Abstract
Climate warming will induce changes in Arctic ecosystem carbon balance, but besides climate, nitrogen availability is a critical controlling factor of carbon cycling. It is therefore essential to obtain knowledge on the influence of a changing climate on nitrogen fixation, as this process is the main source of new nitrogen to arctic ecosystems. In order to gain information on future nitrogen fixation rates in a changing climate, we studied the effects of two decades of warming with passive greenhouses, shading with sackcloth, and fertilization with NPK fertilizer on nitrogen fixation rates. To expand the knowledge on species-specific responses, we measured nitrogen fixation associated with two moss species: Hylocomium splendens and Aulacomnium turgidum. Our expectations of decreased nitrogen fixation rates in the fertilizer and shading treatments were met. However, contrary to our expectation of increased nitrogen fixation in the warming treatment, we observed either no change (Hylocomium) or a decrease (Aulacomnium) in fixation in the warmed plots. We hypothesize that this could be due to moss-specific responses or to long-term induced effects of the warming. For example, we observed that the soil temperature increase induced by the warming treatment was low and insignificant as vegetation height and total vascular plant cover of the warmed plots increased, and moss cover decreased. Hence, truly long-term studies lasting more than two decades provide insights on changes in key biogeochemical processes, which differ from more transient responses to warming in the Arctic.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Arctic ecosystems are exposed to rapid warming, as global climate changes strike hardest at high latitudes (Arctic Climate Impact Assesment (ACIA) 2005; IPCC 2007). It is predicted that temperatures will increase by 3–8°C over the next century and that these changes will have substantial impact on biogeochemical cycling in the Arctic, including accelerated loss of carbon stored in soil organic matter resulting from temperature induced increased microbial activity (Dorrepaal et al. 2009). At the same time, a warmer climate is also believed to induce higher primary productivity, and hence, increase the carbon sink strength of Arctic ecosystems and, to some degree, counteract carbon losses from the soil. However, primary productivity is not only limited by the harsh climate but also by the low availability of nutrients, especially nitrogen (N) (Chapin and Shaver 1996; Jonasson and Shaver 1999; Nadelhoffer et al. 1992), and it is uncertain to which degree productivity will change as a result of increasing temperatures. It is predicted that mineralization will run faster as temperatures increase (Jonasson et al. 1999b; Nadelhoffer et al. 1997), but whether this will lead to higher nutrient availability for plants is uncertain, as microbial immobilization is high (Jonasson et al. 1999a; Schimel et al. 1996; Sorensen et al. 2008a). In the Arctic, biological N2 fixation mainly by cyanobacteria associated with mosses is the main source of new N to the ecosystems (Chapin et al. 1991; Sorensen et al. 2006), and climate induced changes in N2 fixation is a significant control on nutrient availability and, potentially, carbon balance in the Arctic.
Mosses make up a high proportion of the vegetation in most Arctic ecosystems. They influence ecosystem functioning in many ways: by contributing to the net primary productivity (Campioli et al. 2009), by influencing soil temperature and moisture levels (Gornall et al. 2007), and by hosting N2 fixing cyanobacteria, that contribute substantially to the N input to the ecosystems (DeLuca et al. 2002; Solheim and Zielke 2002; Zielke et al. 2005; Sorensen and Michelsen 2011). Moss-associated N2 fixation is not the only source of N2 fixation in the Arctic. Lichens may host cyanobacteria, and N2 fixing, free-living bacteria are found in top soil, especially in areas with surface crusts (Deslippe and Egger 2006; Liengen and Olsen 1997). However, as mosses dominate the vegetation in most of the Arctic, the potential for high amounts of N2 fixed by moss-associated bacteria is significant (Gavazov et al. 2010; DeLuca et al. 2002; Solheim and Zielke 2002). Studies have shown that N2 fixation in the Arctic is pronounced, and in some cases, the amount of incoming N via fixation should be sufficient to sustain the N demand by vegetation (Dickson 2000; Sorensen et al. 2006).
Moss-associated N2 fixation rates are altered by abiotic factors such as temperature, light income, soil water, and nutrient content (Belnap 2001; Chapin et al. 1991; Sorensen et al. 2006; Zielke et al. 2002). Fertilization with high amounts of N reduces N2 fixation rates (Liengen 1999; Zackrisson et al. 2009), while low amounts of extra N and, in particular, P supplied from decaying litter may increase N2 fixation (Sorensen and Michelsen 2011). Warming is reported to either increase (Sorensen and Michelsen 2011) or have no effect (Bjerke et al. 2003; Deslippe et al. 2005; Sorensen and Michelsen 2011) on N2 fixation rates measured in the field under Arctic conditions, depending upon moisture conditions. Laboratory incubations show maximal N2 fixation rates in mosses at temperatures, well above normally observed temperatures in the Arctic (Chapin et al. 1991; Liengen 1999; Zielke et al. 2002). Climate change could also affect N2 fixation through other ways than direct warming. Total vegetation cover is likely to increase, with increased biomass of deciduous shrubs and reduced cover of mosses, (Van Wijk et al. 2003), and as N2 fixation rates are correlated with moss cover (Deslippe et al. 2005), this could lead to decreased N2 fixation rates.
