Abstract
Since Brucella infection mostly occurs through the mucosal surfaces, immune response induced by vaccine that is delivered by a way of mucosal route can be drastically enhanced to control the brucellosis. Omp31is the major outer membrane protein of Brucella, and is considered as a protective antigen against Brucella infection. Accordingly, Lactococcus lactis has been used as an antigen-delivering vector to develop a vaccine-induced mucosal response for having a safer vaccination against brucellosis. A designed omp31 gene fused to the usp45 signal peptide and M6 cell wall anchor was sub cloned in the pNZ7021 expression vector, and a recombinant L. lactis displaying Omp31 was constructed. Omp31 protein expression was confirmed using Western blotting and immunofluorescence analysis. Animals were orally and intraperitoneally immunized with live or killed L. lactis expressing Omp31, respectively. The humoral and cellular immune responses were evaluated by measuring the specific cytokines and antibodies. sIgA, serum IgA, IgM, and total IgG antibodies significantly increased in the mice immunized with live recombinant L. lactis expressing Omp31 and also serum IgM, and total IgG antibodies significantly increased in mice immunized with killed recombinant L. lactis expressing Omp31. Among IgG subtypes, IgG2a response was significantly higher in both groups compared to IgG1. In mice groups immunized with recombinant L. lactis, the IFN-γ and IL-10 level elevated; however, there was no change in the level of IL-4. These results indicated that recombinants L. lactis induce both humoral and cellular immune responses in mice, and also vaccines based on L. lactis-derived live carriers are promising interventions against Brucella melitensis infections.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brucellosis is a widespread zoonotic disease causing more than 500 million cases worldwide; with more than 500,000 new cases each year. The World Health Organization (WHO) considered brucellosis as one of the seven neglected endemic zoonoses like as examples of other endemic zoonoses: anthrax, rabies, human African trypanosomiasis [1,2,3]. Brucellosis is caused by Brueclla spp., which is a Gram-negative coccobacillus lacking capsule, flagella, and endospore [4]. Brueclla melitensis and Brueclla abortus are the main causative agents of brucellosis among animals. The disease caused by B. melitensis has a high rate in developing countries. In humans, this endemic disease is mainly acquired from animals directly or indirectly [2, 5]. Brucella is a facultative intracellular microorganism being able to survive in an extracellular environment, but this pathogen must replicate intracellularly to perpetuate [6].
This bacteria is one of the major etiologic agents responsible for abortion in cattle, sheep, and goats [4]. In addition, infection in humans results in febrile disease (Malta fever) causing economic impacts [7]. The protection against Brucella similar to other facultative intracellular bacterial pathogens depends on a long-lived cellular immune response [5]. The bacterium is able to induce a chronic infection that often makes the treatment and diagnosis difficult [5]. The response to Brucella infection is typically evaluated in mice, and protective immunity seems to be mediated by both cellular and humoral effector mechanisms that are required to prevent the disease [8, 9]. The clearance of intracellular bacteria depends on responses triggered by T helper type 1 cells (Th1 cells), characterized by the production of cytokines, especially IFN-γ and humoral responses based on IgG2a synthesis [5, 10, 11]. Live attenuated vaccines like the B. abortus S19, RB51, and B. melitensis Rev1 can effectively stimulate cell-mediated immunity (CMI) responses against brucellosis that are used to control the disease in domestic animals; however, they have also several disadvantages and are far from the ideal vaccine, e.g., these vaccines induce abortions when applied during pregnancy, elicit antibodies to smooth lipopolysaccharide (LPS) of Brucella interfering in serodiagnosis, and are virulent for humans [10, 11]. Selecting an effective antigen and also a good delivery system are the two most important items that should be considered in the development of an appropriate vaccine. [12]. Some Brucella immunogenic antigens have been found in the outer membrane of this microorganism. Bacterial surface antigens are the first candidates, which display the primary point of contact between the pathogen and host [13]. Among the Brucella antigens, Omp31 has been used as a DNA vaccine against B. melitensis and Brucella ovis challenges. Also, some studies have shown that Omp31 is capable of stimulating cellular and humoral immune responses [12, 14].
Lactic acid bacteria (LAB), particularly Lactococcus spp. and Lactobacillus spp. have been used in some oral immunization trials [15,16,17,18]. Lactococcus lactis, as a model of LAB, is able to survive the intestinal tract without colonizing it [19]. Lactococcus lactis, as a model of LAB, is able to survive the intestinal tract without colonizing it. This microorganism is Gram-positive and is also free of LPS, and lacks endotoxin. In the past decade, LAB have been used in numerous studies as a tool for antigen presentation [20, 21].
Noninvasive and nonpathogenic features in some LAB strains are promising in antigen delivery systems, which can overcome the problems of using attenuated B. abortus strains as antigen. Also, it can provide a means for large-scale and low-cost vaccine administration. Studies have shown that mucosal immunization using modified LAB for the production of viral and bacterial antigens elicits effective humoral and cell-mediated immune responses [18, 19, 22].
In poor or less developed countries, the preparation, costs, and subcutaneously vaccination of livestock with the recombinant Omp31 vaccine in sufficient quantities may be a major problem due to the extensive use of the vaccine. Therefore, designing a cheaper and easier way for the successful delivery of a proper immunoprotective antigen seems to be essential.
In the present study, we analyzed the potential of L. lactis to express Omp31 protein at the cell surface, and its efficacy as an antigen delivery vector in live or killed form for vaccination in the BALB/c mice.
