited by oral administration of S19 or RB51 (Pasquevich et al. 2011). In study conducted by Cassataro et al., it was indicated that i.p. immunization with recombinant Omp31 induces a Th1 response that confers protection against B. ovis and B. melitensis infections (Cassataro et al. 2005). Luo et al., examined the immunogenicity and the protective efficacy of monovalent and divalent fusion DNA vaccines, designated pcDNA3.1-L7/L12-Omp16, pcDNA3.1-L7/L12, or pcDNA3.1-Omp16 in mice. Intramuscular administration of this divalent DNA vaccine into BALB/c mice induced Th1 immune response. The protection level gained by the divalent DNA vaccine was significantly higher than that gained by the univalent DNA vaccines pcDNA3.1-L7/L12 or pcDNA3.1-Omp16. The protection level gained was lower than that elicited by administration of RB51 (Luo et al. 2006). There is a report which shows that intramuscular immunization with Omp25, as DNA vaccine, protects BALB/c mice from virulent B. melitensis (Commander et al. 2007). The antigenicity of recombinant protein Omp25 (rOmp25) was also assessed in mice. The mice were injected intradermally and intraperitoneally with various concentrations of rOmp25 10, 20, 30 and 40 μg. Both i.p. and intradermal (i.d.) administration induced a mixed Th1-Th2 immune response. I.d. immunization elicited protection comparable to that of B. abortus S19 strain. I.d. vaccination showed a high antibody titer with a moderate level of antigen (30 μg) whereas the i.p. vaccination showed a lower antibody titer even with a high level of antigen (40 μg). Moreover, the protection level obtained by i.p. immunization was lower than those elicited by i.d. administration of rOmp25. Low amounts of stimulating cytokines in the i.p. route of vaccination could be one of the reasons behind the low level of protection obtained against the virulent B. abortus 544 challenge compared to i.d. immunization (Goel and Bhatnagar 2012). Goel et al. tested PC-PE liposomes and PLGA microparticles as delivery system for rOmp25. Both liposome and microparticle vaccination elicited a mixed Th1-Th2 immune response. Prime and booster liposome immunization in BALB/c mice rendered high levels of protection against B. abortus 544 compared with B. abortus S19 vaccine strain. However, rOmp25 in PLGA microparticles was not able to generate better protection when compared to B. abortus S19. The reasons behind low protective efficacy of PLGA microparticles included rapid release of Omp25 from microparticles and porous surface morphology (Goel et al. 2013). In contrast to Goel et al., where a single dose of another recombinant antigen (rOmp25) of Brucella delivered with liposomes was sufficient to elicit a high protective response, the results of our work showed that single-dose TMC/Omp19 immunization was not sufficient for that.
Clapp et al. showed that a single oral administration with ∆znuA B. melitensis efficiently induced protection against nasal challenge with virulent B. melitensis 16 M by stimulating both systemic and mucosal Th1 and Th17 cells (Clapp et al. 2011). In another study, nasal vaccination with trigger factor plus BP26 plus CT has also been conducted and stimulated local immune responses and a low level of protection against systemic infection. Similar to our study, the authors did not survey protection against mucosal challenge (Daniel et al. 1993).
In agreement with previous studies, route of administration affected the type of immune responses. In this field, the selection of immunization route can be a key parameter for success or failure of an antigen under development. An improper route of immunization may render an antigen ineffective or mask its potential efficiency, although the antigen within its formulation would be significantly efficient in another route (Johansen et al. 2010; Mohanan et al. 2010). Tabynov et al. showed that imuunogenicity of novel, effective candidate vaccine against B. abortus based on recombinant influenza viruses expressing the Brucella ribosomal protein L7/L12 or Omp16 was evaluated in mice and guinea pigs. Four recombinant influenza A viral constructs of the subtypes Н5N1 or H1N1 expressing the Brucella proteins Omp16 or L7/L12 were gained. The animals were immunized intranasally, conjunctivally or subcutaneously with recombinant influenza A viruses of the Н5N1 subtype (prime vaccination) and H1N1 subtype (booster vaccination) 28 days apart. Their results indicated that the monovalent viral constructs expressing the Omp16 protein and bivalent vaccine formulation expressing the L7/L12 and Omp16 proteins, when administered conjunctivally, were comparable in terms vaccine efficiency and protective responses to the commercial live vaccine produced from B. abortus 19 in guinea pigs. The protection level gained was lower than that elicited by administration of S19 (Tabynov et al. 2014a). Our results indicated a correlation between IL-4 production (Th2 response) and route of immunization (TMC/Omp19 in i.p.). However, protection unit obtained in i.p. route was statistically lower than the one induced in oral route (TMC/Omp19 m Oral) with lower level of IL-4. In the contrary, elevated production of IgA, IFN-γ and IL-12 (Th1 response) was correlated with orally administrated TMC/Omp19. Overall, the protection units obtained indicate that Omp19 administered orally provides more protection than Omp19 injected intraperitoneally. It is noteworthy that protection unit obtained in oral immunization was significantly elevated by TMC nanoparticles comparing to solo antigen (2.28 ver. 1.42 against B. abortus and 2.15 ver. 0.94 against B. melitensis). Since oral administration of U-Omp19 induced a mixed Th1-Th17 immune response, this may be the main reason behind the high level of protection obtained against Brucella spp.