Changes in Arctic vegetation cover after experimentally induced climate change appear to be very slow (Wookey and Robinson 1997), and hence, changes in N2 fixation rates because of a changing climate are believed to be resistant. In this study, we measured rates of N2 fixation associated with two widespread and common moss species in a field experiment after 21 years of warming, shading, and fertilizer addition treatment. Shading was intended to simulate both enhanced cloud cover and the expansion of the forest beyond the current tree-line, and fertilizer addition simulates enhanced soil nutrient availability as a component of warming. We expected that warming would increase N2 fixation if water supply was non-limiting, that shading would reduce N2 fixation and that fertiliser addition would reduce N2 fixation, because the cyanobacteria only fix N2 when this resource is limiting growth.
Materials and methods
Site description
This study was conducted near Abisko Scientific Research Station, Northern Sweden (68°21N, 18°49E). The annual mean temperature is −0.5°C and the temperature is 11°C in July, with a mean annual precipitation at 315 mm (1960–1990) (Abisko Scientific Research Station, www.ans.kiruna.se).
The site is a subarctic dry heath just above the tree-line at 450 m above sea level. The climate is montane subarctic, with a growing season of approximately 3 months, lasting from mid-June to early mid-September. The vegetation is dominated by dwarf shrubs, mainly Cassiope tetragona, Empetrum hermaphroditum, and Vaccinium uliginosum. Total moss cover at the site is 36 ± 4% and the cover of the two mosses used in the N2 fixation measurements in the present experiment, Hylocomium splendens and Aulacomnium turgidum is 11 ± 3 and 4 ± 2%, respectively (mean ± SE, vegetation cover analyzed on 21 July, 2009 in 30 squares that measured 50 × 50 cm placed 5 m apart on a transect just outside the experimental plots). The experimental setup of the site was initiated in 1989, and the measurements of N2 fixation and moss nutrient content for this paper were conducted in 2009 during late spring and summer. It consisted of six treatments: control, shading by sack cloth, warming by open top, transparent, dome-shaped plastic greenhouses, fertilizer addition, fertilizer + shading, and fertilizer + warming. The treatments were replicated across six blocks within an area of ~400 m2. Each year in early June, just after snowmelt and until the end of August, the temperature was raised by erecting dome-shaped plastic greenhouses with a 1.2 × 1.2 m surface area. The greenhouses enhanced mean air temperature in 1991–1995 by 3.9°C, and soil temperature by 0.9–2.0°C (Michelsen et al. 1996; Ruess et al. 1999) above an average growing season air and soil temperature of 10.9 and 7.7°C, respectively, in the control plots, and the greenhouses reduced photosynthetically active radiation by 9% (Havström et al. 1993; Michelsen et al. 1996; Rinnan et al. 2007). NPK fertilizer dissolved in water was added each year soon after snowmelt in June at a dose of 4.9 g m−2 for N, 1.3 g m−2 for phosphorus (P) and 6.0 g m−2 for potassium (K) in 1989. From 1990 to 2009, the additions were 10.0, 2.6, and 9.0 g m−2, respectively, except from three of the 20 years, 1993, 1998, and 2001, where no fertilizer was applied because we wanted to avoid potential interference with ongoing measurements. The fertilizer was dissolved in 2 l water per plot, and after addition, all plots received an additional 2 l water to remove any remaining fertilizer from leaf surfaces.
N2 fixation assays
We used the acetylene reduction assay as an indirect method to estimate N2 fixation (Stewart et al. 1967). Acetylene (C2H2) is reduced to ethylene (C2H4) by nitrogenase in the N2-fixing microorganisms.
All measurements of acetylene reduction were performed on moss cores. This approach was chosen because measurements on entire vegetation surfaces using chambers mounted on flux frames were not feasible on this mesic-dry heath due to unavoidable excessive loss of acetylene and ethylene diffusing through the porous soil. Instead, we measured fluxes from moss cores kept in PVC containers during the assay (Sorensen and Michelsen 2011). The cores were taken with a soil auger, and had a cylindrical shape; 5 cm in diameter and c. 7 cm high, consisting of intact moss vegetation on the surface plus the underlying, highly organic soil collected within the long-term ecosystem manipulation plots. When preparing the cores, we avoided vascular plants, so that the vegetation in the cores consisted solely of mosses. We placed the cores in nylon mesh bags (50 μm mesh size; Sintab, Sweden), and set them back into the hole in the ground, from where the core originated, whenever we were not doing assays. When performing acetylene reduction assays, we placed the cores in separate, closed transparent PVC containers with a volume of 0.5 l. We placed a vial with calcium carbide (CaC2) inside the container and injected water into the vial through a rubber septum placed on top of the chamber. The amount of CaC2 was adjusted to release approximately 10% v/v acetylene to the chamber atmosphere. Syringe samples of 10 ml were taken, 2 min after the injection of water, and again after 2 h. The chambers were placed within the plot from where the core originated, during the assay. After the assay, we measured the core soil temperature at 2 cm depth and weighed the core to calculate the water content. The air-filled syringes were secured with rubber stoppers until analysis of ethylene and acetylene, which took place within 24 h after the sampling. Acetylene and ethylene concentrations were measured on a Shimadzu GC-14B gas chromatograph (GC) equipped with a Porapak N column, a FID detector and with injector, column and detector temperature at 250, 60, and 120°C, using He as carrier gas.
