Abstract
Induced pluripotent stem cells (iPSCs) are thought to be highly beneficial in the field of regenerative medicine and are believed to overcome immunogenic barriers to cell transplantation. However, issues remain regarding their safety and efficiency for medical use. Furthermore, some recent reports have suggested that iPSCs could be targeted by the autologous immune system. To promote practical applications of iPSCs, in depth research using appropriate animal models is needed and porcine species appear to provide an ideal model. Recent studies have focused on the generation of porcine iPSC cells, but no investigations of their immunological properties have been conducted to date. In the present study, we generated putative iPSCs from porcine somatic cells and measured major histocompatibility complex (MHC) expression on the iPSCs and their derivatives. Compact colonies that expressed pluripotent markers appeared 11 days after viral infection. Embryonic bodies (EB) were produced and differentiated into three germ layers in vitro. Karyotyping and swine leukocyte antigen (SLA) typing showed that the iPSCs were identical to parental somatic cells. Porcine iPSCs expressed only low levels of MHC class I and moderately increased levels on their differentiated derivatives, whereas MHC class II was rarely expressed. In the presence of interferon-gamma (IFN-γ), the expression of MHC class I was elevated on differentiated iPSCs, and gradually decreased after withdrawal of the cytokine. Our data suggest that porcine iPSCs could be useful for preclinical studies of the efficiency and viability of iPSCs, and for devising strategies to rescue transplanted cells from the autologous immune system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
iPSCs offer great promise for patient specific cell therapy because they are believed to be free of immunological concerns post-transplantation (Chen and Liu 2009; Kiskinis and Eggan 2010; Lian et al. 2010; Park et al. 2008). Despite these advantages, concerns exist regarding their long term safety and functional efficiency for actual use. Although several studies have shown the therapeutic possibilities of iPSCs in murine models (Hanna et al. 2007; Wernig et al. 2008), more detailed preclinical evaluations using appropriate animal models are needed because mice differ considerably from humans in terms of scale, life span, metabolic rate, and immune system characteristics (Biggar et al. 1985; Dehoux and Gianello 2007; Esteban et al. 2010; Roberts et al. 2009; Wernersson et al. 2005). Pigs comprise an attractive animal model of human diseases because of their anatomical and physiological similarities and availabilities. Moreover, when compared with mice, the porcine innate immune system has been reported to more closely resemble that of human systems in terms of gene regulation and protein sequences, as well as in terms of cellular constituents of circulation and immune pathways (Esteban et al. 2010; Fairbairn et al. 2011; Schook et al. 2005).
The immunogenicity of transplanted cells is of critical concern, but the issue has not been vigorously examined. Some recent studies have shown that pluripotent stem cells might be susceptible targets of the autologous immune system because they express only low levels of MHC class I molecules on their surfaces (Boyd and Wood 2009; Dressel et al. 2010; Drukker et al. 2006; Suarez-Alvarez et al. 2010; Zhao et al. 2011). In contrast to the general expectation that iPSCs might overcome immunological barriers, the missing self hypothesis suggests that cells with no or low of MHC protein on their surfaces could be potential targets of natural killer (NK) cells (de Rham and Villard 2011; Ljunggren and Karre 1990). Thus, to increase safety and beneficial impacts of cell therapy, further investigations of immunological properties of stem cells are required (Dressel et al. 2010).
Some groups have recently generated putative porcine iPSCs, but no studies have been conducted to characterize their MHC expressions. In this study, we generated putative porcine iPSCs and analyzed MHC expression levels on them and their derivatives.
Materials and methods
Cell culture and retroviral infection
Porcine ear fibroblasts (PEF) were obtained from a 4-month-old mixed breed farm pig using a 6 mm skin biopsy punch. To isolate the PEF, the ear tissue was minced with scissors and placed into a 35 mm dish containing high glucose Dulbecco’s modified eagle medium (DMEM, Hyclone, Logan, Utah), 10 % fetal bovine serum (FBS, Hyclone, Logan, Utah), and 10 μg/ml penicillin-streptomycin (Gibco, Carlsbad, CA). After 7–10 days, the outgrowths of fibroblasts appeared and were passaged by trypsinization.
