Abstract
A thorough understanding of the rate of depletion of ungerminated seeds in soil is necessary to understand and model the population dynamics of many plant species. To assess how edaphic conditions influence seed survival over time a long-term field study was set up. Mesh bags of seeds of 12 species were buried under 12 contrasting semi-natural and grassland habitats and retrieved at intervals over 10 years. Seed survival and viability were assessed through germination trials and chemical staining. There were clear differences in the rate of depletion of ungerminated seed between species and also differences in the variability of this measure between habitats. Seed survival was longer in soils with a higher pH, lower moisture content and lower soil C:N. Soil characteristics need to be taken into account within studies of plant populations that depend on regeneration from seed, particularly for species where seed survival is sensitive to edaphic conditions. Ignoring this influence of the dynamics of seeds under different soil conditions may have a serious impact on the success of population modelling.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Seed persistence in the soil is a feature of many species. This strategy is a form of bet hedging, as a proportion of seeds is not used for immediate investment in population growth, but remains available for germination in future years (Venable and Brown 1988). Seedbanks are consequently acknowledged as a key part of understanding plant population dynamics (Saatkamp et al. 2009 and references therein). Seedbanks contribute to population persistence (e.g. Stöcklin and Fischer 1999), species co-existence (e.g. Chesson and Warner 1981) and are key to the dynamics of open or highly disturbed systems (e.g. Thompson et al. 1998).
Much of the direction of seedbank studies has focussed on the differences between species in terms of their persistence in the soil. One type of study has taken a comparative approach by analysing the seedbank beneath different communities (Bossuyt and Hermy 2004; Pakeman and Marshall 1997). Another type has estimated loss rates after vegetation change such as conversion of grassland to forest (e.g. Milberg 1995), heathland to forest (e.g. Pywell et al. 2002) and heathland to bracken (Pakeman and Hay 1996). Others have considered the distribution of seeds with depth as an indicator of persistence (e.g. Bekker et al. 1998a). However, these types of study are potentially inaccurate for a number of reasons and it has been suggested that this type of seedbank data is labelled ‘seed persistence’ (Saatkamp et al. 2009). Actual ‘seed survival’ (Saatkamp et al. 2009) in the soil can be estimated by direct ageing of seeds using 14C dating (e.g. Moriuchi et al. 2000) or by burial experiments (e.g. Granström 1987). In effect survival represents seeds remaining in the soil that have not either germinated or died.
It could be said that many of the approaches used above ignore the possibility of environmental effects on seed survival; this has been the subject of many fewer studies. There is evidence that survival (Davis et al. 2005; Tamado et al. 2005) and persistence (Pakeman et al. 1999) is lower in warmer conditions; though no effect of temperature on persistence was shown in another study (Akinola et al. 1998). Similarly, warmer and wetter conditions reduced survival in three Australian weed species (Long et al. 2009). Reduced survival in wetter conditions has been put down to the increased hazard that fungi represent in these soils (Schafer and Kotanen 2003) as saprophytic fungi exercise an important control on seed mortality (Wagner and Mitschunas 2008). However, seed-attacking fungi may have the most significant impact at intermediate levels of soil moisture (Kiewnick 1964) whilst at very high levels of soil moisture seed death is a result of autolysis/anoxia (Bekker et al. 1998b). Unidentified site effects also affected the survival of buried seeds of fen meadow species (Bekker et al. 1998b, c).
Given so little is known about how edaphic conditions might influence seed survival in the soil, a long-term field study using natural variation in soil conditions between different sites was set up to address the question whether soil characteristics alter the rate of depletion of ungerminated seeds in the soil. To do this, a nine and a half year burial experiment was carried out with a range of species across different habitats within a small area to factor out climatic and geological effects. To date the only similar work has been done in arable systems, where soil nitrogen accelerated the mortality of Abutilon theophrasti (velvetleaf, Davis 2007). The focus on less disturbed habitats, agricultural grasslands and semi-natural communities, in the current study allowed a long-term study as the sites were not subject to soil disturbance.