On May 15, 2009, we established cores dominated by the moss Hylocomium splendens in all treatments, by choosing places with high cover of Hylocomium. We performed the acetylene reduction assay in the field on these cores on May 15, June 13, July 9, July 19, and 27, and August 4, 18, and 21. Furthermore, on July 11, 2009, we established cores in places with dense cover of the moss Aulacomnium turgidum in the control, shade, warmed, and fertilizer treatments, to do additional acetylene reduction measurements with a different moss species. In the Aulacomnium cores, we did not measure ethylene production rates in the combined treatments, and only for a part of the season, due to time constraints on GC analysis of gas samples. In order to provide optimal moisture conditions for N2 fixation, these cores were watered with 30 mL of water from a near-by stream 2 days prior to acetylene reduction assays, which took place on July 11, 21, and 29, and on August 6 and 10, 2009.
Plant cover and nutrient content
On July 16, 2008, vegetation cover in control and warmed plots was estimated in 100 points per plot using a 50 × 50 cm pin-point frame.
On August 30, 2009, after the last acetylene reduction assay, the cores were dried at 80°C, weighed, and brought to the laboratory in Copenhagen. We counted moss shoots and separated the cores into a green moss fraction i.e., the top 2 cm of each core, and a brown moss fraction i.e., the layer from 2 to 7 cm depth. The samples were acid digested and analyzed for total N and P using a Fiastar 5000 flow injection analyzer (Foss Tecator, Sweden).
Statistical analysis
Data means were compared by analyses of variance (ANOVAs) with type III sum of squares by the GLM procedure (SAS Institute v.9.1 2002–03; SAS Institute, Cary, NC). Results with P ≤ 0.05 were regarded as statistically significant, although marginally significant (P ≤ 0.1) results were also reported. For Hylocomium, soil temperature, soil water content, and ethylene production rates were analyzed using three-factor repeated measurements ANOVA, with shade, warming, and fertilizer as main factors, with interactions between shade and fertilizer and between warming and fertilizer included, and with time as the repeated effect. For Aulacomnium, the model was a repeated measurement three-way ANOVA with time as the repeated factor. In addition, we also analyzed each measurement occasion (date) separately using three-factor ANOVAs, with shade, warming, and fertilizer and (Hylocomium only) the combined treatments. The effect of block was included in the models whenever the block effect was below P = 0.20, to account for any underlying gradients at the site not related to treatments. In addition, we included the effect of water content in the cores as a covariate in the models whenever the water content effect was below P = 0.20, to account for any changes in water content by the treatments, on the ethylene production. Before performing the ANOVAs, we analyzed the data for homogeneity of variances by Levene’s test and, if necessary, carried out the appropriate transformations. Models were improved by stepwise removal of interactions far from significance (P ≥ 0.20). Spearman correlations were used to test for relationships between ethylene production and soil temperatures and soil water content.
Results
Field measurements of ethylene production
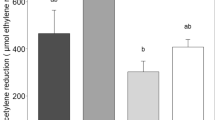
The overall growing season ethylene production over the measurement campaign in the Hylocomium cores ranged between 0.8 μmol ethylene m−2 h−1 in the shade + fertilized plots to 7.6 μmol ethylene m−2 h−1 in the control plots (Fig. 1a). In the Aulacomnium cores the range was from 1.7 μmol ethylene m−2 h−1in the fertilized plots to 12.0 μmol ethylene m−2 h−1 in the control plots (Fig. 1b). The ethylene production was generally higher in the Aulacomnium cores, probably because we added water to the Aulacomnium cores before the measurements and not to the Hylocomium cores.
Seasonal and treatment effects on ethylene production rates of a Hylocomium and b Aulacomnium cores in the control, shade, warmed, fertilized, and combined treatments (mean ± SE, n = 6) 21 years after the experiment was initiated in a subarctic heath. The overall effects treatment factors, measurement date, and interactions were analyzed with repeated measurements ANOVA, and effects of factors were in addition analyzed for each date separately *P < 0.05, **P < 0.01, ***P < 0.001
In the Hylocomium cores the ethylene production rates, analyzed over the whole measurement period with repeated measurement ANOVA, were reduced significantly (P = 0.0002) by 89% in the fertilized plots compared to the control plots, and this effect was also significant on most of the individual measurement dates (Fig. 1a). The repeated measurement ANOVA did not show more main effects of the treatments, but there was a significant interaction between the shade and the fertilizer treatment, caused by a non-significant increase of the ethylene rate in the shaded + fertilized plots. Surprisingly, we found few effects of warming, except for a near-significant positive effect on June 13 and a significant negative effect on August 21.