Materials and Methods
Bacterial Strains and Growth Conditions
Escherichia coli Top10 and BL21 (DE3) were grown in Luria–Bertani medium (LB) (Sigma-Aldrich, St. Louis, MO) at 37 °C with shaking at 200 rpm and L. lactis NZ9000 was grown in the M17 medium (Quelab, Montreal, Canada) supplemented with 0.5% (w/v) glucose at 30 °C without shaking. Plasmids were selected by the addition of antibiotics, 100 μg/ml ampicillin (Bio Basic, Markham, Canada), and 10 μg/ml chloramphenicol (Bio Basic, Markham, Canada).
Expression and Purification of Omp31 Protein
In short, DNA was extracted from B. melitensis strain Rev1using accuPrep® genomic DNA extraction kit (Bioneer, Daejeon, Korea). Omp31 was cloned into pTZ57R/T vector and transformed into E. coli Top10F, then this plasmid was digested using EcoRI and BamHI endonucleases and was subcloned into pET-32a (+). Next E. coli BL21 (DE3) was used to synthesize Omp31 protein. After reaching the culture OD to 0.5, induction by 1 mM isopropyl β-d-1-thiogalactopyranoside (IPTG, Thermo Fisher Scientific, Waltham, Massachusetts, USA) was done for 6 h, at 37 °C. Expressed recombinant Omp31 (rOmp31) protein was purified by Ni-NTA agarose (QIAGEN, Hilden, Germany) according to the manufacturer’s protocol. Purity and identity of the purified protein were surveyed using SDS-PAGE coomassie blue staining and Western blotting. The Bradford method was used to determine the concentration of recombinant protein [23, 24]. Finally, Western blotting was performed with anti-6xHis peroxidase (Sigma, USA) (1:2000) to confirm the rOmp31 protein.
Production of Polyclonal Antibodies Against Purified Recombinant Omp31 Protein
A white female rabbit was immunized intradermally with 200 μg of purified recombinant Omp31 (rOmp31) protein emulsified with complete Freund’s adjuvant (CFA, Sigma-Aldrich., St. Louis, MO, USA). After 2 weeks, the rabbit was immunized with 200 μg of rOmp31 and incomplete Freund’s adjuvant (IFA, Sigma-Aldrich) then 2 weeks later, the rabbit serum was collected and stored at − 80 °C for further studies.
Construction of Recombinant L. lactis
A gene cassette was designed and synthesized in pGH vector (Generay Biotechnology, Shanghai, China) to encode the precursor protein SPusp45-Omp31-CWA M6, composed by the signal peptide Usp45, the major secreted protein in lactococci, fused with Omp31 protein, and the M6 protein, a cell wall anchor from Streptococcus pyogenes commonly used for cell wall anchoring of heterologous proteins in L. lactis.
The designed gene construct was subcloned to pNZ7021 (MoBiTec, Goettingen, Germany) using SphI and SacI restriction enzymes (Thermo Fisher, USA), resulting in recombinant pNZ7021-Omp31. The recombinant plasmid was finally electrotransformed into the L. lactis strain NZ9000 (MoBiTec), as previously described [25, 26]. The L. lactis transformants containing pNZ7021-Omp31 were cultured in M17 agar supplemented with glucose, containing 10 μg/ml chloramphenicol and incubated at 30 °C, 24 to 48 h. Positive clones were selected and confirmed by colony PCR using pNZ primers. Recombinant expression vectors were extracted from positive clones and confirmed through restriction digestion, and DNA sequencing (data not shown). The bacterial strains, plasmids, and primers used in this study have been represented in Tables 1 and 2.
Expression and Identification of the Omp31 Protein in L. lactis by Western Blotting
Briefly, recombinant L. lactis (rL. lactis) (pNZ7021-Omp31) was cultured at 30 °C overnight and harvested by centrifugation at 12,000 rpm for 10 min at 4 °C. The cells were washed three times with PBS 1X (pH 7.2) and suspended in 50 μl PBS 1× (pH 7.2). For disruption of the cell wall, 100 μl of 10 mg/ml lysozyme (Sinaclon, Tehran, Iran) was added and incubated at 55 °C for 1 h. Finally, the bacterial protein supernatant examined by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was electrotransferred onto the nitrocellulose membrane. After overnight blocking with Tris-buffered saline, 0.05% Tween-20 (TBST) buffer containing 5% skimmed milk at 4 °C, the membrane was incubated with a rabbit anti Omp31 polyclonal antibody (1:200) in tris-buffered saline (TBS) for 2 h. Then, the nitrocellulose membrane was washed three times and incubated with 1:5000 goat anti-rabbit IgG-peroxidase (Sigma-Aldrich) for 60 min and Omp31 was analyzed after adding diaminobenzidine (DAB, Sigma-Aldrich) [25].
Immunofluorescence Microscopy
rL. lactis (pNZ7021-Omp31) was cultured in the M17 medium at 30 °C, harvested by centrifugation, and washed three times with PBS 1× (pH 7.2), and then 20 μl of the sample was put on slides precoated with poly-l-lysine and also incubated for 15 min. Finally, slides were fixed with 4% paraformaldehyde for 20 min. For blocking, the cells were incubated with 4% bovine serum albumin (BSA) in PBS 1X (pH 7.2) for 30 min at room temperature (RT). After washing three times with PBS 1× (pH 7.2), the cells were incubated with rabbit anti-Omp31 polyclonal antibody 1:250 at RT for 1.5 h. The cells were washed three times with PBS 1X (pH 7.2) and incubated with FITC-conjugated goat anti-rabbit antibody (Sigma-Aldrich) 1:1000 at RT for 1.5 h. Finally, slides were washed three times with PBS 1× (pH 7.2) and mounted with glycerol. The labeled slides were then analyzed by immunofluorescence microscopy (EUROStar III Plus, Germany) [25].