Materials and methods
Species selection, bag construction and burial location
Twelve species of flowering plants (Table 1) were selected whose seeds were classified as showing a small proportion of seeds as persistent in the soil (type 3 of Grime et al. 1988) and whose seed was available commercially (Emorsgate Seeds, Tilney All Saints, Norfolk, UK). Species with a very short-lived seedbank (except for Anthoxanthum odoratum and Galium verum) and species with a very long-lived seedbank were not selected as their response would have been unlikely to be uninformative at the temporal resolution of our sampling. Ellenberg (1988) indicator values for these species are shown in Table A1 in the Supplementary material.
One hundred seeds of each species were counted out and placed in a fine nylon mesh bag (315 μm mesh to prevent seed loss but allow access to soil fungi and smaller soil invertebrates). To reduce effort, three species (random combinations) were placed in the same bag along with 2 g of fine, sterile sand as a packer to reduce direct seed to seed contact. As there were three species in a bag, ‘Bag’ was used as a fixed factor in the statistical analysis. It was never significant suggesting that species behaviour was independent of the other species it was mixed with. At each site 40 bags were placed, 10 bags of each of the four groups of three species. These were located at random positions in a 10 × 4 grid (roughly 5 × 2 m) placed at 10 locations within each of 12 sites at the Glensaugh Research Station of the Macaulay Institute (56°53′52′′N, 2°31′44′′W) in October 1999. Sites were all within 3.5 km and 230 m in altitude to reduce differences due to soil parent material and climate, and covered the full range of communities which were not subject to soil disturbance such as ploughing. There was no replication of sites or replication within sites. Bags were placed at 5 cm depth, their locations triangulated and marked by a short length (ca. 3 cm) of 10-cm diameter plastic drainpipe placed with its short axis vertical, central over the bag and with its top surface level to the soil surface. Each site had a different vegetation type (Table 2); it was assumed that vegetation could be used as a proxy for soil conditions in order to get variation in soil between sites. It is acknowledged that densities in the bags are high compared to natural densities and hence seeds may well have been at risk of seed to seed contamination by fungi (van Mourik et al. 2005). However, the conditions were the same for all species so this should not affect interpretation of the data if all species were similarly affected.
After burial, soil samples were taken, ten 5 cm diameter by 10 cm depth cores from each site mixed to give a composite sample. Samples were analysed for: pH (1:5 slurry in deionised water), moisture content (24 h at 105°C), loss on ignition (mass loss at 450°C), total carbon and nitrogen (automated Dumas combustion technique using a Flash EA1112 Elemental Analyser from ThermoFinnigan, Milan, Italy) and their ratio (C:N), extractable phosphate (acetic acid extraction followed by inductively coupled plasma optical emission spectroscopy), and exchangeable nitrate and ammonium (shaken with 2 M KCl and then analysed calorimetrically).
Removal and viability testing
One sample of each species (4 bags with 3 species per bag) was removed at each of 10 time points from each site: April 2000, October 2000, April 2001, October 2001, and April of 2002, 2003, 2004, 2005, 2006 and 2009. Sampling was more intensive at the start of the experiment as it was expected that the fastest depletion of ungerminated seed would occur then. Of the 480 bags set out in 1999 only 23 were not recovered.
The contents of each bag were sorted into species and whole seeds removed and counted. Empty seed coats were assumed to have germinated or been affected by fungal infection and seeds with no mechanical strength (tested by squeezing gently with forceps) were assumed to be dead. The whole seeds were sown on sand and kept in a heated greenhouse for 6 months and germination scored. Any remaining seeds were removed and their viability tested directly using red tetrazolium and indigo carmine (Hendry and Grime 1993). Viable seed at each time point was taken as the sum of the seeds that germinated and those shown to be viable by staining.
At the start of the experiment five 100 seed samples of each species were tested in the same way. All but one species had an initial viability between 90.8 and 97.4%. The exception was Lapsana communis whose initial viability was only 40%. These data were used as the zero time point in the analysis.
Statistical analysis
The initial analysis focussed on assessing if species and sites, and their interaction, were important factors to include in the analysis linking longevity to edaphic factors. This was assessed using generalised linear models (GLM), assuming a logit transformation and binomial errors for the viable seed count with the fixed factors being time, species and site. Season and bag were also trialled in these models but were not significant.
