Abstract
Tree bark characteristics influence epiphyte establishment and survival and consequently the way in which epiphytes are distributed on trees. Tree species with peeling bark have been reported as poor epiphyte hosts. We analyzed the distribution and seedling mortality of two Tillandsia species (Bromeliaceae) in relation to rate of bark peeling of Bursera fagaroides (Burseraceae). The highest peeling rate (0.12% per day) took place on the trunk and the lowest rate on twigs (0.04% per day; branches ≤2 cm in diameter). The highest proportion of Tillandsia plants appeared on twigs. The distributions of juvenile and adult plants on twigs were higher than those expected based on the distribution of first-year seedlings, suggesting that on twigs, survival could be greater than on trunks and branches, canopy areas where peeling is faster. On the trunk and branches, in contrast, the proportion of juveniles and adults were similar to or less than that expected for first-year seedlings. The main cause of mortality was peeling and the area of minor overall mortality was the trunk, suggesting that this area should be favored as the main distribution area for the Tillandsia species but is not. Our results show that the peeling rate of B. fagaroides depends on branch size and suggest that the Tillandsia distribution depends not only on peeling rate but also on seed dispersion. We suggest that to colonize B. fagaroides epiphytes would either have adaptations to counteract the peeling rate or should occur in the areas of lowest peeling rate located in the exterior crown of trees.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plant–plant interactions are of prime importance to understand the maintenance of biodiversity, especially in tropical areas where plant diversity is high. Epiphytes are plants wholly dependent on other plants, and it has been suggested that epiphytes should evolve mechanisms permitting generalist establishment, making the host species redundant and allowing the epiphytes to use any host (Callaway et al. 2002). In general, epiphyte plants can colonize many tree species and only a few epiphyte species have been considered as host specific (e.g. Ackerman et al. 1989; Tremblay et al. 1998; Mehltreter et al. 2005). However, the number of epiphyte species and epiphyte abundance that a host can harbor vary greatly; some hosts can carry as many as 50 epiphyte species, while other species have no epiphytes (Freiberg 1996), suggesting that some host species are especially difficult for epiphytes to colonize.
Features of the bark have been suggested as important in determining the number and composition of epiphyte species that grow on a particular tree species. Trees having bark with low water-holding capacity, alellochemicals, or that peels off, may carry fewer epiphyte species than those trees with bark that has a high capacity for holding water, lacks alellochemicals or durable bark (Frei 1974; Benzing 1990; Talley et al. 1996; Castro-Hernández et al. 1997; Callaway et al. 2001, 2002; Mehltreter et al. 2005).
Based on published reports of tree species abundances and composition, trees with peeling bark make up between 9 and 70% of the individuals found in dry tropical forest (Rico-Gray et al. 1988; Ackerman et al. 1989; Zimmerman and Olmsted 1992; Valencia-Díaz 1995; Martínez-García 1999). Peeling bark is one of the most thoroughly documented factors influencing low species richness and abundance of epiphytes and vines on trees (Todzia 1986; Kiew and Anthonysamy 1987; ter Steege and Cornelissen 1989; Brown 1990; Talley et al. 1996; Zimmerman and Olmsted 1992). Despite the proposed role of bark in limiting epiphyte abundance, some epiphytes grow on trees with peeling bark and may be specialized on these tree species (e.g. Psygmorchis glossomystax (Rchb. f.) Dodson & Dressler (Orchidaceae) on Psidium guajava L. (Myrtaceae); Benzing 1990).
Although exfoliation is supposedly a characteristic that helps trees to lower epiphyte and vine invasions (Todzia 1986; Kiew and Anthonysamy 1987; Stevens 1987; ter Steege and Cornelissen 1989; Brown 1990; Talley et al. 1996; Zimmerman and Olmsted 1992), there are no data on the rate at which peeling occurs. Recurrent observations that tree species with peeling bark may host epiphytes suggest that peeling rates vary in the crown, making establishment of epiphytes possible. We analyzed the peeling rate of Bursera fagaroides Engl. var. purpusii (Brandegee) McVaugh & Rzed. (Burseraceae) and related it to the distribution and seedling mortality of the epiphytic bromeliads: Tillandsia palmasolana Matuda and T. paucifolia Baker. We hypothesized that:
-
a.
if bark peeling rate influences the distribution of epiphyte species, then the majority of the epiphytes inhabiting bark peeling trees should be in canopy zones with the lowest peeling rate; and
-
b.
if bark peeling rate is the main mortality cause, we expected that in canopy zones of high peeling rate should be the major mortality and the proportion of adult plants should be reduced because the transition from seedling to adult plants will be the lowest.