Animals
The 6- to 8-week-old female BALB/c mice acclimated in the animal facility and randomly distributed into experimental groups. Mice were kept under optimal conditions of temperature, humidity, light (cycles of 12 h dark/light), and hygiene with free access to food and water during the experiment. All experimental procedures on animals were approved by the ethical committee of Zanjan University of Medical Sciences (ZUMS.REC.1396.146). Mice were assigned in seven groups (5 mice per group). rL. lactis (pNZ7021-Omp31) and rL. lactis (pNZ7021) were grown as described earlier. The test and control groups were orally immunized with the 1010 colony-forming unit (CFU) of rL. lactis (pNZ7021-Omp31) and rL. lactis (pNZ7021) respectively. The negative control group was immunized orally with PBS 1X (pH 7.2). Oral immunization was performed for 4 weeks (2 times per week) by using a feeding tube. The other two groups were intraperitoneally immunized with 1010 CFU heat-killed rL. lactis (pNZ7021-Omp31) and rL. lactis (pNZ7021) respectively. Killed bacteria were prepared heating the culture of L. lactis in a water bath at 60 °C for 20 min. Intraperitoneally immunization was performed three times: days 0, 15, and 30. The positive control group was immunized intraperitoneally with purified rOmp31, 30 μg protein emulsified with CF adjuvant (Sigma-Aldrich) on day 0, and IF adjuvant (Sigma-Aldrich) on days 15 and 30. The negative control group was immunized intraperitoneally with PBS 1× (pH 7.2) on days 0, 15, and 30.
Humoral Immune Response Assessment (ELISA Assay)
Mice sera were obtained prior to immunization and 2 weeks after the last immunization from tail bleed and stored at − 70 °C. The presence of serum Omp31-specific immunoglobulin G (total IgG), IgG1, IgG2a, IgM, and IgA was determined by indirect enzyme-linked immunosorbent assay (ELISA). The purified rOmp31 protein (1 μg/well) in carbonate buffer (pH 9.6) was coated in 96-well high binding plates (Greiner-bio-one, Frickenhausen, Germany) and incubated for 16 h at 4 °C. Then, the wells were washed three times with PBST wash buffer (PBS 1× (pH 7.2) containing 0.05% Tween 20) and blocked for 1 h at 37 °C with 1% BSA in PBS 1X (pH 7.2). Plates were then incubated with mouse sera (1:200) for 2 h at RT and washed three times with PBST. In the next step, wells were incubated with 100 μl of a 1:1000 dilution of polyclonal goat anti-mouse IgG (Fc):horseradish peroxidase (HRP) conjugated (Bio-Rad, USA) for 1 h at 37 °C. For measurement of IgG1, IgG2a subclasses, and IgM, IgA isotypes, the wells were incubated with 100 μ; of a 1:1000 dilution of anti isotype monoclonal antibody (Sigma-Aldrich), for 1 h at 37 °C. Then the wells were washed three times with PBST. At the next step, 100 μl of a 1:5000 dilution of peroxidase-conjugated rabbit anti-goat IgG whole molecule (Sigma, USA) was added for 1 h at 37 °C. After a final washing step, color development was triggered by the addition of 100 μl/well of the enzyme-substrate ABST (2,2′-Azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt) (KPL-SeraCare Life Sciences, Milford, MA, USA) for 30 min. The reaction was stopped by adding 100 μl of 1% SDS in each well. Optical density (OD) at 405 nm was measured using an ELISA plate reader (BioTek, Winooski, VT, USA). We used fecal pellets for the measurement of mucosal IgA (sIgA) levels. The fecal pellet samples were collected 2 weeks after the last immunization, weighed, homogenized at a final concentration of 100 mg per 0.5 ml of PBS 1× (pH 7.2) containing 1% BSA. Then, the samples were incubated for 16 h at 4 °C, centrifuged at 15,000 rpm for 5 min at 4 °C, and finally, the supernatants were used to detect sIgA.
ELISA was performed using purified rOmp31 protein for the detection of specific sIgA. Further, 96-well microtiter high binding plates (Greiner-bio-one) were coated with 2 μg/well Omp31 protein in 100 μl carbonate buffer (pH 9.6) and incubated 16 h at 4 °C. Afterward, the wells were washed three times with PBST and blocked for 1 h at 37 °C with 1% BSA in PBS 1× (pH 7.2). Plates were then incubated with 100 μl fecal supernatants for 2 h at room temperature and washed three times with PBST. A goat monoclonal anti-mouse IgA (Sigma, USA) and peroxidase-conjugated rabbit anti-goat IgG whole molecule (Sigma, USA) was used for detection of sIgA.