As the time × species × site interaction was significant, the time course of survival for each species × site combination was modelled independently. This was done using the slope of the decay function generated by the GLM for each combination. As the values were skewed (absolute value left skewed) and negative, the data had their absolute value log-transformed and the negative sign restored. This kept the data in the form that a more negative value represented a steeper depletion of ungerminated seed. The transformed slopes were then used as the dependent variable in a linear mixed effect model fitted with maximum likelihood with species and the soil variables as fixed factors and site as the random factor. As there were a relatively large number of correlated soil variables, an initial principal components analysis was carried out and the number of variables reduced by choosing only one from each set of correlated measures. For instance, total C, total N, loss on ignition and extractable ammonium were all highly correlated (r > 0.9); only loss on ignition was kept for the modelling as it had a higher loading in the PCA (loadings shown in Table A2 in the Supplementary material). Consequently only pH, moisture content, loss on ignition, the ratio of total carbon to nitrogen (C:N) and extractable phosphate were kept in the initial model alongside species identity and the interactions between the soil variables and species identity. This initial model was simplified by removing interaction terms and factors that did not contribute to its explanatory power. At each stage of the deletion process the model was checked with the previous the one by testing the likelihood ratio and by comparing AIC values (Crawley 2007). The new model was preferred if AIC fell. As an alternative measure of site conditions, weighted mean Ellenberg indicator values (Hill et al. 1999) were calculated for the vegetation at each site (visual estimates of cover in four 1 × 1 m quadrats) and the model fitting procedure repeated.
As germination (and possibly other forms of mortality) during the initial 6-month burial period represented a significant proportion of the seeds for many species, the statistical modelling was repeated without the data relating to initial viability. This approach may therefore represent a more appropriate estimate of long-term depletion rates.
All analyses were carried out in R version 2.10.0 (R Development Core Team 2009). The mixed effect modelling was carried out using the nlme package (Pinheiro et al. 2009).
Results
There were clear differences in the behaviour between species. As might have been expected from the classification in Grime et al. (1988) and the data in Thompson et al. (1997), the number of viable seeds of Anthoxanthum odoratum declined rapidly (Table 3). However, the decline was not as rapid as that of Lapsana communis which had the second highest longevity index. Also, these data sources predicted Galium verum should have depleted quickly. It was relatively rapid, but was not exceptional.
Final survival was highest in Silene dioica followed by Plantago lanceolata (Table 3). There was also substantial survival (ca. 5%) in Medicago lupulina, Myosotis arvensis, Poa trivialis, Prunella vulgaris and Viola tricolor. However, the loss of viable seeds from the bags followed different trajectories, as species that showed a slow rate of initial loss (time to 50% mortality) such as P. trivialis and Agrostis capillaris were not ranked as highly by the end of the experiment.
Some species such as Daucus carota and Medicago lupulina showed little variability between sites (Fig. 1c, f, respectively), whilst others seem to have a rate of loss of viable seeds very dependent on the site of placement. This was particularly true of P. lanceolata and P. vulgaris (Fig. 1h, j, respectively).
Proportion of ungerminated seeds of a Agrostis capillaris, b Anthoxanthum odoratum, c Daucus carota, d Galium verum, e Lapsana communis, f Medicago lupulina, g Myosotis arvensis, h Plantago lanceolata, i Poa trivialis, j Prunella vulgaris, k Silene dioica and l Viola tricolor against time since burial. Site codes are Bracken dotted line, Burn grey dashed dotted line, Conifer solid line, Deciduous dashed line, Flush grey dotted line, Heather grey dashed line, Loch Reseed light grey dashed dotted line, Nardus light grey dashed line, Rhododendron dashed dotted line, Rush light grey dotted line, Thorter Reseed light grey solid line and Vaccinium grey solid line
Removal of the initial viability data increased the resulting half-life (Table A4). This included substantially longer half-lifes for Anthoxanthum odoratum, Silene dioica and Viola tricolor indicating that once the initial depletion due to germination was accounted for then these species were relatively long-lived.
The GLM showed that each of the main factors (time, species and site) was significant and so were their interactions. No model simplification resulted in a decrease in AIC values either. Bag or season was not significant in explaining any variation. This indicated that the strategy of using the rate of loss of ungerminated seed for each species × site combination as the independent variable in the rest of the analysis was justified. The same pattern of significant variables (time, species and site and their interactions) was also seen if the initial viability data were removed from the analysis.