For the Aulacomnium cores, the overall effects of the fertilization were similar to that of the Hylocomium (Fig. 1b), with a significant (P = 0.0003) reduction of the ethylene production after fertilization by 85% compared to the control plots, and furthermore the ethylene production halved in the shaded and warmed plots, compared to control plots. The rates varied with time, with very low rates on July 21 and August 6, compared to the other measurement times. At these two measurement dates we found no effects of either the shade, warming, or fertilizer treatment with three-way ANOVAs (P > 0.36), which probably caused the observed significant interactions between date of measurement and the treatments. Analyses for effects of treatments on the different measurement dates separately showed that there was a negative effect of fertilization on July 11, July 29, and on August 10. Furthermore, we found negative effects of both shading and warming on July 11 and on July 29.
Temperature, nutrient, and water content of moss cores
The number of moss shoots in the Hylocomium cores was 43.3 (±2.9) in the control plots, and slightly lower, 33.0 (±8.5) and 37.3 (±3.8) in the shaded and warmed plots, respectively, while in the fertilized plots alone we found more, 61 ± 30.2 moss shoots. In the Aulacomnium cores, the number of moss shoots were half as many in warmed (35.7 ± 10.0) than in control (71.3 ± 14.6) plots, and also here we found that in the fertilized cores the moss shoot number were higher; 94.3 ± 37.2. The N concentration in the Hylocomium cores generally increased after fertilization and in the brown moss also after warming (Table 1). Also the P concentration increased after fertilization, and furthermore, we found positive effects of shading on P concentrations. In the Aulacomnium cores, fertilizer had a significant, positive effect on both N and P content in both depths (Table 1).
We had expected that warming would increase the soil temperature in 2 cm depth at the 2009 measurement dates, as earlier observations (1991–1995) has shown increased temperatures in the warmed plots. However, this was not what we observed, neither in Hylocomium nor in Aulacomnium (P = 0.978 and 0.2 for the Hylocomium and Aulacomnium cores, respectively (Fig. 2a, c); tested with repeated measurement analyses. However, in the Hylocomium cores we found a near-significant interaction between the warming treatment and the fertilizer treatment (P = 0.09). In addition, for the individual measurement occasions we found that in the warmed plots the temperature increased significantly on June 13, July 27, and August 18. Hence, the lack of warming effect observed when analyzing soil temperature data could be caused by decreased temperature in the warmed + fertilized plots due to increased vascular plant cover. In consistence with this, in the Hylocomium cores we found that the shade treatment had a significant, negative effect on soil temperature.
Seasonal and treatment’s effects measured in the Hylocomium and Aulacomnium cores in a, c core soil temperature and b, d core water content in the control, shade, warmed, fertilized, and combined treatments (mean ± SE, n = 6). The effects of treatment factors, measurement date and interactions were analyzed with repeated measurements ANOVA: *P < 0.05, **P < 0.01, ***P < 0.001
The water content of the cores was measured every time we did acetylene reduction measurements. The water content of the Hylocomium cores varied significantly with time (P ≤ 0.0001) and was the highest in mid May, only a few days after snowmelt, and the lowest at July 9 and on August 4 (Fig. 2b). The water content was, analyzed over the whole season, reduced significantly with 15% in the warmed plots compared to the control plots. However, when we included water content as a covariate in the statistical tests of treatment effects on ethylene production, we found no effects of these changes. The Aulacomnium cores were watered 2 days before the measurements, and we therefore did not find any effects of treatments on soil water content either over the whole measurement period or on the individual measurement days (Fig. 2d). However, the water content varied slightly with time, with lowest soil water content on July 21 and August 6.
In the unfertilized plots, the N2 fixation rate increased significantly with increasing water content in both species, whereas in the fertilized plots, there was no correlation between N2 fixation and water content (Fig. 3).
Relationships between core water content and ethylene production rates in a Hylocomium and b Aulacomnium cores. Each dot represents data from one date. Filled dots are from un-fertilized plots (control, shade and warming, n = 24 in Hylocomium and n = 15 in Aulacomnium) and unfilled dots are from fertilized plots (Hylocomium cores: fertilized, warmed + fertilized, shade + fertilized, n = 24, Aulacomnium: fertilized alone plots, n = 5). The correlation coefficients (r 2) and P values are in the Hylocomium plots: r 2 = 0.77 and P < 0.0001 in the unfertilized plots and r 2 = 0.11 and P = 0.11 for the fertilized plots and in Aulacomnium plots: r 2 = 0.69 and P = 0.07 for the unfertilized and r 2 = 0.70 and P < 0.0001 for the fertilized plots
Vegetation cover of the plots
The analysis of the control and warmed plots showed that warming had increased both the coverage of vascular plants and plant height in the warmed plots significantly (Table 2). The cover of deciduous shrubs tended to increase, and the dominant evergreen dwarf shrubs increased significantly with warming, whereas the cover of graminoids and forbs were relatively low and unchanged. The cover of mosses was in the warmed plots reduced to one-third of the coverage in control plots. In addition, the coverage of lichens decreased with warming to only 25% of that in control plots.