Determination of Cytokine Levels
To evaluate the cellular immune response, 2 weeks after the last immunization, mice were euthanized, and under aseptic conditions, their spleens were removed. Single-cell suspensions were prepared from the spleens by mechanical dissociation and homogenization on ice. Briefly, red cells lysis was performed by ACK solution (0.1 mM Na2EDTA, 150 mM NH4Cl, 10 mM KHCO3) and then cells were washed three times with PBS 1× (pH 7.2). Finally, splenocytes were cultured in RPMI 1640 (Inoclon, Karaj, Iran) supplemented with 10% heat-inactivated fetal bovine serum (FBS, Gibco, Paisley, UK), 0.05 M 2-Mercaptoethanol, 2 mM l-glutamine, and 1% antibiotic solution (penicillin, streptomycin) (Sigma-Aldrich). Splenocytes viability was evaluated by Trypan blue solution (Sigma-Aldrich). Splenocytes were seeded a total number of 3 × 106 cells in a 48-well plate. Cells were incubated in vitro at 37 °C in 5% CO2 with rOmp31 (10 μg/ml). Positive control wells received 5 μg/ml concanavalin A (Con A, Sigma-Aldrich) and non-stimulated cells were considered as a negative control. Cell culture supernatants were collected, 72 h after stimulation, and stored at − 80 °C. Levels of interferon-gamma (IFN-γ), interleukin-10, and interleukin-4 were measured in culture supernatants by sandwich ELISA according to the manufacturer’s instructions (R&D Systems, Minneapolis, USA).
Lymphocyte Proliferation Assay (MTT Assay)
We used 3-(4,5dimethylthiazole-2yl)-2,5 diphenyl tetrazolium bromide (MTT, 5 mg/ml) (Sigma-Aldrich (assay to investigate the splenocytes proliferative response. The supernatant was removed and 20 μl MTT was added to each well. After 4 h of incubation at 37 °C, the plate was centrifuged in 1000×g for 5 min at RT, the supernatant was removed, and 150 μl of dimethyl sulfoxide (DMSO) (Merck, Darmstadt, Germany) was added to dissolve the formazan crystals for 20 min at 37 °C. The test was read at 570 nm wavelength (BioTek). Lymphocyte proliferation was defined by proliferation index (PI) which was calculated as follows: the ratio of the mean optical density of stimulated splenocyte cultures to mean optical density of the non- stimulated splenocyte cultures.
Statistical Analysis
The data corresponding to the evaluation of the level of antibodies, cytokines, and lymphocyte proliferation was analyzed by two-way ANOVA and one-way ANOVA. All experiments were performed in duplicate and results were reported as mean ± standard deviation (SD). A p value of 0.05 or less was considered statistically significant.
Results
Expression of Omp31 in E. coli
The size of the recombinant Omp31 protein after expression and purification was verified by SDS-PAGE and Western blotting and the expected 51 kDa rOmp31 was confirmed (Fig. S1).
Cloning and Expression of a Cell Wall-Anchored Omp31 in L. lactis
The schematic map of the pNZ7021-Omp31 plasmid is shown in Fig. 1 (by Snap Gene). Recombinant vector (pNZ7021-Omp31) was validated by PCR amplification using pNZ primers and Sanger Sequencing. The omp31 gene was amplified by PCR and the expected size of the amplified fragment corresponded to 1395 bp (Fig. S2). Restriction digestion was performed for more confirmation (Fig. S3).
Immunoblotting revealed 42 kDa Omp31 protein in the supernatant of the recombinant L. lactis, corresponding to the expected size of precursor SP Usp45- Omp31-CWAM (Fig. S4).
Immunofluorescence Microscopy
The immunofluorescence assay is essential for the determination of the Omp31 protein localization on the L. lactis. Recombinant L. lactis (pNZ7021-Omp31) cells showed green fluorescence signals on the cell surface (Fig. 2).
Evaluation of Humoral Immune Responses
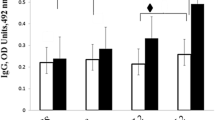
A significant increase in serum IgM and IgG was seen in mice groups which were immunized with the killed and live form of L. lactis pNZ7021-Omp31 compared to the control groups (p < 0.05, Figs. 3 and 4).
Mice group which orally immunized with live and intraperitoneally immunized with killed rL. lactis showed higher IgG1 and IgG2a levels compared to the control group (p < 0.05, Fig. 5).
sIgA antibody level in mice group orally immunized with live rL. lactis (pNZ7021-Omp31) was significantly higher compared to the control group(p < 0.05, Fig. 6a). Also immunization of mice with live recombinant L. lactis expressing Omp31 induced significant serum IgA responses (p < 0.05, Fig. 6b).
Omp31-specific IgA antibodies. a Anti-Omp31 mucosal IgA antibody responses in mice orally immunized with live recombinant rL. lactis (pNZ7021-Omp31) and control rL. lactis (pNZ7021). Fecal samples were assessed for Omp31-specific IgA by ELISA. b Sera samples from mice immunized with live and killed recombinant L. lactis expressing Omp31were tested for serum IgA (*p < 0.05;**p < 0.01). Data are shown as mean ± SD of duplicate experiments
Proliferative Responses of Splenocytes
The MTT assay results showed that the proliferative response of splenocytes from mice immunized with killed and live L. lactis were significantly increased compared to the control group (p < 0.05, Fig. 7).
Proliferative responses of mice splenocytes stimulated with rOmp31 antigen in vitro.MTT assay was used to analyze splenocyte proliferation in response to rOmp31 antigen following 72 h stimulation with 10 μg/ml of rOmp31or 5 mg/ml of Con A as Tcell mitogen. The magnitude of the proliferative response is expressed as the proliferative index (PI) defined as the ratio of the mean absorption of cells incubated with antigen to the mean absorption of cells incubated with medium alone
Evaluation of Cytokine Levels
Both oral or intraperitoneal immunized mice groups with recombinant L. lactis demonstrated significant secretion of IFN-γ and IL-10 compared to the control (p < 0.05, Fig. 8a, b) but there was no significant change in the level of IL-4 (p > 0.05, Fig. 8c).