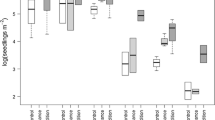
Model simplification removed total phosphate and loss on ignition to give a minimally adequate model that contained species identity, as well as soil pH, soil moisture loss and soil C:N ratio and their interaction terms with species identity (Table 4). In fact the interaction terms were significant indicating that species behaviours differed along the environmental gradients tested. Longer survival was, however, generally a feature of soils with a higher pH (Fig. 2a) that held less water (Fig. 2b) and had a lower C:N (Fig. 2c). Removing the initial viability data from the model resulting in a similar selection of variables, but C:N ratio was eliminated from the model (Table A5). Replacing individual soil variables with weighted mean Ellenberg indicator values was less successful (higher AIC) but resulted in a similar model (Table A3). Survival was longer in soils of higher pH, higher nutrient and lower water content, and there were significant interactions between species identity and both nutrient status and soil pH.
Box plots of calculated seed half-life (time at 50% survival) versus a soil pH, b soil moisture loss and c soil C:N. Thick line corresponds to the median, the upper and lower limits of the box to the 75th and 25th percentiles, respectively, the whiskers to 1.5 × the interquartile range. Outliers are shown as circle
Discussion
The study described here clearly shows that the rate of depletion of ungerminated of seeds in the soil is affected by the characteristics of the soil. The best model that included single environmental variables indicated that higher pH, lower soil moisture and lower soil C:N (and hence likely higher fertility) were associated with a slower decay in survival. This model was largely supported (with the exception of soil C:N) even if the initial viability data was removed. It was also supported by the similar result from fitting the model using Ellenberg indicator values. These findings supports that of Long et al. (2009) who showed that there were considerable differences in seed survival between three different soils over two growing seasons. However, they attributed this to be an effect of soil moisture and temperature. Other studies have shown that survival depends on other soil characteristics including clay content and organic matter (Davis et al. 2005) and soil type (Narwal et al. 2008). This study, however, contradicts the findings of Bekker et al. (1998c) who found no differences in the effects of fertility on seed survival in fen meadows, though their study was only 2 years in duration. It partly supports the findings of Davis (2007) who showed nitrogen-driven effects on seed decay: high fertility, and in particular high nitrate levels, in arable soils might have a strong, negative impact on longevity as nitrate promotes germination in many weed seeds (Benech-Arnold et al. 2000; Egley 1986) but through enhancing microbial activity it may increase mortality. However, here the best variable associated with fertility was the C:N ratio of the soil, though this was correlated to soil ammonium (r = 0.41, n = 12), the main form or inorganic nitrogen in soils of the pHs used in this study. This was backed up by the significance of the Ellenberg Nitrogen indicator in the alternative model, and this has been shown to be more appropriately identified as a general fertility indicator (Hill and Carey 1997). However, there may also be ‘no unifying mechanism relating soil fertility to seed mortality’ (Davis 2007).
However, the data also showed that species are not affected into the same degree by differences in these soils conditions. Certain species appear to have similar depletion rates across the different sites, whilst others appear more sensitive (Figs. 1, 2). The former are the two species, Daucus carota and Medicago lupulina, that do not show physiological dormancy (Table 1). The physical dormancy of M. lupulina appears to make it less sensitive to the environment than the species with physiological dormancy. This suggests that even the overall conclusion drawn here, that seed survival is sensitive to soil pH and nutrient status, is species specific and dependent on the dormancy mechanism present in the seeds. Differences may be due to differences in seed coat characteristics or responses to environmental cues that result in germination at depth. The previous storage of the seeds after harvest may well have contributed to the rapid initial depletion of live seeds in the bags. As it was commercially sourced seed it is difficult to establish possible impacts of storage on initial likelihood of germination after burial, but accounting for this did not substantially change the fitted models. It is possible that the lower survival of seeds at low pH may be due to toxicity from aluminium or other metals. Given that the majority of species shared the same dormancy mechanism (Table 1) it was not possible to test whether depletion rates and dormancy are related.