Retroviral polycistronic vector (pE4-OSKM), which includes the human OCT4, SOX2, KLF4, and cMYC sequences linked with picornaviral 2A plasmid (generously provided by In-Hyun Park, Yale University, CT, USA) was used for reprogramming (Loh et al. 2012). Vesicular stomatitis virus G protein vector (VSV-G, plasmid 8454, R. Weinberg) and Gag-Pol vectors (plasmid 8455, R. Weinberg) were acquired from Addgene. The retrovirus was produced by transfection of 14 μg pE4-OSKM, 1.4 μg VSV-G, and 12.6 μg Gag-Pol vectors using 28 μl of 18 mM polyethylenimine (Sigma, St. Louis, MO) into the human embryonic kidney cell line 293 T (HEK293T) in a T175 flask as described previously with some modifiactions (Toledo et al. 2009). Retrovirus-containing medium was collected at day 2 and 3 after transfection, filtered (0.45 μm pore size), and then centrifuged at 23,000 rpm at 4 °C for 90 min to obtain viral pellets.
To produce porcine iPSCs, 5 × 104 PEF were incubated in a 35 mm dish at 37 °C overnight and then infected with retrovirus containing human OCT4, SOX2, KLF4, and cMYC with a multiplicity of infection of 10. Protamine sulfate (5 μg/ml) was also added to increase the infection rate. The day after retroviral infection, cells were washed three times with PBS and then incubated in the same culture media for an additional 72 h. Infected cells were subsequently transferred to a 10 cm dish with 1 × 106 mouse embryonic fibroblast (MEF) feeder cells. Subsequently, cells were maintained in a 2:1 mixture of medium for livestock embryo [Low glucose Dulbecco’s modified eagle medium (Welgene, Daegu, Korea) / Ham’s F10 (Invitrogen, Carlsbad, CA) (1:1) containing 15 % defined FBS (Hyclone, Logan, Utah), 2 mM L-glutamine (Invitrogen, Carlsbad, CA), 0.1 mM non essential amino acid (Invitrogen, Carlsbad, CA), 0.1 mM 2-mercaptoethanol (Sigma, St. Louis, MO), 20 ng/ml human recombinant basic fibroblast growth factor (Millipore, Billerica, MA), and 10 μg/ml penicillin-streptomycin (Gibco, Carlsbad, CA)] to medium for human embryonic stem cells (ES) [High glucose DMEM / Ham’s F12 (Invitrogen, Carlsbad, CA) (1:1) containing 20 % knockout serum replacement (Invitrogen, Carlsbad, CA), 2 mM L-glutamine, 0.1 mM non essential amino acid, 0.1 mM 2-mercaptoethanol, 20 ng/ml human recombinant basic fibroblast growth factor and 10 μg/ml penicillin-streptomycin] at 37 °C in 5 % CO2 (Fig. 1A) (Son et al. 2009; Park et al. 2008). The media was changed daily and MEF feeder cells were obtained as previously described (Kim et al. 2010).
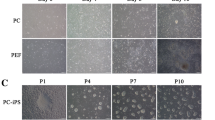
Generation of porcine iPSCs and pluripotent marker expression A Process for the generation of porcine iPSCs from fibroblasts to colony formation. PEFs were infected with retroviruses encoding the Yamanaka 4 factors. Four days post-infection, the cells were seeded onto feeder layers. From the following day, the cells were maintained with iPSCs culture media. iPSC colonies appeared after 11 days of viral infection. B Phase image of porcine fibroblasts (a) and porcine iPSCs on MEF feeder cells (b and c). Porcine iPSC colonies stained positively for alkaline phosphatase (AP) (d). Scale bars = 200 μm. C Immunofluorescence microscopy images of porcine iPSCs show expression of the pluripotent marker SOX2, OCT4, SSEA1, SSEA 4, Tra-1-60, and Tra-1-81. Scale bars = 200 μm. D RT-PCR analysis shows pluripotent gene expression of porcine iPSC compared to PEF and reprogramming vector (pE4-OSKM). Total indicates both exogenous and endogenous genes
Alkaline phosphatase (AP) staining and immunostaining
AP staining was performed using an AP detection kit (Chemicon, Billerica, MA). Immunostaining was accomplished using anti-OCT4 (SC-9081, 1:300) from Santa Cruz (Dallas, TX), anti-SOX2 (AB5603, 1:300), anti-stage-specific embryonic antigen-1 (SSEA1, MAB4301, 1:50), anti-SSEA4 (MAB4301, 1:50), anti-Tra-1-60 (MAB4360, 1:50), and anti-Tra-1-81 (MAB4381, 1:50) from Millipore (Billerica, MA). FITC conjugated secondary antibodies were purchased from Millipore. A LSAB+System-HRP kit (K0679, Dako, Glostrup, Denmark) was used to examine differentiation into the three germ layers. The primary antibodies used were anti-neurofilament (MAB1615, Millipore, Billerica, MA) for ectoderm, anti-alpha smooth muscle actine (ab5694, AbCam, Cambridge, UK) for mesoderm, and anti-keratin (MAB1625, Chemicon, Billerica, MA) for endoderm, all of which were diluted 1:300 in PBS.