Discussion
The use of live vector vaccine systems using probiotics has been considered by many researchers as an effective antigen delivery system. Among these, the LAB has been used as a safe model in many studies [27, 28].
In our study, to anchor the Omp31 protein in the cell wall, we used native usp45 secretion signal at the N-terminus, and the M6 CWA from Streptococcus pyogenes at the C-terminus of the protein, and finally the gene cassette was subcloned into the pNZ7021, which is an effective expression vector in L. lactis [25, 29]. Here, we demonstrated that Omp31 can be efficiently displayed at the surface of L. lactis using Western blotting and immunofluorescence analysis (Fig. S4 and Fig. 2). Also, studies had shown that heterologous expression of anchored proteins in the cell wall of L. lactis can act as an adjuvant and boost the host’s immune response [19]. Another advantage of the membrane-bound expression is that the protein is less prone to be inactivated by degrading and/or denaturing agents, such as proteinases and pH variation from gastrointestinal tract [19]. We demonstrated that oral immunization of mice with live rL. lactis expressing Omp31 could induce significant Omp31-specific mucosal IgA secretion (Fig. 6a). In a study by Stabel et al. (1990), the researchers used an attenuated Salmonella typhimurium to present the 31-KDa Brucella abortus BCSP31 antigen. Immunological studies also showed that oral administration of the vaccine resulted in a slight increase in IgA level in the sera of immunized mice besides an undetectable IgA in saliva [30]. In a study by Villena et al. (2008), the researchers used rL. Lactis to present a pneumococcal protective protein A (PppA). Immunological studies also showed that oral administration of the vaccine resulted in an elevated level of serum and mucosal IgA [31]. Also in our study, serum IgA level in the mice immunized with live rL. lactis (pNZ7021-Omp31) significantly increased compared to the control group (Fig. 6b). Results from other two similar studies using rL. lactis as delivery system of SOD and L7/L12 antigens from B. abortus showed that antigen-specific IgA secretion was comparable to our study [15, 32]. Despite the significant increase in total IgG and IgM levels in serum of both mice groups immunized with pNZ7021-Omp31 orally or intraperitoneally, low IgM/IgG ratios can be due to IgM isotype switching to IgG (Figs. 34) [31]. An increased IgG2a/IgG1 ratio was detected in the mice sera that were orally or intraperitoneally immunized with pNZ7021-Omp31 (Fig. 5). The results of some similar studies showed that using Omp31 protein can improve the IgG2a/IgG1 ratio. Which could be indicative of an activation of the Th1-type immune response [33,34,35,36]. IgG2a plays an important role in immune responses since its Fc domain binds to a receptor on phagocytes which subsequently results in the stimulation of a vast spectrum of anti-microbial responses [34, 37].
Induction of an immunoglobulin class switching to IgG2 and the activation of cytotoxic T cells are indicated as Th1-type immune response in mice, while the production of IgM, IgG1, IgA, and IgE class antibodies indicate a Th2-type response [38]. Our study showed that oral and intraperitoneal immunization with the live or killed forms of rL. lactis expressing Omp31 was able to induce a humoral immune response in mice.
Similar to all intracellular pathogens, immunity against brucellosis depends mainly on a suitable immune response. The activation of T lymphocytes and cellular immunity plays a pivotal role in the induction of protective immunity against brucellosis [39,40,41]. The stimulation of cellular immunity was evaluated by the proliferative measure of splenocytes beside the cytokines profile after the stimulation of splenic cells using rOmp31. Lymphocyte proliferation in conjunction with IFN-γ and IL-10 production in the groups that received rL. lactis-expressing Omp31 (orally and intraperitoneally) indicates an effective stimulation of Th1-type cellular immune response. In this regard, studies about immunizations models using rOmp31, similar to our control group (rOmp31), had confirmed these results and indicated an increase in IFN-γ synthesis compared to IL-4 and IL-10 [34, 36, 41]. IFN-γ is one of the most important components of Th1 cells that stimulate macrophages and mononuclear cells to produce IL-12 that helps in the differentiation of Th1 cells. In turn, Th1-type cells secrete IFN-γ and induces class switching to IgG2a, which facilitates phagocytosis via opsonization and antibody-dependent cell-mediated cytolysis (ADCC) phenomenon [42].
In this study, mice immunized with live or killed rL. lactis-expressing Omp31 showed a predominant Th1 polarization as indicated by the cytokine profile and by the synthesis of IgG2a isotype antibody [43]. In our study, an increase in the IL-10 levels indicated that L. lactis can induce IL-10 production by Th2. Studies by Ghasemi et al. (2015) also showed that after immunization with Omp31 antigen, BALB/c mice had an increased production of IFN-γ and IL-10 [44]. Induction of IFN-γ and IL-10 in BALB/c mice can influence T cell activation of both Th1 and Th2 responses. IL-10 and IFN-γ act as isotype switch factors for the B cell production of IgG1 and IgG2a, respectively [45, 46]. There is some other evidence implying that L. lactis, as an antigen delivery platform, can induce both Th1 and Th2 responses with Th1 dominance [15, 47, 48].
Conclusion
The current study is the first report of the efficient expression of B. melitensis Omp31 protein by L. lactis as a delivery vector. Our results showed that oral or intraperitoneal administration of live or killed form of rL. lactis displaying Omp31antigen of Brucella can induce both humoral and cellular immune response and can be also a potential vaccine candidate for the prevention of brucellosis.