The differences between species survival in the soil were not predicted by either seed mass or persistence data. There was no correlation (r = −0.02, n = 12) between time to a 50% fall in survival and the seed longevity index of Thompson et al. (1998), calculated as the proportion of records classifying species as short- or long-term persistent out of all records. Similarly, there was no correlation between survival and seed mass (r = −0.33, P > 0.29, n = 12), though the direction of the correlation did indicate higher survival of smaller seeds. With the initial viability data removed the same absence of significant correlation was observed. This lack of correlation does not, however, contradict the conclusions of Saatkamp et al. (2009) that there was no relationship between experimental seed survival and estimates of seedbank persistence in emergence studies as the latter is correlated to seed production. Habitat level conclusions about the significance of seedbanks need to be treated differently to assessments for population dynamics of individual species.
In conclusion, there appears to be good evidence from this and other studies that soil characteristics need to be taken into account within studies of plant populations that depend on regeneration from seed. For species that are particularly sensitive to edaphic conditions, ignoring the dynamics of seeds under different soil conditions may have a serious impact on the accuracy of population modelling. The promotion of seed survival at high pH and high fertility conflicts with some published evidence (see above). However, if one of the major determinants of seed survival is fungal attack (Wagner and Mitschunas 2008) then in more fertile and neutral soils the shift to bacterial dominance in the microbial community (Bardgett and McAlister 1999) may exclude some of the saprotrophs responsible for seed mortality.
References
Akinola MO, Thompson K, Hillier SH (1998) Development of soil seed banks beneath synthesized meadow communities after seven years of climate manipulations. Seed Sci Res 8:493–500
Bardgett RD, McAlister E (1999) The measurement of soil fungal:bacterial biomass ratios as an indicator of ecosystem self-regulation in temperate meadow grasslands. Biol Fert Soils 29:282–290
Baskin CC, Baskin JM (1998) Seeds. Ecology, biogeography, and evolution of dormancy and germination. Academic Press, San Diego
Bekker RM, Oomes MJM, Bakker JP (1998a) The impact of groundwater level on soil seed bank survival. Seed Sci Res 8:399–404
Bekker RM, Bakker JP, Grandin U, Kalamees R, Milberg P, Poschlod P, Thompson K, Willems JH (1998b) Seed size, shape and vertical distribution in the soil: indicators of seed longevity. Funct Ecol 12:834–842
Bekker RM, Knevel IC, Tallowin JBR, Troost EML, Bakker JP (1998c) Soil nutrient input effects on seed longevity: a burial experiment with fen-meadow species. Funct Ecology 12:673–682
Benech-Arnold RL, Sánchez RA, Forcella F, Kruk BC, Ghersa CM (2000) Environmental control of dormancy in weed seed banks in soil. Field Crops Res 67:105–122
Bossuyt B, Hermy M (2004) Seed bank assembly follows vegetation succession in dune slacks. J Veg Sci 15:449–456
Chesson PL, Warner RR (1981) Environmental variability promotes coexistence in lottery competitive systems. Am Nat 117:923–943
Crawley MJ (2007) The R book. Wiley, Chichester
Davis AS (2007) Nitrogen fertilizer and crop residue effects on seed mortality and germination of eight annual weed species. Weed Sci 55:123–128
Davis AS, Cardina J, Forcella F, Johnson GA, Kegode G, Lindquist JL, Luschie EC, Renner KA, Sprague CL, Williams MM III (2005) Environmental factors affecting seed persistence of annual weeds across the US corn belt. Weed Sci 53:860–868
Egley GH (1986) Stimulation of weed seed germination in soil. Rev Weed Sci 2:67–89
Ellenberg H (1988) Vegetation ecology of Central Europe, 4th edn. Cambridge University Press, Cambridge
Granström A (1987) Seed viability of fourteen species during five years of storage in a forest soil. J Ecol 75:321–331
Grime JP, Hodgson JG, Hunt R (1988) Comparative plant ecology. Unwin Hyman, London
Hendry GAF, Grime JP (eds) (1993) Methods in comparative plant ecology: a laboratory manual. Chapman and Hall, London
Hill MO, Carey PD (1997) Prediction of yield in the Rothamsted Park Grass Experiment by Ellenberg indicator values. J Veg Sci 8:579–586