Indeed the cell proliferative response obtained in Omp19-vaccinated mice indicates the activation of cellular immune responses which is considered to be important for controlling Brucella infections. Data obtained from the cell proliferation assay demonstrates that the vaccination with Omp19 elicits a vigorous antigen specific cell proliferative response which could be further increased after oral immunization with TMC/Omp19.
The results obtained in this study indicate the importance of immunization route in protective efficiency of Omp19. The protection units obtained show that U-Omp19 when administered orally confers more protection, which may be due to the elicited Th17 response.
References
Abkar M, Amani J, Sahebghadam Lotfi A, Nikbakht Brujeni G, Alamian S, Kamali M (2015) Subcutaneous immunization with a novel immunogenic candidate (urease) confers protection against Brucella abortus and Brucella melitensis infections. APMIS: Acta Pathol Microbiol Immunol Scand 123:667–675. doi:10.1111/apm.12400
Amidi M et al (2007) N-trimethyl chitosan (TMC) nanoparticles loaded with influenza subunit antigen for intranasal vaccination: biological properties and immunogenicity in a mouse model. Vaccine 25:144–153. doi:10.1016/j.vaccine.2006.06.086
Ariza J, Gudiol F, Pallares R, Viladrich PF, Rufi G, Corredoira J, Miravitlles MR (1992) Treatment of human brucellosis with doxycycline plus rifampin or doxycycline plus streptomycin. A randomized, double-blind study. Ann Intern Med 117:25–30
Bal SM, Slutter B, Jiskoot W, Bouwstra JA (2011) Small is beautiful: N-trimethyl chitosan-ovalbumin conjugates for microneedle-based transcutaneous immunisation. Vaccine 29:4025–4032. doi:10.1016/j.vaccine.2011.03.039
Baumann U (2008) Mucosal vaccination against bacterial respiratory infections. Expert Rev Vaccines 7:1257–1276
Brown DM, Kamperschroer C, Dilzer AM, Roberts DM, Swain SL (2009) IL-2 and antigen dose differentially regulate perforin- and FasL-mediated cytolytic activity in antigen specific CD4+ T cells. Cell Immunol 257:69–79. doi:10.1016/j.cellimm.2009.03.002
Calvo P, Remunan-Lopez C, Vila-Jato JL, Alonso MJ (1997) Chitosan and chitosan/ethylene oxide-propylene oxide block copolymer nanoparticles as novel carriers for proteins and vaccines. Pharm Res 14:1431–1436
Cassataro J et al (2005) Vaccination with the recombinant Brucella outer membrane protein 31 or a derived 27-amino-acid synthetic peptide elicits a CD4+ T helper 1 response that protects against Brucella melitensis infection. Infect Immun 73:8079–8088
Chen F, Zhang ZR, Yuan F, Qin X, Wang M, Huang Y (2008) In vitro and in vivo study of N-trimethyl chitosan nanoparticles for oral protein delivery. Int J Pharm 349:226–233. doi:10.1016/j.ijpharm.2007.07.035
Clapp B, Skyberg JA, Yang X, Thornburg T, Walters N, Pascual DW (2011) Protective live oral brucellosis vaccines stimulate Th1 and th17 cell responses. Infect Immun 79:4165–4174. doi:10.1128/IAI.05080-11
Cloeckaert A, Verger JM, Grayon M, Paquet JY, Garin-Bastuji B, Foster G, Godfroid J (2001) Classification of Brucella spp. isolated from marine mammals by DNA polymorphism at the omp2 locus. Microbes Infect 3:729–738
Commander NJ, Spencer SA, Wren BW, MacMillan AP (2007) The identification of two protective DNA vaccines from a panel of five plasmid constructs encoding Brucella melitensis 16M genes. Vaccine 25:43–54. doi:10.1016/j.vaccine.2006.07.046
Daniel LW, Huang C, Strum JC, Smitherman PK, Greene D, Wykle RL (1993) Phospholipase D hydrolysis of choline phosphoglycerides is selective for the alkyl-linked subclass of Madin-Darby canine kidney cells. J Biol Chem 268:21519–21526
Fiorentino MA, Campos E, Cravero S, Arese A, Paolicchi F, Campero C, Rossetti O (2008) Protection levels in vaccinated heifers with experimental vaccines Brucella abortus M1-luc and INTA 2. Vet Microbiol 132:302–311. doi:10.1016/j.vetmic.2008.05.003
Fooks AR (2000) Development of oral vaccines for human use. Curr Opin Mol Ther 2:80