Reverse transcription polymerase chain reaction (RT-PCR)
RNA was extracted using RNeasy Mini kits (Qiagen, Valencia, CA) and PCR was carried out using a TProfessional standard 96 gradient machine (Biometra, Goettingen, Germany). The sequences of the primers used are shown in Supplementary Table 1.
EB formation
Porcine iPSC colonies were collected manually and transferred into differentiation media (DMEM containing 10 % FBS) in a bacterial-grade petri dish, after which they were cultured for 10 days. EBs were then replated in a tissue culture plate and cultured for 2 weeks in differentiation media.
Karyotyping and SLA typing
Karyotype assays were carried out at GenDix (Seoul, Korea) using standard protocols by high resolution G-banding. Genomic PCR and direct-sequencing methods were used to determine SLA class I (SLA-1, SLA-2, and SLA-3) and SLA class II (SLA-DQB1 and SLA-DRB1) types of PEF and porcine iPSCs (Thong et al. 2011). Briefly, each locus was amplified by locus specific PCR using genomic DNA prepared from cells. For SLA class I and II genes, genotyping results were determined by sequencing exons 2 and 3 and exon 2, respectively. Sequencing was performed using an ABI Bigdye Terminator kit V3.1 (Applied Biosystems, Foster City, CA) and ABI 3730 automatic DNA analyzer (Applied Biosystems, Foster City, CA). Analyses were conducted following the SLA nomenclature system established by the SLA Nomenclature Committee of the International Society for Animal Genetics (Ho et al. 2009; Smith et al. 2005a, b).
Fluorescence activated cell sorting (FACs) analysis and antibodies
Analysis was performed on a FACSCalibur (Becton Dickinson, Franklin Lakes, NJ) with a Vis. 488 laser optics system using the CELLQUEST software (Becton Dickinson, Franklin Lakes, NJ). Cells were stained with anti SLA class I (MCA2261), SLA class II DQ (MCA1335), and SLA class II DR (MCA2314) primary antibodies, washed, and treated with FITC-conjugated secondary antibody. All antibodies were purchased from AbD Serotec (Kidlington, UK). To obtain porcine macrophages, we collected peripheral blood mononuclear cells from porcine blood and induced macrophage differentiation as previously described (Wulster-Radcliffe et al. 2004).
IFN-γ treatment
To determine the effects of porcine IFN-γ (ProSpec, Est Brunswick, NJ) on MHC expression of 4-week-differentiated porcine iPSCs, different concentrations (0–10 ng/ml) of IFN-γ were added to cell culture media for 48 h. In withdrawal experiments, 0.6 ng/ml of IFN-γ was added to cell culture media for 48 h, after which the cells were washed with PBS and transferred to media without cytokines.
Results
Generation of porcine iPSCs
Eleven days after retroviral infection, initial colonies appeared and presented ES-like morphologies with compact cell adhesion and clear colony borders (Fig. 1B,b–c). The colonies were selected manually and subcultured every 7 days. After initial passage, 1 mg/ml collagenase type IV was used for subculture at a 1:5 ratio. Many non-ES-like cells with loose cell adhesion and no clear border also appeared, but these were easily differentiated. Porcine iPSCs were maintained over 25 passages and their doubling time was 24.9 h.
Pluripotent marker expression
Putative porcine iPSCs stained positively for AP (Fig. 1B, d) and for the pluripotent markers including SOX2 and OCT4 (Fig. 1C and Supplementary Fig. 1 A and B). Colonies were also positive for surface antigens SSEA-1, SSEA-4, Tra-1-60, and Tra-1-81 (Fig. 1C). RT-PCR demonstrated that putative porcine iPSCs expressed many endogenous pluripotent genes, including SOX2, OCT4, NANOG, LIN28, REX1, CDH1, and DNMT. These pluripotent genes were not expressed in the porcine fibroblasts and retroviral vector (Fig. 1D). These findings suggest that reprogramming with Yamanaka 4 factors and our culture conditions could induce endogenous pluripotent gene expression, but that exogenous transgenes also continued to be expressed.