References
Pappas G, Papadimitriou P, Akritidis N, Christou L, Tsianos EV (2006) The new global map of human brucellosis. Lancet Infect Dis 6(2):91–99. https://doi.org/10.1016/S1473-3099(06)70382-6
World Health O, United Kingdom. Dept. for International Development. Animal Health P, Food, Agriculture Organization of the United N, World Organisation for Animal H (2006) The control of neglected zoonotic diseases : a route to poverty alleviation : report of a joint WHO/DFID-AHP meeting, 20 and 21 September 2005, WHO headquarters, Geneva, with the participation of FAO and OIE. World Health Organization, Geneva http://apps.who.int/iris/handle/10665/43485. Accessed 10 Mar 2020
Seyedalizadeh N, Alesheikh A, Ahmadkhani M (2019) Spatio-statistical modeling of human brucellosis using environmental parameters: a case study of northern Iran. ISPRS Archives 42:969–973. https://doi.org/10.5194/isprs-archives-XLII-4-W18-969-2019
Moreno E, Moriyón I (2002) Brucella melitensis: a nasty bug with hidden credentials for virulence. Proc Natl Acad Sci U S A 99(1):1–3. https://doi.org/10.1073/pnas.022622699
Pappas G, Akritidis N, Bosilkovski M, Tsianos E (2005) the b. melitensis genome. N Engl J Med 352:2325–2336. https://doi.org/10.1056/NEJMra050570
Guzmán-Verri C, Manterola L, Sola-Landa A, Parra A, Cloeckaert A, Garin J, Gorvel J-P, Moriyón I, Moreno E, Lopez-Goñi I (2002) The two-component system BvrR/BvrS essential for Brucella abortus virulence regulates the expression of outer membrane proteins with counterparts in members of the Rhizobiaceae. Proc Natl Acad Sci U S A 99(19):12375–12380. https://doi.org/10.1073/pnas.192439399
Boschiroli ML, Foulongne V, O'Callaghan D (2001) Brucellosis: a worldwide zoonosis. Curr Opin Microbiol 4(1):58–64. https://doi.org/10.1016/s1369-5274(00)00165-x
Araya LN, Elzer PH, Rowe GE, Enright FM, Winter AJ (1989) Temporal development of protective cell-mediated and humoral immunity in BALB/c mice infected with Brucella abortus. J Immunol 143(10):3330–3337
Olsen SC (2013) Recent developments in livestock and wildlife brucellosis vaccination. Rev Sci Tech 32(1):207–217. https://doi.org/10.20506/rst.32.1.2201
Guilloteau LA, Laroucau K, Vizcaı́no N, Jacques I, Dubray G (1999) Immunogenicity of recombinant Escherichia coli expressing the omp31 gene of Brucella melitensis in BALB/c mice. Vaccine 17(4):353–361. https://doi.org/10.1016/S0264-410X(98)00205-9
Vitry MA, Hanot Mambres D, De Trez C, Akira S, Ryffel B, Letesson JJ, Muraille E (2014) Humoral immunity and CD4+ Th1 cells are both necessary for a fully protective immune response upon secondary infection with Brucella melitensis. J Immunol 192(8):3740–3752. https://doi.org/10.4049/jimmunol.1302561
Nazifi N, Tahmoorespur M, Sekhavati MH, Haghparast A, Behroozikhah AM (2019) In vivo immunogenicity assessment and vaccine efficacy evaluation of a chimeric tandem repeat of epitopic region of OMP31 antigen fused to interleukin 2 (IL-2) against Brucella melitensis in BALB/c mice. BMC Vet Res 15(1):1–11. https://doi.org/10.1186/s12917-019-2074-7
DelVecchio VG, Alefantis T, Ugalde RA, Comerci D, Marchesini MI, Khan A, Lubitz W, Mujer CV (2006) Identification of protein candidates for developing bacterial ghost vaccines against Brucella. Methods Biochem Anal 49:363–377. https://doi.org/10.1002/0471973165.ch19
Cassataro J, Velikovsky CA, de la Barrera S, Estein SM, Bruno L, Bowden R, Pasquevich KA, Fossati CA, Giambartolomei GH (2005) A DNA vaccine coding for the Brucella outer membrane protein 31 confers protection against B. melitensis and B. ovis infection by eliciting a specific cytotoxic response. Infect Immun 73(10):6537–6546. https://doi.org/10.1128/IAI.73.10.6537-6546.2005
Sáez D, Fernández P, Rivera A, Andrews E, Oñate A (2012) Oral immunization of mice with recombinant Lactococcus lactis expressing Cu, Zn superoxide dismutase of Brucella abortus triggers protective immunity. Vaccine 30(7):1283–1290. https://doi.org/10.1016/j.vaccine.2011.12.088
Miyoshi A, Bermúdez-Humarán LG, Ribeiro LA, Le Loir Y, Oliveira SC, Langella P, Azevedo V (2006) Heterologous expression of Brucella abortus GroEL heat-shock protein in Lactococcus lactis. Microb Cell Factories 5(1):14. https://doi.org/10.1186/1475-2859-5-14
Liu J-K, Hou X-L, Wei C-H, Yu L-Y, He X-J, Wang G-H, Lee J-S, Kim C-J (2009) Induction of immune responses in mice after oral immunization with recombinant Lactobacillus casei strains expressing enterotoxigenic Escherichia coli F41 fimbrial protein. Appl Environ Microbiol 75(13):4491–4497. https://doi.org/10.1128/AEM.02672-08