Discussion
N2 fixation and climatic changes
Our study provides novel information on the environmental controls and temporal changes in N input through N2 fixation to arctic, N-limited ecosystems. This study includes data for most of the snow-free season, from within 1 week after snow melt until late summer at the onset of leaf fall. In addition, the 20 years of continued treatments offer a unique possibility to predict long-term effects on ecosystem N cycling.
Among the treatments, fertilization gave the most pronounced effects, with a doubling of the N concentration in the top moss shoots of both Hylocomium and Aulacomnium, and a clear reduction in N2 fixation rates in both moss core types, despite a higher number of moss shoots in the fertilized cores. The mechanism behind this decrease is not fully clear. The added amount of fertilizer, which was 10 g N m−2 year−1, highly exceeds both the present atmospheric deposition of N in the area, which is estimated to 0.23 g N m−2 year−1 (Karlsson et al. 2009) and it is also much higher than predictions for future N deposition. The added amount of fertilizer also exceeds the N mineralization rate of the plots, which is less than 1 g N m−2 year−1 (Schmidt et al. 1999, 2002). Nevertheless, in experiments with 15N pulse labeling of soil, in or just below the green moss, up to 90% of the pulse-added N is quickly immobilized in soil microbial biomass and the non-microbial soil organic matter pool, and less than 0.5% retained in moss (Sorensen et al. 2008c). Hence, only a small fraction of added N may affect cyanobacteria associated with mosses. DeLuca et al. (2007) observed a higher number of cyanobacteria on feather mosses growing in areas with low N deposition, compared to mosses in areas with high N deposition. A study on N2 fixation in a boreal forest showed that both the number of cyano-bacteria and the rate of N2 fixation associated with the feather moss Pleurozium schreberi declined when adding as little as 0.3 g N m−2 year−1 (Gundale et al. 2011). We observed increased P concentration in both Hylocomium and Aulacomnium after fertilization, but this had no positive effect on N2 fixation rates, likely because the NPK fertilizer also increased the N content of the moss and repressed N2 fixation. In experiments from the boreal zone to the Arctic with P-fertilization solely, or increased natural P-availability, either no effects of P on N2 fixation (Zackrisson et al. 2009) or increased N2 fixation with increased P-availability (Chapin et al. 1991; DeLuca and Zackrisson 2007; Liengen 1999; Weiss et al. 2005) has been reported.
Warming had no effect on N2 fixation in Hylocomium and a negative effect in Aulacomnium. In an earlier experiment with warming at a wet heath near Abisko, we observed either no response or occasionally even a tendency towards a negative effect on N2 fixation in Hylocomium and Sphagnum warnstorfii cores exposed to field warming. However, when we measured on whole-ecosystem level, N2 fixation rates doubled or tripled after warming for ten years (Sorensen and Michelsen 2011). The reason for the discrepancy in results obtained with the two methods in the earlier study was likely a warming-induced drying of the small moss cores, which may reduce N2 fixation (Sorensen and Michelsen 2011). In the current study, we had expected the N2 fixation rates to increase with increasing temperature in the warmed plots, at least in the Aulacomnium moss cores, in which a potential water limitation was counteracted by frequent watering. This is because, it is generally observed that N2 fixation is temperature limited in the Arctic, and the warming treatment in the dry heath experiment has earlier led to an air warming of 3–4°C and a soil warming of 1–2°C (Michelsen et al. 1996; Ruess et al. 1999). However, at the measurement dates of this study, we only occasionally observed elevated soil temperatures in the warmed plots (Fig. 2a, c), and this moderate response could explain the lack of a positive response of N2 fixation to warming. One explanation to why there was no temperature increase in the soil could be that the amount of litter and the vegetation height in the warmed plots has increased significantly (Table 2), leading to a more closed vascular plant canopy, and hence, a lower light intensity at moss surface level after two decades of warming. Also, the observed changes in vegetation cover could possibly have direct effects on N2 fixation rates, as both the number of moss shoots in the cores and the frequency of mosses decreased in the warmed plots (Table 2). Another study in the high Arctic has shown that N2 fixation rates correlates positively with the number of moss shoots (Deslippe et al. 2005).
Water limitation could offer an additional explanation for the decreased N2 fixation in the warmed plots. We found that the ethylene production correlated significantly with the core water content as long as there was no fertilizer addition (Fig. 3), and furthermore, the water content decreased significantly in the warmed plots with Hylocomium (Fig. 2b), but not in the watered Aulacomnium cores (Fig. 2d). We visually observed an increased drying of the moss surface in the warmed plots, which could affect the N2 fixation rates, as previous studies observed that N2 fixation associated with mosses declined with decreased water availability (Zielke et al. 2002; Sorensen et al. 2006; Gundale et al. 2010; Stewart et al. 2011). Our results not only indicate that water is an important factor in determining the rate of N2 fixation associated with both species, but also that N2 fixation rates are unrelated to the core water content when the plots are fertilized and the N2 fixation rates are much lower (Fig. 3). An additional explanation for the negative effect of warming on N2 fixation could be that the N availability increased because of warming, and hence, the energy expensive N2 fixation process was reduced. Mineralization rates increased in the warmed plots after 5 years of treatments (Schmidt et al. 1999), and the availability of inorganic N was higher in warmed plots after 11 years of treatment (Sorensen et al. 2008b). In this study, we observed a tendency toward increased N concentration in both Hylocomium and Aulacomnium in warmed plots, although only significant in Hylocomium cores in the 2–7 cm layer (Table 1). This is known to reduce N2 fixation rates (Gundale et al. 2010; Zackrisson et al. 2004), as we also observed in the fertilized plots.