EB formation and differentiation
To examine their differentiation abilities, putative iPSCs were collected and cultured with differentiation media. EBs were formed in 3–5 days and maintained for 10 days (Fig. 2A, a and b). EBs were subsequently replated into tissue culture plates and incubated for 2 weeks. The cells attached and proliferated continuously and some produced cystic-like structures (Fig. 2A,c). Immunostaining showed that putative porcine iPSCs have the ability to differentiate into 3 germ layers (Fig. 2A,d–i). RT-PCR also demonstrated differentiation ability by showing the expression of Amylase for endoderm, Enolase for mesoderm, and Beta-III tubulin for ectoderm (Fig. 2B).
In vitro differentiation and karyotype analysis. A Formation of EBs and differentiation into three germ layers. Porcine iPSCs produced EBs for 10 days (a and b). EBs were replated into the cell culture dish under differentiation conditions and maintained for 2 weeks (c). The differentiated cells contained various types of cells including three germ layers, the ectoderm (d and e), mesoderm (f and g), and endoderm (h and i). Abbreviations: diff2w, 2-week-differentiated cells; NF, neurofilament; αSMA, alpha smooth muscle actin; CK, pan cytokeratin. Scale bars = 200 μm. B RT-PCR analysis of EBs, diff2w, and iPSCs. EBs and diff2w cells demonstrate gene expression of three germ layers. Abbreviations: AMYL, Amylase; ENO, Enolase; TUB, Beta-III tubulin. C Karyotype analysis demonstrated identical chromosomal characteristics (38, XY) of iPSCs compared to fibroblasts. The black arrow indicates a deletion of q11 of chromosome 17
Karyotyping
To test chromosomal translocation during reprogramming, karyotype and G banding analysis were performed. Porcine iPSCs showed a normal 38, XY karyotype, but displayed a q11 deletion in chromosome 17 (Fig. 2C). However, the same chromosomal abnormality was present in the porcine fibroblasts prior to reprogramming, and integration of the transgene did not induce chromosomal translocation.
SLA typing
PCR analysis was performed to confirm that the SLA genotype of putative porcine iPSCs was identical to that of parental somatic cells. SLA typing showed identical compositions in both cell types. Integrated exogenous genes and the reprogramming process did not alter the SLA genotype (Table 1).
Expression levels of MHC on porcine iPSCs and their differentiated derivatives
MHC protein expression was assessed in two different putative porcine iPSCs lines. The timeline of cell differentiation and FACs analysis is summarized in Fig. 3A. For analysis of MHC class I expression, anti-SLA class I antibodies were used. In contrast to parental fibroblasts, putative iPSCs expressed only low levels of MHC class I. To examine whether the differentiation process increased MHC class I expression, we also evaluated levels of SLA class I on EBs. FACs data showed that MHC class I expression was slightly increased on EBs. We subsequently replated EBs into cell culture dishes and cultured them for 2 weeks in differentiation media. Next, we trypsinized the cells and subcultured them for an additional 2 weeks to give a total of 4 weeks in differentiation media. The expression of MHC class I on the surface of 4-week-differentiated cells was slightly higher than that on EBs, but markedly lower than on parental fibroblasts. We next examined MHC class II expression using SLA class II DQ and DR antibodies. Porcine monocyte-derived macrophages stained positively for MHC class II protein, but porcine iPSCs and their differentiated derivatives rarely expressed MHC class II. No expression of MHC class II on porcine fibroblasts was observed (Fig. 3B). The differentiated cells showed loss of pluripotent markers, but they expressed markers of the three germ layers (Fig. 3C).
MHC protein expression of undifferentiated and differentiated porcine iPSCs A Timeline of cell differentiation and FACs analysis. EBs were induced from iPSCs for 10 days with differentiation media. Next, the EBs were transferred into cell culture dishes (Day 0) and cultured for 2 weeks under the same conditions. The cells were subsequently trypsinized and sub-cultured for an additional 2 weeks, giving a total of 4 weeks. Scale bars = 200 μm B FACs analysis of MHC proteins expression in porcine iPSCs and their derivatives. The level of MCH class I expression was measured using SLA class I antibody. The level of MHC class II expression was measured using SLA class II DR and SLA class II DQ antibodies. The black lines indicate negative controls and grey indicates antibody expression of each cell group. C RT-PCR analysis of differentiated iPSCs (diff3w and diff4w) and undifferentiated iPSCs. Diff3w and diff4w cells demonstrated gene expression of three germ layers. SOX2 and OCT4 were expressed in undifferentiated iPSCs, but not in diff3w and diff4w cells. Abbreviation: diff3w, 3-week-differentiated cells; diff4w, 4-week-differentiated cells
IFN-γ increased MHC class I expression
After treatment of 4-week-differentiated iPSCs with IFN-γ for 48 h, a gradual elevation of MHC class I expression was observed, with the greatest increase being observed at an IFN-γ level of 0.6 ng/ml. Higher concentrations of IFN-γ did not induce any further elevation of MHC class I expression level (Fig. 4A). Additionally, MHC class II expression was not upregulated by the addition of IFN-γ (data not shown). After withdrawing IFN-γ from the culture media, MHC class I expression decreased with time, returning to baseline levels at 14 days after withdrawal (Fig. 4B).