Wegmann U, O'Connell-Motherway M, Zomer A, Buist G, Shearman C, Canchaya C, Ventura M, Goesmann A, Gasson MJ, Kuipers OP (2007) Complete genome sequence of the prototype lactic acid bacterium Lactococcus lactis subsp. cremoris MG1363. J Bacteriol 189(8):3256–3270. https://doi.org/10.1128/JB.01768-06
Ribeiro LA, Azevedo V, Le Loir Y, Oliveira SC, Dieye Y, Piard JC, Gruss A, Langella P (2002) Production and targeting of the Brucella abortus antigen L7/L12 in Lactococcus lactis: a first step towards food-grade live vaccines against brucellosis. Appl Environ Microbiol 68(2):910–916. https://doi.org/10.1128/AEM.68.2.910-916.2002
Bahey-El-Din M, Gahan CG (2011) Lactococcus lactis-based vaccines: current status and future perspectives. Human vaccines 7(1):106–109. https://doi.org/10.4161/hv.7.1.13631
Bahey-El-Din M (2012) Lactococcus lactis-based vaccines from laboratory bench to human use: an overview. Vaccine 30(4):685–690. https://doi.org/10.1016/j.vaccine.2011.11.098
Berlec A, Ravnikar M, Štrukelj B (2012) Lactic acid bacteria as oral delivery systems for biomolecules. Pharmazie 67(11):891–898. https://doi.org/10.1691/ph.2012.1705
Stoscheck CM (1990) Quantitation of protein. Methods Enzymol 182:50–68. https://doi.org/10.1016/0076-6879(90)82008-p
Yousefi S, Sekhavati MH, Tahmoorespur M, Abbassi-Daloii M (2016) Cloning and molecular characterization of Omp31 gene from Brucella melitensis rev 1 strain. Arch Razi Inst 71(2):117–124. https://doi.org/10.22034/ARI.2016.106450
Bohlul E, Hasanlou F, Taromchi AH, Nadri S (2019) TRAIL-expressing recombinant Lactococcus lactis induces apoptosis in human colon adenocarcinoma SW 480 and HCT 116 cells. J Appl Microbiol 126(5):1558–1567. https://doi.org/10.1111/jam.14237
Wegkamp A, van Oorschot W, de Vos WM, Smid EJ (2007) Characterization of the role of para-aminobenzoic acid biosynthesis in folate production by Lactococcus lactis. Appl Environ Microbiol 73(8):2673–2681. https://doi.org/10.1128/AEM.02174-06
Takiishi T, Korf H, Van Belle TL, Robert S, Grieco FA, Caluwaerts S, Galleri L, Spagnuolo I, Steidler L, Van Huynegem K, Demetter P, Wasserfall C, Atkinson MA, Dotta F, Rottiers P, Gysemans C, Mathieu C (2012) Reversal of autoimmune diabetes by restoration of antigen-specific tolerance using genetically modified Lactococcus lactis in mice. J Clin Invest 122(5):1717–1725. https://doi.org/10.1172/JCI60530
Braat H, Rottiers P, Hommes DW, Huyghebaert N, Remaut E, Remon JP, van Deventer SJ, Neirynck S, Peppelenbosch MP, Steidler L (2006) A phase I trial with transgenic bacteria expressing interleukin-10 in Crohn's disease. Clin Gastroenterol Hepatol 4(6):754–759. https://doi.org/10.1016/j.cgh.2006.03.028
Cortes-Perez NG, Azevedo V, Alcocer-González JM, Rodriguez-Padilla C, Tamez-Guerra RS, Corthier G, Gruss A, Langella P, Bermúdez-Humarán LG (2005) Cell-surface display of E7 antigen from human papillomavirus type-16 in Lactococcus lactis and in Lactobacillus plantarum using a new cell-wall anchor from lactobacilli. J Drug Target 13(2):89–98. https://doi.org/10.1080/10611860400024219
Stabel TJMJ, Tabatabai LB, Wannemuehler MJ (1990) Oral immunization of mice with attenuated Salmonella typhimurium containing a recombinant plasmid which codes for production of a 31-kilodalton protein of Brucella abortus. Infect Immun 58(7):2048–2055. https://doi.org/10.1128/IAI.58.7.2048-2055.1990
Villena J, Medina M, Raya R, Alvarez S (2008) Oral immunization with recombinant Lactococcus lactis confers protection against respiratory pneumococcal infection. Can J Microbiol 54(10):845–853. https://doi.org/10.1139/W08-077
Pontes DSDF, Ribeiro LA, Miyoshi A, LeLoir Y, Gruss A, Oliveira SC, Langella P, Azevedo V (2003) Induction of partial protection in mice after oral administration of Lactococcus lactis producing Brucella abortus L7/L12 antigen. J Drug Target 11(8–10):489–493. https://doi.org/10.1080/10611860410001670035
Cassataro JES, Pasquevich KA, Velikovsky CA, de la Barrera S, Bowden R, Fossati CA, Giambartolomei GH (2005) Vaccination with the recombinant Brucella outer membrane protein 31 or a derived 27-amino-acid synthetic peptide elicits a CD4+ T helper 1 response that protects against Brucella melitensis infection. Infect Immun 73(12):8079–8088. https://doi.org/10.1128/iai.73.12.8079-8088.2005
Abbassi-Daloii T, Yousefi SSM, Tahmoorespur M (2018) Impact of heat shock protein 60KD in combination with outer membrane proteins on immune response against Brucella melitensis. APMIS 126(1):65–75. https://doi.org/10.1111/apm.12778
Gupta VKRG, Harms J, Splitter G (2012) Invasive Escherichia coli vaccines expressing Brucella melitensis outer membrane proteins 31 or 16 or periplasmic protein BP26 confer protection in mice challenged with B. melitensis. Vaccine 30(27):4017–4022. https://doi.org/10.1016/j.vaccine.2012.04.036