In the shaded plots, we found that the N2 fixation rates tended to decrease, in both Hylocomium and Aulacomnium. This was probably caused by a combination of the decreased light in the shaded plots and the decrease in temperature, which was significant in Hylocomium cores (Fig. 2a, c). Hylocomium mortality increased after shading with sack cloth in boreal forest (Startsev et al. 2008), and we observed a tendency toward decreasing numbers of moss shoots both in Aulacomnium and Hylocomium cores. Stewart et al. (2011) observed that the increased number of vascular plants decreased the number of mosses, and therefore, also the rate of N2 fixation. Hence, as we also observed an increase in plant height and vascular plant cover combined with decreased moss cover in the warmed plots (Table 2); we cannot rule out that increased shading of the sub-canopy in warmed plots led to decreased N2 fixation of these plots. We found that the P concentration in the moss cores increased in the shade + fertilized plots, even more than in the fertilized plots alone (see Table 1, significant effect of shade, but with a significant interaction between shade and fertilizer). At the same time, the N concentration in the shade + fertilized plots was at the same level as in the fertilized alone plots. This is probably because decreased plant cover in the shaded plots (Graglia et al. 2001) has decreased the total N and P demand by plants, leading to higher concentrations in deciduous and evergreen plants (Jonasson et al. 1999b), while shading specifically reduces N2 fixation, and hence, moss N concentration to the level in fertilized plots.
Conclusion
Our expectations that the warming treatment, and hence, a warmer climate, should lead to higher N2 fixation rates, were not met. This was probably because the long-term treatments had led to changes in vegetation, and soil biogeochemical and physical properties that restrained N2 fixation rates: (i) Increased vegetation height meant that the soil temperatures of the warmed plots generally did not increase, counter to our expectation for the warmed plots. (ii) Moss abundance decreased, which directly leads to lower N2 fixation rates. (iii) N2 fixation and soil moisture content are strongly positively correlated, and warming may lead to periodical drying of the moss surface. (iv) On top of this, increased mineralization rates in warmed plots further restrict N2 fixation rates. All these side effects of warming are likely to take place in the warmer Arctic. Hence, in conclusion, it is probable that a warmer climate in the Arctic will lead to higher moss-associated N fixation rates in a transitional phase, at least in wet heath types, as previously shown after one decade of warming of a subarctic wet heath (Sorensen and Michelsen 2011). We cannot exclude that moss core based field estimates of nitrogen fixation may be negatively affected by increased drying of moss, beyond the natural side effect caused by warming. However, our results on negative effects of warming on moss-associated N fixation, even in frequently watered moss cores, suggest that N2 fixation rates likely decrease due to increase in vascular plant cover and biogeochemical N cycles changes with increased N mineralization and N availability. The current experiment, which demonstrates reduced N2 fixation rates after more than two decades of warming of this dry subarctic heath, suggests that warming may have profound consequences for ecosystem carbon storage. Depending upon (unresolved) long-term effects on N mineralization, progressive N limitation after a few decades could lead to stagnant plant production, while carbon losses from organic matter accumulated in the past may continue due to accelerated microbial activity.