Methods
Study area
This research was conducted at La Mancha Coastal Research Center (CICOLMA, 19°35´12′′ to 19°36′18′′ N, 96°22′18′′ to 96°23′24′′ O, 0–50 m a.s.l.) in Central Veracruz, Mexico. The climate is warm sub-humid, with a mean minimum temperature of 18°C and a maximum of 34°C. Rains are concentrated in the summer, with total annual rainfall varying from 1,200 to 1,500 mm (Castillo-Campos and Medina-Abreo 2002). At CICOLMA, there are eight types of vegetation and this research was done in savannah (coastal dune brushland; Castillo-Campos and Medina-Abreo 2002). This community is an ecotone between tropical dry forest and the herbaceous communities found on dunes; isolated dwarf trees (3–6 m in height) are abundant and the most characteristic are Bursera fagaroides, Byrsonima crassifolia (L.) H. B. K. (Malpighiaceae) Coccoloba barbadensis Jacq. (Polygonaceae), Chrysobalanus icaco L. (Chrysobalanaceae), Fraxinus schiedeana Schltdl. & Cham. (Oleaceae), and Lysiloma divaricata Hook. & Jackson Marchr. (Fabaceae). In this brushland, the most frequent epiphytes are the narrow Mexican endemic T. palmasolana and the widespread T. paucifolia; other epiphyte bromeliad species are nearly absent (García-Franco 1996).
Data collection
Fieldwork was carried out from November 2001 to April 2002. During that period, 10 B. fagaroides var. purpusii (hereafter B. fagaroides) dwarf trees were selected from the windward slope of a dune. Trees measured 2.8 ± 0.2 m in height (hereafter \( \ifmmode\expandafter\bar\else\expandafter\=\fi{x} \) ± SE) and 305.8 ± 55.6 cm2 in basal area; they had 1.5 ± 0.2 small trunks and were isolated from all other trees.
Bark peeling rate
To evaluate the B. fagaroides bark peeling rate, three canopy zones were defined: (1) twigs, i.e. exterior branchlets of the tree crown with a diameter ≤2 cm; (2) branches, i.e. limbs of 2.1–10 cm in diameter; and (3) trunks, i.e. basal sections of the tree ≥10 cm in diameter. From these canopy zones we randomly chose a total of 30 sections in each of the selected trees. Each section was measured (diameter). All sections were classified into one of seven categories of 2-cm intervals. On each section, a plot 5 cm long × 1 cm wide (20 0.5 × 0.5 cm2) was painted with commercial vinyl paint. After 3 months the exfoliated area was measured; to accomplish this, a transparent grid of 20 0.5 × 0.5 cm2 was placed on the painted plots and we counted the number of exfoliated squares. With a randomized block analysis of variance (Zar 1996), we analyzed if the percentage of exfoliation after 3 months was different among the seven diameter categories. In this analysis the trees were considered as the blocks. To avoid pseudoreplication, we used as the response variable the mean exfoliated area of the plots of each diameter category for each tree.
Distribution and population structure of Tillandsia spp.
In each tree, Tillandsia palmasolana and T. paucifolia plants were counted on the whole trunk and in a sample of branches randomly selected (50–100% of the branches). We measured the branch diameter where each Tillandsia plant was found, and we recorded the length of the longest leaf for each Tillandsia, the number of rosettes, and the presence or absence of inflorescences. To create a static population structure based on plant size, Benzing’s (1981) age-size classification system for T. paucifolia (≈ circinnata) was employed in a slightly modified version (Table 1). The age-size system proposed for T. paucifolia (Benzing 1981) was also used for T. palmasolana to facilitate comparison of results, as has been done with other Tillandsia species (e.g. Mondragon et al. 1999, 2004).
We created a contingency table to determine bromeliad distribution on B. fagaroides. In this table rows were Tillandsia age-size categories and columns were the seven diameter categories of branches-trunks described previously (see previous section; Tables 2, 3). Because Tillandsia category A corresponds to first-year seedlings (Table 1), we considered their abundance as an indicator of the establishment success of bromeliads in different canopy zones. If seed establishment and survival were similar throughout the canopy zones, the larger categories of plant age size should exhibit a distribution similar to that of first-year seedlings, as has been suggested previously by Zotz (1997). For each Tillandsia’s age-size category we calculated the expected number of plants in each trunk-branch category following the observed distribution of first-year seedlings. To calculate the expected values we multiplied the observed total number of plants in each size category with the proportions found for first-year seedling in each trunk-branch category (Tables 1–3). Using the entire cross tabulation matrix, a χ 2 test was applied to determine if size categories were distributed along trunks-branches in accordance with the pattern observed for first-year seedlings, and a test of residuals was used to identify individual differences between observed and expected abundances (Haberman 1973). In the case of T. palmasolana, categories A and B were pooled, as there were no first-year seedlings for some trunk sizes, making it impossible to calculate expected values.