Effect of IFN-γ on the MHC expression level of porcine iPSCs-derived differentiated cells A In the FACs histogram, black lines indicate negative controls and grey indicates MHC class I expression of each group. After adding IFN-γ (0–10 ng/ml) to the 4-week-differentiated porcine iPSCs for 48 h, MHC class I expression gradually increased. B Withdrawal of IFN-γ from the culture media induced a gradual decrease in MHC class I protein expression. At 14 days after withdrawal, the MHC class I level represented was similar to that before treatment. w/d withdrawal
Discussion
The pig is a useful animal model for preclinical studies because it is physiologically and immunologically more closely related to humans than rodents (Esteban et al. 2010; Fairbairn et al. 2011; Montserrat et al. 2011; Roberts et al. 2009; Wernersson et al. 2005). Although no convincing porcine ES lines have been produced to date, putative porcine iPSCs have been recently generated by some research groups (Esteban et al. 2009; Ezashi et al. 2009; Montserrat et al. 2011; Wu et al. 2009). However, immunogenicity has not been examined; thus, we generated putative porcine iPSCs and evaluated MHC protein expression in the undifferentiated iPSCs and their differentiated derivatives.
In the present study, we employed a retrovirus containing four reprogramming factors (human OCT4, SOX2, KLF4 and cMYC), which is the traditional method for generating iPSCs. The human genomic sequences of these factors showed great similarity with the porcine sequences. Specifically, homologies of 90 % for OCT4 (NM_001113060.1), 94 % for SOX2 (NM_001123197.1), 90 % for KLF4 (EU669074.2), and 90 % for MYC (NM_001005154.1) were observed. These findings suggest that porcine iPSCs could be generated using human factors. However, comparative approaches to the generation of porcine iPSCs using the four human and porcine factors might help determine the reprogramming efficiency and differences between these two factors. Our putative iPSCs contained exogenous genes in their genome, which is similar to previous findings that most reported porcine iPSCs possessed and expressed exogenous reprogramming genes in their genomes (Esteban et al. 2009; Ezashi et al. 2009; Montserrat et al. 2011; Telugu et al. 2010; Wu et al. 2009). However, further research is needed to establish transgene free porcine iPSCs.
Previously reported putative porcine iPSCs exhibited pluripotent characteristics, although their surface antigen expressions differed greatly (Esteban et al. 2009; Ezashi et al. 2009; West et al. 2010; Wu et al. 2009). Our putative iPSCs also expressed SSEA-1, SSEA-4, Tra-1-60, and Tra-1-81, which matches the characteristics of putative porcine ES (Kim et al. 2010). However, the lack of defined culture conditions and reprogramming protocols for porcine iPSCs and a lack of porcine specific antibodies are likely to cause variations; thus, further investigations are required to optimize these issues.
Evaluation of MHC expression levels on the cells surface is critical for cell therapy. Even though the transplanted cells are of self origin, low expression of MHC could trigger immune rejection by NK cells via inhibitory receptors on the cells (Ljunggren and Karre 1990). Although it is not well understood, the immunogenicity of iPSCs and their derivatives should be determined to increase their viabilities and functional efficiencies for cell therapy. Many preclinical trials have been conducted using iPSC-derived differentiated cells; however, the immunogenicity of these cells has not been sufficiently investigated (Si-Tayeb et al. 2010; Song et al. 2009; Xu et al. 2009; Zhang et al. 2009). We demonstrated MHC expression on the differentiated iPSCs as well as undifferentiated iPSCs because undifferentiated iPSCs would never be used therapeutically. We found that 4-week-differentiated iPSCs expressed only half of the MHC class I as parental cells. In previous reports, MHC protein expression levels were evaluated on human and mouse ES and were found to be low in both (Boyd and Wood 2009; Drukker et al. 2006). Lack of antigen presentation machinery transcription and transport molecules on pluripotent stem cells could induce the absence or down regulation of MHC protein (Suarez-Alvarez et al. 2010). After differentiation, MHC class I expression increased by approximately two to ten times on human ES. For analysis, we used randomly differentiated porcine iPSCs because no efficient protocols have been devised for their differentiation to specific organ lineages during our studies. Since the level of MHC expression could be dependent on differentiated cell conditions, further studies using various cell types are required.