References
ACIA Arctic Climate Impact Assesment (2005) Cambridge University Press, Cambridge
Belnap J (2001) Factors influencing nitrogen fixation and nitrogen release in biological soil crusts. In: Belnap J, Lange OL (eds) Biological soil crusts: structure, function, and management. Springer/Verlag, Berlin/Heidelberg, pp 240–261
Bjerke JW, Zielke M, Solheim B (2003) Long-term impacts of simulated climatic change on secondary metabolism, thallus structure and nitrogen fixation activity in two cyanolichens from the Arctic. New Phytol 159:361–367
Campioli M, Samson R, Michelsen A, Jonasson S, Baxter R, Lemeur R (2009) Nonvascular contribution to ecosystem NPP in a subarctic heath during early and late growing season. Plant Ecol 202:41–53
Chapin FS III, Shaver GR (1996) Physiological and growth responses of arctic plants to a field experiment simulating climatic change. Ecology 77:822–840
Chapin DM, Bliss LC, Bledsoe LJ (1991) Environmental regulation of nitrogen fixation in a high arctic lowland ecosystem. Can J Bot 69:2744–2755
DeLuca TH, Zackrisson O (2007) Enhanced soil fertility under Juniperus communis in arctic ecosystems. Plant Soil 294:147–155
DeLuca TH, Zackrisson O, Nilsson M-C, Sellstedt A (2002) Quantifying nitrogen-fixation in feather moss carpets of boreal forests. Nature 419:917–920
DeLuca TH, Zackrisson O, Gentili F, Sellstedt A, Nilsson M-C (2007) Ecosystem controls on nitrogen fixation in boreal feather moss communities. Oecologia 152:121–130
Deslippe JR, Egger KN (2006) Molecular diversity of nifH genes from bacteria associated with high arctic dwarf shrubs. Microb Ecol 51:516–525
Deslippe JR, Egger KN, Henry GHR (2005) Impacts of warming and fertilization on nitrogen-fixing microbial communities in the Canadian high Arctic. Microbiol Ecol 53:41–50
Dickson LG (2000) Constraints to nitrogen fixation by cryptogamic crusts in a polar desert ecosystem, Devon Island, N.W.T., Canada. Arct Antarct Alp Res 32:40–45
Dorrepaal E, Toet S, van Logtestijn RSP, Swart E, van de Weg MJ, Callaghan TV, Aerts R (2009) Carbon respiration from subsurface peat accelerated by climate warming in the subarctic. Nature 460:616–679
Gavazov KS, Soudzilovskaia NA, van Logtestijn RSP, Braster M, Cornelissen JHC (2010) Isotopic analysis of cyanobacterial nitrogen fixation associated with subarctic lichen and bryophyte species. Plant Soil 333:501–517
Gornall JL, Jónsdottir IS, Woodin SJ, Van der Wal R (2007) Arctic mosses govern below-ground environment and ecosystem processes. Oecologia 153:931–941
Graglia E, Jonasson S, Michelsen A, Schmidt IK, Havström M, Gustavsson L (2001) Effects of environmental pertubations on abundance of subarctic plants after three, seven and ten years of treatments. Ecography 24:5–12
Gundale MJ, Wardle DA, Nilsson MC (2010) Vascular plant removal effects on biological N fixation vary across a boreal forest island gradient. Ecology 9:1704–1714
Gundale MJ, DeLuca TH, Nordin A (2011) Bryophytes attenuate anthropogenic nitrogen inputs in boreal forests. Glob Change Biol 17:2743–2753
Havström M, Callaghan TV, Jonasson S (1993) Differential growth responses of Cassiope tetragona, an arctic dwarf-shrub, to environmental pertubations among three contrasting high- and subarctic sites. Oikos 66:389–402
IPCC (2007) Climate change 2007, intergovernmental panel on climate change fourth assessment report. Cambridge University Press, New York
Jonasson S, Shaver GR (1999) Within-stand nutrient cycling in arctic and boreal wetlands. Ecology 80:2139–2150
Jonasson S, Michelsen A, Schmidt IK (1999a) Coupling of nutrient cycling and carbon dynamics in the Arctic, integration of soil microbial and plant processes. Appl Soil Ecol 11:135–146
Jonasson S, Michelsen A, Schmidt IK, Nielsen EV (1999b) Responses in microbes and plants to changed temperature, nutrient and light regimes in the Arctic. Ecology 80:1828–1843
Karlsson GP, Akselsson C, Hellsten S, Karlsson PE and Malm G (2009) Övervakning av luftföroreningar norra sverige—mätningar och moddellering. Svenska Miljöinstitut IVL rapport B1851. Lund Universitet, Lund
Liengen T (1999) Environmental factors influencing the nitrogen fixation activity of free-living terrestrial cyanobacteria from a high arctic area, Spitsbergen. Can J Microbiol 45:573–581
Liengen T, Olsen RA (1997) Nitrogen fixation by free-living cyanobacteria from different coastal sites in a high arctic tundra, Spitsbergen. Arct Alp Res 29:470–477
Michelsen A, Jonasson S, Sleep D, Havström M, Callaghan TV (1996) Shoot biomass, δ13C, nitrogen and chlorophyll responses of two arctic dwarf shrubs to in situ shading, nutrient application and warming simulating climatic change. Oecologia 105:1–12
Nadelhoffer KJ, Giblin AE, Shaver GR (1992) Microbial processes and plant nutrient availability in arctic soils. In: Chapin FS III, Jefferies RL, Reynolds JF, Shaver GR, Svoboda J (eds) Arctic ecosystems in a changing climate: an ecophysiological perspective. Academic Press, San Diego, pp 281–300