Doosti A, Ghasemi-Dehkordi P, Javadi GR, Sardari S, Shokrgozar MA (2009) DNA vaccine encoding the Omp31 gene of Brucella melitensis induces protective immunity in BALB/c mice. Res J Biol Sci 4(1):126–131 rjbsci.2009.126.131
Unkeless JC, Scigliano E, Freedman VH (1988) Structure and function of human and murine receptors for IgG. Annu Rev Immunol 6(1):251–281. https://doi.org/10.1146/annurev.iy.06.040188.001343
Shibaki AKS (2002) Induction of skewed Th1/Th2 T-cell differentiation via subcutaneous immunization with Freund's adjuvant. Exp Dermatol 11(2):126–134. https://doi.org/10.1034/j.1600-0625.2002.110204.x
Murphy EA, Sathiyaseelan J, Parent MA, Zou B, Baldwin CL (2001) Interferon-gamma is crucial for surviving a Brucella abortus infection in both resistant C57BL/6 and susceptible BALB/c mice. Immunology 103(4):511–518. https://doi.org/10.1046/j.1365-2567.2001.01258.x
Zhan Y, Liu Z, Cheers C (1996) Tumor necrosis factor alpha and interleukin-12 contribute to resistance to the intracellular bacterium Brucella abortus by different mechanisms. Infect Immun 64(7):2782–2786. https://doi.org/10.1128/IAI.64.7.2782-2786.1996
Clausse M, Diaz AG, Ibanez AE, Cassataro J, Giambartolomei GH, Estein SM (2014) Evaluation of the efficacy of outer membrane protein 31 vaccine formulations for protection against Brucella canis in BALB/c mice. Clin Vaccine Immunol 21(12):1689–1694. https://doi.org/10.1128/cvi.00527-14
Yoshimoto T, Okada K, Morishima N, Kamiya S, Owaki T, Asakawa M, Iwakura Y, Fukai F, Mizuguchi J (2004) Induction of IgG2a class switching in B cells by IL-27. J Immunol 173(4):2479–2485. https://doi.org/10.4049/jimmunol.173.4.2479
Tewari AK, Kurup SP, Baidya S, Barta JR, Sharma B (2015) Protective antibody and cytokine responses in mice following immunization with recombinant beta-tubulin and subsequent Trypanosoma evansi challenge. Parasites Vectors 8(1):580. https://doi.org/10.1186/s13071-015-1189-3
Ghasemi A, Jeddi-Tehrani M, Mautner J, Salari MH, Zarnani A-H (2015) Simultaneous immunization of mice with Omp31 and TF provides protection against Brucella melitensis infection. Vaccine 33(42):5532–5538. https://doi.org/10.1016/j.vaccine.2015.09.013
Mathers AR, Cuff CF (2004) Role of Interleukin-4 (IL-4) and IL-10 in serum immunoglobulin G antibody responses following mucosal or systemic Reovirus infection. J Virol 78(7):3352–3360. https://doi.org/10.1128/jvi.78.7.3352-3360.2004
Mosmann TR, Coffman R (1989) TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol 7(1):145–173. https://doi.org/10.1146/annurev.iy.07.040189.001045
Aliramaei MR, Khorasgani MR, Rahmani MR, Esfahani SHZ, Emamzadeh R (2020) Expression of Helicobacter pylori CagL gene in Lactococcus lactis MG1363 and evaluation of its immunogenicity as an oral vaccine in mice. Microb Pathog 142:103926. https://doi.org/10.1016/j.micpath.2019.103926
Liu X, Qi L, Lv J, Zhang Z, Zhou P, Ma Z, Wang Y, Zhang Y, Pan L (2020) The immune response to a recombinant Lactococcus lactis oral vaccine against foot-and-mouth disease virus in mice. Biotechnol Lett:1–11. https://doi.org/10.1007/s10529-020-02900-6
Acknowledgments
The present study was supported by the grant from Zanjan University of Medical Sciences (grant NO. A- 12-873-7).
The authors thank Dr. Negar Seyed, Dr.Yeganeh Talebkhan at Pasteur Institute of Iran and Dr.Narges Nazifi, Dr.Soheil Yousefi at Ferdowsi University of Mashhad for their technical guidance and constant support.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Amirhossein Taromchi and Hoda Shirdast: Material preparation; Methodology: Hoda Shirdast and Fatemeh Ebrahimzadeh; data collection and analysis: Hoda Shirdast and Amirhossein Taromchi; Rabbit immunization: Esmat Mirabzadeh; Mice immunization: Hoda Shirdast and Keivan Nedaei; Writing - original draft preparation: Hoda Shirdast; Writing - review and editing: Amirhossein Taromchi, Yousef Mortazavi, Mohammad Hadi Sekhavati and Abdolreza Esmaeilzadeh; Supervision: Amirhossein Taromchi. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
The study was approved by the Animal Experimentation Ethics Committee of Zanjan University of Medical Sciences (ZUMS.REC.1396.146).
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shirdast, H., Ebrahimzadeh, F., Taromchi, A.H. et al. Recombinant Lactococcus Lactis Displaying Omp31 Antigen of Brucella melitensis Can Induce an Immunogenic Response in BALB/c Mice. Probiotics & Antimicro. Prot. 13, 80–89 (2021). https://doi.org/10.1007/s12602-020-09684-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-020-09684-1