In a previous study, endogenous IFN- γ could not elevate MHC class I after ES transplantation (Boyd and Wood 2009). Pretreatment with IFN-γ could be a possible strategy to reduce cytolysis by autologous NK cells and therefore increase MHC expression of transplanted cells (Boyd and Wood 2009; Drukker et al. 2002). However, the mechanisms through which the in vivo interaction between cytokine treated cells and host response occurs are still unclear. Furthermore, the overexpression of some cytokines could diminish functional abilities of the transplanted cells or induce autoimmune or inflammatory diseases (Baldeon et al. 1997; Nagy et al. 2008; Theofilopoulos et al. 2001). Thus, further research is required and pigs could be a valuable animal model for such investigations.
Conclusions
In summary, we generated putative iPSCs from porcine somatic cells and provided the first report evaluating MHC expression on porcine iPSCs and their derivatives. Putative porcine iPSCs showed pluripotent characteristics and similar MHC expressional patterns as those shown by human ES. Despite the need for future studies, we suggest that porcine iPSCs are likely to be useful for preclinical investigations of the safety, efficiency, and viability of transplanted cells and that they could promote actual applications of iPSCs for medical use.
References
Baldeon ME, Neece DJ, Nandi D, Monaco JJ, Gaskins HR (1997) Interferon-gamma independently activates the MHC class I antigen processing pathway and diminishes glucose responsiveness in pancreatic beta-cell lines. Diabetes 46:770–778
Biggar WD, Barker C, Bohn D, Kent G (1985) An experimental model to study blood and inflammatory neutrophils. Immunol Investig 14:473–477
Boyd AS, Wood KJ (2009) Variation in MHC expression between undifferentiated mouse ES cells and ES cell-derived insulin-producing cell clusters. Transplantation 87:1300–1304
Chen L, Liu L (2009) Current progress and prospects of induced pluripotent stem cells. Sci China C Life Sci 52:622–636
de Rham C, Villard J (2011) How to cross immunogenetic hurdles to human embryonic stem cell transplantation. Semin Immunopathol 33:525–534
Dehoux JP, Gianello P (2007) The importance of large animal models in transplantation. Front Biosci 12:4864–4880
Dressel R, Nolte J, Elsner L, Novota P, Guan K, Streckfuss-Bomeke K, Hasenfuss G, Jaenisch R, Engel W (2010) Pluripotent stem cells are highly susceptible targets for syngeneic, allogeneic, and xenogeneic natural killer cells. FASEB J 24:2164–2177
Drukker M, Katz G, Urbach A, Schuldiner M, Markel G, Itskovitz-Eldor J, Reubinoff B, Mandelboim O, Benvenisty N (2002) Characterization of the expression of MHC proteins in human embryonic stem cells. Proc Natl Acad Sci U S A 99:9864–9869
Drukker M, Katchman H, Katz G, Even-Tov Friedman S, Shezen E, Hornstein E, Mandelboim O, Reisner Y, Benvenisty N (2006) Human embryonic stem cells and their differentiated derivatives are less susceptible to immune rejection than adult cells. Stem Cells 24:221–229
Esteban MA, Xu J, Yang J, Peng M, Qin D, Li W, Jiang Z, Chen J, Deng K, Zhong M, Cai J, Lai L, Pei D (2009) Generation of induced pluripotent stem cell lines from Tibetan miniature pig. J Biol Chem 284:17634–17640
Esteban MA, Peng M, Deli Z, Cai J, Yang J, Xu J, Lai L, Pei D (2010) Porcine induced pluripotent stem cells may bridge the gap between mouse and human iPS. IUBMB Life 62:277–282
Ezashi T, Telugu BP, Alexenko AP, Sachdev S, Sinha S, Roberts RM (2009) Derivation of induced pluripotent stem cells from pig somatic cells. Proc Natl Acad Sci U S A 106:10993–10998
Fairbairn L, Kapetanovic R, Sester DP, Hume DA (2011) The mononuclear phagocyte system of the pig as a model for understanding human innate immunity and disease. J Leukoc Biol 89:855–871