Seedling mortality
To evaluate seedling mortality we marked 96 sections of trunk, branches, and twigs with flagging tape and counted the number of seedlings on each section. The seedlings were 3–10 mm in height and were clearly rooted to the bark. As in other studies (Winkler 2005) we assumed that all the seedlings we observed were either T. palmasolana or T. paucifolia, although occasionally T. ionantha Planch. plants could be found. Every 15 days we counted the number of living and dead seedlings. Cause of mortality was assumed to be from either lack of moisture when desiccated seedlings remained attached to the bark or from bark peeling if the seedling had disappeared. Herbivory and branch falling could be another possible cause of mortality; in the case of herbivory, Tillandsia roots remain attached to the bark but the leaves disappear; however, we did not observe either herbivore damage or branch falling and all the seedling mortality could be determined as caused by drought or peeling. As we found a low number of seedlings on trunks, we used the total number of seedlings found on all the marked sections of trunk (n = 50 seedlings), branches (n = 283 seedlings), and twigs (n = 544 seedlings) for the analysis. With three χ2 tests (Zar 1996) we tested whether each mortality source (drought, peeling, and total) was different among the three canopy zones (trunk, branches, and twigs) (Zar 1996). When one test revealed significant differences we performed a Tukey-type multiple comparison test among proportions (Zar 1996) to isolate the canopy zone where mortality differed.
The low mortality of plants located on the trunk prevented us from determining whether sources of mortality (drought vs. peeling), differed. To resolve this problem we used the pooled mortality (all canopy zones together) and with a χ 2 test we compared whether mortality through drought or peeling differed (assuming an expected 1:1 ratio). As this test revealed significant differences we tested if the observed mean ratio were constant between the three canopy zones; for this analysis we used a χ 2 test of heterogeneity with a ratio of 1:1.7 (assuming a mean mortality ratio of 1:1.7 between mortality caused by drought and by peeling, see Results).
Results
Bark peeling rate
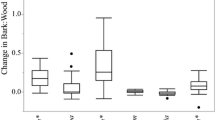
The bark peeling rate was different among size categories of trunks and branches (F 6, 54 = 6.6, P < 0.0001) (Fig. 1a). The peeling rate followed an ascending gradient from the twigs to the trunk (Fig. 1a). The trunk peeling rate (10.8 ± 1.1 after 91 days) was three times faster than the peeling rate of twigs (3.2 ± 1.1 after 91 days) (Fig. 1a).
Percent of peeled bark after 91 days (a) and the distribution of two Tillandsia species on Bursera fagaroides (b, c). In (a) the values are means obtained from 10 different trees, and the error bars are ±1 SE. Different letters correspond to significant differences (Tukey test, P < 0.05) in the percent of peeled bark
Distribution and population structure of Tillandsia spp.
A total of 1,193 plants were counted, 411 T. palmasolana and 782 T. paucifolia (Table 1). For T. palmasolana, the population structure showed that most plants belonged to categories C and H; while for T. paucifolia, most were from the first three size categories (A–C) as well as sub-adults (H) (Table 1).
The greatest numbers of plants of both species were found on twigs, diminishing toward larger branches and the trunk (Fig. 1b, c). This pattern was consistently found in all age-size categories for both species (Tables 2, 3) with the exception of T. palmasolana seedlings, which were more abundant on branches measuring 2.1–8 cm in diameter. We found no T. paucifolia CA or SA plants on trunks of over 10 cm in diameter.
For T. palmasolana the distribution of the plants differed from that observed in first-year seedlings (χ 2 = 409.4, P < 0.001) (Table 2). The proportion of C-SA plants on twigs ≤ 2 cm in diameter was greater than that expected according to first-year-seedling distribution (Table 2). On branches with a diameter of 2.1–8 cm, C-SA plant distribution was similar to or less than that expected on the basis of first-year-seedling distribution; while on branches with a diameter of over 8 cm, C-SA plant distribution was similar to and in four cases greater than that expected on the basis of first-year-seedling distribution (Table 2).