Fu S et al (2012) Immunization of mice with recombinant protein CobB or AsnC confers protection against Brucella abortus infection. PLoS One 7:e29552. doi:10.1371/journal.pone.0029552
Garg NK, Mangal S, Khambete H, Tyagi RK (2010) Mucosal delivery of vaccines: role of mucoadhesive/biodegradable polymers. Recent Pat Drug Deliv Formul 4:114–128
Gebert A, Steinmetz I, Fassbender S, Wendlandt KH (2004) Antigen transport into Peyer’s patches: increased uptake by constant numbers of M cells. Am J Pathol 164:65–72. doi:10.1016/S0002-9440(10)63097-0
Ghasemi A, Jeddi-Tehrani M, Mautner J, Salari MH, Zarnani AH (2014a) Immunization of mice with a novel recombinant molecular chaperon confers protection against Brucella melitensis infection. Vaccine. doi:10.1016/j.vaccine.2014.09.013
Ghasemi A, Zarnani AH, Ghoodjani A, Rezania S, Salari MH, Jeddi-Tehrani M (2014b) Identification of a new immunogenic candidate conferring protection against Brucella melitensis infection in Mice. Mol Immunol 62:142–149. doi:10.1016/j.molimm.2014.06.017
Giambartolomei GH, Zwerdling A, Cassataro J, Bruno L, Fossati CA, Philipp MT (2004) Lipoproteins, not lipopolysaccharide, are the key mediators of the proinflammatory response elicited by heat-killed Brucella abortus. J Immunol 173:4635–4642
Goel D, Bhatnagar R (2012) Intradermal immunization with outer membrane protein 25 protects Balb/c mice from virulent B. abortus 544. Mol Immunol 51:159–168. doi:10.1016/j.molimm.2012.02.126
Goel D, Rajendran V, Ghosh PC, Bhatnagar R (2013) Cell mediated immune response after challenge in Omp25 liposome immunized mice contributes to protection against virulent Brucella abortus 544. Vaccine 31:1231–1237. doi:10.1016/j.vaccine.2012.12.043
Golding B et al (2001) Immunity and protection against Brucella abortus. Microbes Infect 3:43–48
Gregory AE, Titball R, Williamson D (2013) Vaccine delivery using nanoparticles. Front Cell Infect Microbiol 3:13. doi:10.3389/fcimb.2013.00013
Hall JB, Dobrovolskaia MA, Patri AK, McNeil SE (2007) Characterization of nanoparticles for therapeutics. Nanomedicine (Lond) 2:789–803. doi:10.2217/17435889.2.6.789
Jabbal-Gill I, Watts P, Smith A (2012) Chitosan-based delivery systems for mucosal vaccines. Expert Opin Drug Deliv 9:1051–1067. doi:10.1517/17425247.2012.697455
Johansen P, Mohanan D, Martinez-Gomez JM, Kundig TM, Gander B (2010) Lympho-geographical concepts in vaccine delivery. J Control Release 148:56–62. doi:10.1016/j.jconrel.2010.05.019
Kammona O, Kiparissides C (2012) Recent advances in nanocarrier-based mucosal delivery of biomolecules. J Control Release 161:781–794. doi:10.1016/j.jconrel.2012.05.040
Kostrzak A et al (2009) Oral administration of low doses of plant-based HBsAg induced antigen-specific IgAs and IgGs in mice, without increasing levels of regulatory T cells. Vaccine 27:4798–4807
Kumar P, Chen K, Kolls JK (2013) Th17 cell based vaccines in mucosal immunity. Curr Opin Immunol 25:373–380. doi:10.1016/j.coi.2013.03.011
Luna-Martinez JE, Mejia-Teran C (2002) Brucellosis in Mexico: current status and trends. Vet Microbiol 90:19–30
Luo D et al (2006) Protective immunity elicited by a divalent DNA vaccine encoding both the L7/L12 and Omp16 genes of Brucella abortus in BALB/c mice. Infect Immun 74:2734–2741. doi:10.1128/IAI.74.5.2734-2741.2006
Mahapatro A, Singh DK (2011) Biodegradable nanoparticles are excellent vehicle for site directed in-vivo delivery of drugs and vaccines. J Nanobiotechnol 9:55. doi:10.1186/1477-3155-9-55
Mobley HL, Island MD, Hausinger RP (1995) Molecular biology of microbial ureases. Microbiol Rev 59:451–480
Mohanan D et al (2010) Administration routes affect the quality of immune responses: a cross-sectional evaluation of particulate antigen-delivery systems. J Control Release 147:342–349. doi:10.1016/j.jconrel.2010.08.012
Montejo JM, Alberola I, Glez-Zarate P, Alvarez A, Alonso J, Canovas A, Aguirre C (1993) Open, randomized therapeutic trial of six antimicrobial regimens in the treatment of human brucellosis. Clin Infect Dis 16:671–676