Hanna J, Wernig M, Markoulaki S, Sun CW, Meissner A, Cassady JP, Beard C, Brambrink T, Wu LC, Townes TM, Jaenisch R (2007) Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science 318:1920–1923
Ho CS, Lunney JK, Ando A, Rogel-Gaillard C, Lee JH, Schook LB, Smith DM (2009) Nomenclature for factors of the SLA system, update 2008. Tissue Antigens 73:307–315
Kim S, Kim JH, Lee E, Jeong YW, Hossein MS, Park SM, Park SW, Lee JY, Jeong YI, Kim HS, Kim YW, Hyun SH, Hwang WS (2010) Establishment and characterization of embryonic stem-like cells from porcine somatic cell nuclear transfer blastocysts. Zygote 18:93–101
Kiskinis E, Eggan K (2010) Progress toward the clinical application of patient-specific pluripotent stem cells. J Clin Invest 120:51–59
Lian Q, Chow Y, Esteban MA, Pei D, Tse HF (2010) Future perspective of induced pluripotent stem cells for diagnosis, drug screening and treatment of human diseases. Thromb Haemost 104:39–44
Ljunggren HG, Karre K (1990) In search of the ‘missing self’: MHC molecules and NK cell recognition. Immunol Today 11:237–244
Loh YH, Yang JC, De Los Angeles A, Guo C, Cherry A, Rossi DJ, Park IH, Daley GQ (2012) Excision of a viral reprogramming cassette by delivery of synthetic Cre mRNA. Curr Protoc Stem Cell Biol Chapter 4:Unit4A.5
Montserrat N, Bahima EG, Batlle L, Hafner S, Rodrigues AM, Gonzalez F, Belmonte JC (2011) Generation of pig iPS cells: a model for cell therapy. J Cardiovasc Transl Res 4:121–130
Nagy ZB, Gergely P, Donath J, Borgulya G, Csanad M, Poor G (2008) Gene expression profiling in Paget’s disease of bone: upregulation of interferon signaling pathways in pagetic monocytes and lymphocytes. J Bone Miner Res 23:253–259
Park IH, Lerou PH, Zhao R, Huo H, Daley GQ (2008) Generation of human-induced pluripotent stem cells. Nat Protoc 3:1180–1186
Roberts RM, Telugu BP, Ezashi T (2009) Induced pluripotent stem cells from swine (Sus scrofa): why they may prove to be important. Cell Cycle 8:3078–3081
Schook L, Beattie C, Beever J, Donovan S, Jamison R, Zuckermann F, Niemi S, Rothschild M, Rutherford M, Smith D (2005) Swine in biomedical research: creating the building blocks of animal models. Anim Biotechnol 16:183–190
Si-Tayeb K, Noto FK, Nagaoka M, Li J, Battle MA, Duris C, North PE, Dalton S, Duncan SA (2010) Highly efficient generation of human hepatocyte-like cells from induced pluripotent stem cells. Hepatology 51:297–305
Smith DM, Lunney JK, Ho CS, Martens GW, Ando A, Lee JH, Schook L, Renard C, Chardon P (2005a) Nomenclature for factors of the swine leukocyte antigen class II system, 2005. Tissue Antigens 66:623–639
Smith DM, Lunney JK, Martens GW, Ando A, Lee JH, Ho CS, Schook L, Renard C, Chardon P (2005b) Nomenclature for factors of the SLA class-I system, 2004. Tissue Antigens 65:136–149
Son H, Kim J, Lee S, Kim H, Lee E, Park J, Ka H, Kim H, Lee C (2009) Efficient Derivation and Long Term Maintenance of Pluripotent Porcine Embryonic Stem-like Cells. Asian-Aust J Anim Sci 22:26–34
Song Z, Cai J, Liu Y, Zhao D, Yong J, Duo S, Song X, Guo Y, Zhao Y, Qin H, Yin X, Wu C, Che J, Lu S, Ding M, Deng H (2009) Efficient generation of hepatocyte-like cells from human induced pluripotent stem cells. Cell Res 19:1233–1242
Suarez-Alvarez B, Rodriguez RM, Calvanese V, Blanco-Gelaz MA, Suhr ST, Ortega F, Otero J, Cibelli JB, Moore H, Fraga MF, Lopez-Larrea C (2010) Epigenetic mechanisms regulate MHC and antigen processing molecules in human embryonic and induced pluripotent stem cells. PLoS One 5:e10192
Telugu BP, Ezashi T, Roberts RM (2010) Porcine induced pluripotent stem cells analogous to naive and primed embryonic stem cells of the mouse. Int J Dev Biol 54:1703–1711