Hill MO, Mountford JO, Roy DB, Bunce RGH (1999) Ellenberg’s indicator values for British plants. ECOFACT vol 2, technical annex. Institute of Terrestrial Ecology, Huntingdon
Kiewnick I (1964) Untersuchungen über den Einfluss der Samen- und Bodenmikroflora auf die Lebensdauer der Spelzfrüchte des Flughafers (Avena fatua L.). II. Zum Einfluss der Mikroflora auf die Lebensdauer der Samen im Boden. Weed Res 4:31–43
Long RL, Steadman KJ, Panetta FD, Adkins SW (2009) Soil type does not affect seed ageing when soil water potential and temperature are controlled. Plant Soil 320:131–140
Milberg P (1995) Soil seed bank after eighteen years of succession from grassland to forest. Oikos 72:3–13
Moriuchi KS, Venable DL, Pake CE, Lange T (2000) Direct measurement of the seed bank age structure of a Sonoran desert annual plant. Ecology 81:1133–1138
Narwal S, Sindel BM, Jessop RS (2008) Dormancy and longevity of annual ryegrass (Lolium rigidum) as affected by soil type, depth, rainfall, and duration or burial. Plant Soil 310:225–234
Pakeman RJ, Hay E (1996) Heathland seedbanks under bracken Pteridium aquilinum (L.) Kuhn and their importance for re-vegetation after bracken control. J Environ Manage 47:329–339
Pakeman RJ, Marshall AG (1997) The seedbanks of the Breckland heaths and heath grasslands, and their relationship to the vegetation and the effects of management. J Biogeogr 24:375–390
Pakeman RJ, Cummins RP, Miller GR, Roy DB (1999) Potential climatic control of seedbank density. Seed Sci Res 9:101–110
Pinheiro J, Bates D, DebRoy S, Sarkar D, the R Core team (2009) nlme: Linear and nonlinear mixed effects models. R package version 3.1-96
Pywell RF, Pakeman RJ, Allchin EA, Bourn NAD, Warman EA, Walker KJ (2002) The potential for lowland heath regeneration following plantation removal. Biol Conserv 108:247–255
R Development Core Team (2009) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. ISBN 3-900051-07-0, http://www.R-project.org
Rodwell JS (ed) (1991a) British plant communities. Volume 1. Woodlands and scrub. Cambridge University Press, Cambridge
Rodwell JS (ed) (1991b) British plant communities. Volume 2. Mires and heath. Cambridge University Press, Cambridge
Rodwell JS (ed) (1992) British plant communities. Volume 3. Grassland and montane communities. Cambridge University Press, Cambridge
Saatkamp A, Affre L, Dutoit T, Poschlod P (2009) The seed bank longevity index revisited: limited reliability evident from a burial experiment and database analyses. Ann Bot 104:715–724
Schafer M, Kotanen PM (2003) The influence of soil moisture on losses of buried seeds to fungi. Acta Oecol 24:255–263
Stöcklin J, Fischer M (1999) Plants with longer-lived seeds have lower local extinction rates in grassland remnants 1950–1985. Oecologia 120:539–543
Tamado T, Schutz W, Milberg P (2005) Germination ecology of the weed Parthenium hysterophorus in eastern Ethiopia. Ann Appl Biol 140:263–270
Thompson K, Bakker JP, Bekker RM (1997) Soil seed banks of North West Europe: methodology, density and longevity. Cambridge University Press, Cambridge
Thompson K, Bakker JP, Bekker RM, Hodgson JG (1998) Ecological correlates of seed persistence in soil in the north-west European flora. J Ecol 86:163–169
Van Mourik TA, Stomph TJ, Murdoch AJ (2005) Why high seed densities within buried mesh bags may overestimate depletion rates of soil seed banks. J Appl Ecol 42:299–305
Venable DL, Brown JS (1988) The selective interactions of dispersal, dormancy and seed size as adaptations for reducing risks in variable environments. Am Nat 131:360–384
Wagner M, Mitschunas N (2008) Fungal effects on seed bank persistence and potential applications in weed biocontrol: a review. Basic Appl Ecol 9:191–203
Acknowledgments
Thanks to Jo Denyer for her help in the middle part of the experiment and to Andrew Scobie for checking and formatting the data. Thanks also to Donald Barrie for use of the research farm at Glensaugh. This study was funded by the Scottish Government’s Rural and Environmental Research and Analysis Directorate. The comments of three referees improved the article.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pakeman, R.J., Small, J.L. & Torvell, L. Edaphic factors influence the longevity of seeds in the soil. Plant Ecol 213, 57–65 (2012). https://doi.org/10.1007/s11258-011-0006-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-011-0006-0