Murphy EA, Sathiyaseelan J, Parent MA, Zou B, Baldwin CL (2001) Interferon-gamma is crucial for surviving a Brucella abortus infection in both resistant C57BL/6 and susceptible BALB/c mice. Immunology 103:511–518
Neutra MR, Kozlowski PA (2006) Mucosal vaccines: the promise and the challenge. Nat Rev Immunol 6:148–158
Pappas G, Papadimitriou P, Akritidis N, Christou L, Tsianos EV (2006) The new global map of human brucellosis. Lancet Infect Dis 6:91–99. doi:10.1016/S1473-3099(06)70382-6
Pasquevich KA et al (2009) Immunization with recombinant Brucella species outer membrane protein Omp16 or Omp19 in adjuvant induces specific CD4+ and CD8+ T cells as well as systemic and oral protection against Brucella abortus infection. Infect Immun 77:436–445. doi:10.1128/IAI.01151-08
Pasquevich KA et al (2011) An oral vaccine based on U-Omp19 induces protection against B. abortus mucosal challenge by inducing an adaptive IL-17 immune response in mice. PLoS One 6:e16203
Perkins SD, Smither SJ, Atkins HS (2010) Towards a Brucella vaccine for humans. FEMS Microbiol Rev 34:379–394
Perrie Y, Mohammed AR, Kirby DJ, McNeil SE, Bramwell VW (2008) Vaccine adjuvant systems: enhancing the efficacy of sub-unit protein antigens. Int J Pharm 364:272–280. doi:10.1016/j.ijpharm.2008.04.036
Saraiva M, Christensen JR, Veldhoen M, Murphy TL, Murphy KM, O’Garra A (2009) Interleukin-10 production by Th1 cells requires interleukin-12-induced STAT4 transcription factor and ERK MAP kinase activation by high antigen dose. Immunity 31:209–219. doi:10.1016/j.immuni.2009.05.012
Shakir RA (1986) Neurobrucellosis. Postgrad Med J 62:1077–1079
Slutter B et al (2009) Mechanistic study of the adjuvant effect of biodegradable nanoparticles in mucosal vaccination. J Control Release 138:113–121. doi:10.1016/j.jconrel.2009.05.011
Subbiah R, Ramalingam P, Ramasundaram S, Kim do Y, Park K, Ramasamy MK, Choi KJ (2012) N,N,N-Trimethyl chitosan nanoparticles for controlled intranasal delivery of HBV surface antigen. Carbohydr Polym 89:1289–1297. doi:10.1016/j.carbpol.2012.04.056
Tabynov K et al (2014a) Influenza viral vectors expressing the Brucella OMP16 or L7/L12 proteins as vaccines against B. abortus infection. Virol J 11:69. doi:10.1186/1743-422X-11-69
Tabynov K, Yespembetov B, Sansyzbay A (2014b) Novel vector vaccine against Brucella abortus based on influenza A viruses expressing Brucella L7/L12 or Omp16 proteins: evaluation of protection in pregnant heifers. Vaccine 32:5889–5892. doi:10.1016/j.vaccine.2014.08.073
Vemulapalli R et al (2000) Characterization of specific immune responses of mice inoculated with recombinant vaccinia virus expressing an 18-kilodalton outer membrane protein of Brucella abortus. Clin Diagn Lab Immunol 7:114–118
Verheul RJ, Slutter B, Bal SM, Bouwstra JA, Jiskoot W, Hennink WE (2011) Covalently stabilized trimethyl chitosan-hyaluronic acid nanoparticles for nasal and intradermal vaccination. J Control Release 156:46–52. doi:10.1016/j.jconrel.2011.07.014
Vitry MA et al (2012) Crucial role of gamma interferon-producing CD4+ Th1 cells but dispensable function of CD8+ T cell, B cell, Th2, and Th17 responses in the control of Brucella melitensis infection in mice. Infect Immun 80:4271–4280. doi:10.1128/IAI.00761-12
Acknowledgments
This work was supported by INSF (No: 91000787): Iran National Science Foundation. We would like to thank Dr. Bram Slutter and Dr. Nima Khoramabadi for their helpful advice.
Conflict of interest
The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Abkar, M., Lotfi, A.S., Amani, J. et al. Survey of Omp19 immunogenicity against Brucella abortus and Brucella melitensis: influence of nanoparticulation versus traditional immunization. Vet Res Commun 39, 217–228 (2015). https://doi.org/10.1007/s11259-015-9645-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11259-015-9645-2