Theofilopoulos AN, Koundouris S, Kono DH, Lawson BR (2001) The role of IFN-gamma in systemic lupus erythematosus: a challenge to the Th1/Th2 paradigm in autoimmunity. Arthritis Res 3:136–141
Thong LM, Choi H, Kwon OJ, Kim JH, Kim YB, Oh JW, Seo K, Yeom SC, Lee WJ, Park C (2011) Systematic analysis of swine leukocyte antigen-DRB1 nucleotide polymorphisms using genomic DNA-based high-resolution genotyping and identification of new alleles. Tissue Antigens 77:572–583
Toledo JR, Prieto Y, Oramas N, Sanchez O (2009) Polyethylenimine-based transfection method as a simple and effective way to produce recombinant lentiviral vectors. Appl Biochem Biotechnol 157:538–544
Wernersson R, Schierup MH, Jorgensen FG, Gorodkin J, Panitz F, Staerfeldt HH, Christensen OF, Mailund T, Hornshoj H, Klein A, Wang J, Liu B, Hu S, Dong W, Li W, Wong GK, Yu J, Wang J, Bendixen C, Fredholm M, Brunak S, Yang H, Bolund L (2005) Pigs in sequence space: a 0.66X coverage pig genome survey based on shotgun sequencing. BMC Genomics 6:70
Wernig M, Zhao JP, Pruszak J, Hedlund E, Fu D, Soldner F, Broccoli V, Constantine-Paton M, Isacson O, Jaenisch R (2008) Neurons derived from reprogrammed fibroblasts functionally integrate into the fetal brain and improve symptoms of rats with Parkinson’s disease. Proc Natl Acad Sci U S A 105:5856–5861
West FD, Terlouw SL, Kwon DJ, Mumaw JL, Dhara SK, Hasneen K, Dobrinsky JR, Stice SL (2010) Porcine induced pluripotent stem cells produce chimeric offspring. Stem Cells Dev 19:1211–1220
Wu Z, Chen J, Ren J, Bao L, Liao J, Cui C, Rao L, Li H, Gu Y, Dai H, Zhu H, Teng X, Cheng L, Xiao L (2009) Generation of pig induced pluripotent stem cells with a drug-inducible system. J Mol Cell Biol 1:46–54
Wulster-Radcliffe MC, Ajuwon KM, Wang J, Christian JA, Spurlock ME (2004) Adiponectin differentially regulates cytokines in porcine macrophages. Biochem Biophys Res Commun 316:924–929
Xu D, Alipio Z, Fink LM, Adcock DM, Yang J, Ward DC, Ma Y (2009) Phenotypic correction of murine hemophilia A using an iPS cell-based therapy. Proc Natl Acad Sci U S A 106:808–813
Zhang D, Jiang W, Liu M, Sui X, Yin X, Chen S, Shi Y, Deng H (2009) Highly efficient differentiation of human ES cells and iPS cells into mature pancreatic insulin-producing cells. Cell Res 19:429–438
Zhao T, Zhang ZN, Rong Z, Xu Y (2011) Immunogenicity of induced pluripotent stem cells. Nature 474:212–215
Acknowledgments
The authors thank Dr. In-Hyun Park (Yale University, USA) for providing the retroviral vector, Dr. Chankyu Park (Konkuk University, Korea) for SLA typing analysis, and Dr. Jung-Im Yun for technical assistance. This study was supported by a grant (Project Code No. Z-1541745-2013-14-01, Z-1541745-2013-14-02) from Animal and Plant Quarantine Agency, Ministry of Agriculture, Food and Rural Affairs (MAFRA), Republic of Korea in 2013. This work was also supported by grants from Next-Generation BioGreen 21 program (No.PJ009069), Rural Development Administration, Korea.
Ethical standard
The experiments comply with the current laws of the Republic of Korea and Institutional Animal Care and Use Committee (Kangwon National University, Korea).
Conflict of interest
The authors have no conflict of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
(PDF 780 kb)
Supplementary Table 1
(DOCX 19 kb)
Rights and permissions
About this article
Cite this article
Park, KM., Cha, SH., Ahn, C. et al. Generation of porcine induced pluripotent stem cells and evaluation of their major histocompatibility complex protein expression in vitro. Vet Res Commun 37, 293–301 (2013). https://doi.org/10.1007/s11259-013-9574-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11259-013-9574-x