Nadelhoffer KJ, Shaver GR, Giblin AE, Rastetter EB (1997) Potential impacts of climate change on nutrient cycling, decomposition, and productivity in arctic ecosystems. In: Oechel WC, Callaghan TV, Gilmanov T, Holten JI, Maxwell B, Molau U, Sveinbjörnsson B (eds) Global change and arctic terrestrial ecosystems. Springer, New York, pp 349–364
Rinnan R, Michelsen A, Bååth E, Jonasson S (2007) Fifteen years of climate change manipulations alter soil microbial communities in a subarctic heath ecosystem. Glob Change Biol 13:28–39
Ruess L, Michelsen A, Jonasson S (1999) Simulated climate change in subarctic soils: responses in nematode species composition and dominance structure. Nematology 1:513–526
Schimel JP, Kielland K, Chapin FS III (1996) Nutrient availability and uptake by tundra plants. In: Reynolds JF, Tenhunen JD (eds) Landscape function and disturbance in arctic tundra. Springer, Berlin, pp 203–221
Schmidt IK, Jonasson S, Michelsen A (1999) Mineralization and microbial immobilization of N and P in arctic soils in relation to season, temperature and nutrient amendment. Appl Soil Ecol 11:147–160
Schmidt IK, Jonasson S, Shaver GR, Michelsen A, Nordin A (2002) Mineralization and distribution of nutrients in plants and microbes in four arctic ecosystems: responses to warming. Plant Soil 242:93–106
Solheim B, Zielke M (2002) Associations between cyanobacteria and mosses. In: Rai AN, Bergman B, Rasmussen U (eds) Cyanobacteria in symbiosis. Kluwer Academic Publishers, Dordrecht, pp 137–152
Sorensen PL, Michelsen A (2011) Long-term warming and litter affects nitrogen fixation in a sub-arctic heath. Glob Change Biol 17:528–537
Sorensen PL, Jonasson S, Michelsen A (2006) Nitrogen fixation, denitrification, and ecosystem nitrogen pools in relation to vegetation development in the Subarctic. Arct Antarct Alp Res 38:263–272
Sorensen PL, Clemmensen KE, Michelsen A, Jonasson S, Ström L (2008a) Plant and microbial uptake and allocation of organic and inorganic nitrogen related to plant growth forms and soil conditions at two subarctic tundra sites in Sweden. Arct Antarct Alp Res 40:171–180
Sorensen PL, Michelsen A, Jonasson S (2008b) Ecosystem partitioning of 15N-glycine after long-term climate and nutrient manipulations, plant clipping, and addition of labile carbon in a subarctic heath tundra. Soil Biol Biochem 40:2344–2350
Sorensen PL, Michelsen A, Jonasson S (2008c) Nitrogen uptake during one year in subarctic plant functional groups and in microbes after long-term warming and fertilization. Ecosystems 11:223–233
Startsev N, Lieffers VJ, Landhäusser SM (2008) Effects of leaf litter on the growth of boreal feather mosses: Implications for forest floor development. J Veg Sci 19:253–260
Stewart WPD, Fitzgerald GP, Burris RH (1967) In situ studies on N2 fixation using the acetylene reduction technique. Proc Nat Acad Sci USA 58:2071–2078
Stewart KJ, Lamb EG, Darwyn SC, Siciliano SD (2011) Bryophyte-cyanobacterial associations as a key factor in N2-fixation across the Canadian Arctic. Plant Soil 344:335–346
Van Wijk MT, Clemmensen KE, Shaver GR, Williams M, Callaghan TV, Chapin FS III, Cortina J, Gough L, Hobbie SE, Jonasson S, Lee JA, Michelsen M, Press MC, Richardson SJ, Rueth H (2003) Long-term ecosystem level experiments at Toolik Lake, Alaska, and at Abisko, Northern Sweden: generalizations and differences in ecosystem and plant type responses to global change. Glob Change Biol 10:105–123
Weiss MS, Hobbie SE, Gettel GM (2005) Contrasting responses of nitrogen-fixation in arctic lichens to experimental and ambient nitrogen and phosphorus availability. Arct Antarct Alp Res 37:396–401
Wookey PA, Robinson CH (1997) Responsiveness and resilience of high arctic ecosystems to environmental change. Opera Bot 132:215–231
Zackrisson O, DeLuca TH, Nilsson M-C, Sellstedt A, Berglund LM (2004) Nitrogen fixation increases with successional age in boreal forests. Ecology 85:3327–3334
Zackrisson O, DeLuca TH, Gentili F, Sellstedt A, Jäderlund A (2009) Nitrogen fixation in mixed Hylocomium splendens moss communities. Oecologia 160:309–319
Zielke M, Ekker AS, Olsen RA, Spjelkavik S, Solheim B (2002) The influence of abiotic factors on biological nitrogen fixation in different types of vegetation in the high arctic, Svalbard. Arct Antarct Alp Res 34:293–299
Zielke M, Solheim B, Spjelkavik S, Olsen RA (2005) Nitrogen fixation in the high Arctic: role of vegetation and environmental conditions. Arct Antarct Alp Res 37:372–378
Acknowledgments
PLS was supported by postdoctoral grants from the University of Copenhagen and the Carlsberg Foundation. The long-term field experiment was maintained with support from the Danish Research Council/Nature and Universe and Abisko Scientific Research Station. The authors are grateful for invaluable laboratory assistance by Gosha Sylvester.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sorensen, P.L., Lett, S. & Michelsen, A. Moss-specific changes in nitrogen fixation following two decades of warming, shading, and fertilizer addition. Plant Ecol 213, 695–706 (2012). https://doi.org/10.1007/s11258-012-0034-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-012-0034-4