For T. paucifolia, the distribution of plants differed from that observed in first-year seedlings (χ 2 = 239.2, P < 0.001) (Table 3). The number of C-H plants on twigs (≤2 cm in diameter) was greater than that expected on the basis of first-year-seedling distribution (Table 3). The number of B-SA plants growing on branches 2.1–4 cm was greater than or similar to that of first-year seedlings. The number of B-H plants growing on branches 4.1–12 cm in diameter was lower than or similar to that expected on the basis of first-year seedlings (Table 3). Only the number of B and H plants growing on trunks with a diameter >12 cm was greater than expected (Table 3).
Seedling mortality
The proportion of seedlings that died by drought (χ 2 = 3.72, P = 0.16) or through bark peeling (χ 2 = 4.77, P = 0.09) did not differ between canopy zones (Fig. 2a, b). However, total mortality (drought plus peeling) was different between canopy zones (χ 2 = 7.82, P = 0.02) (Fig. 2c). In the trunk the total mortality was the lowest (20.0%) but only differed from the total mortality on twigs (39.3%) (Tukey-type test for proportions, P < 0.05), and the total mortality was similar among twigs and branches (35.3%).
Percent of bromeliads seedling mortality caused by drought (a), peeling bark (b), or total (c). The percent was obtained after a 91-day observation period of seedling survival on Bursera fagaroides trees. Sample size was 50 seedlings on trunk, 283 seedlings on branches, and 544 seedlings on the twigs. Different letters correspond to significant differences (Tukey-type test for multiple comparisons among proportions, P < 0.05)
Independent of canopy zone, total number of dead seedlings (324) was mainly (58.6%) caused through bark peeling (χ 2 = 9.68, P < 0.05). The observed ratio obtained from the total of seedlings that died by drought and those died through peeling bark was 1:2.3 in the trunk (3:7 seedlings), 1:1.1 in branches (47:53 seedlings), and 1:1.5 in twigs (84:130 seedlings) giving a mean ratio of 1:1.7 for the whole tree. The ratio of the three canopy zones did not differ (χ 2 of heterogeneity = 1.73, P > 0.05).
Discussion
As expected, we found that exfoliation rate varied inside the crown of B. fagaroides. For epiphytes, substrate age and stability are factors that determine community composition and succession (Catling and Lefkovitch 1989; Nadkarni 2000); if bark from older areas exfoliates faster, the epiphyte community suffers a disturbance rate that can influence its development. According to our hypotheses we noted that regardless of Tillandsia plant size, a lower abundance occurs near the trunk and many plant size classes were less abundant than expected on the trunk, where the peeling rate is three times higher than on twigs; however, the fact that mortality was the lowest in the trunk suggests that the distribution of the two Tillandsia species is not only determined by the peeling rate.
Two main mortality factors have been found in population of epiphytes: drought (Zotz and Tyree 1996; Zotz and Andrade 1998) and dislodgement from the anchorage position (Martínez and García-Franco 2004; Mondragon et al. 2004; Zotz 2004; Winkler 2005; Zotz et al. 2005). We measured both sources of mortality, and as expected bark peeling was the main cause of mortality of epiphytes. It could be that some seedlings died by drought and fell off with the bark before we carry out our observations; we believe that this bias had low importance in our observations because we made observations with a high periodicity, but future studies should take this problem into account. The overall seedling mortality found is similar to that reported for authors working with epiphytic bromeliads of lowland forests (Mondragon et al. 2004; Zotz et al. 2005) and minor to that found in epiphytic bromeliads of montane forest (Winkler 2005). Contrary to our expectations lower overall mortality was found in the trunk, and some adult-sized plants were more abundant than expected in the trunk. Our results differ from those found for the bromeliads Guzmania monostachya (L.) Rusby ex Mez, Tillandsia fasciculata Sw., and Werauhia sanguinolenta (Cogn. & Marchal) J.R. Grant, in which first-year-seedling survival was the same throughout the crown of Annona glabra L. (Annonaceae) (Zotz and Vollrath 2002). In these species and in the epiphytic orchid Tolumnia variegata (Sw.) Braem, the distribution of different-sized plants was similar throughout the host crowns (Ackerman et al. 1996; Zotz 1997), suggesting that mortality is similar throughout the crown. Winkler (2005) found that bromeliad seedling survival increased with canopy openness in a lower montane cloud forest. Seedling mortality could differ inside the canopy, and then different canopy zones could be favored as epiphyte microhabitats. If the trunk of B. fagaroides is an area of low mortality then this canopy zone should be favored as epiphyte habitat, but it was the area with the lowest abundance of Tillandsia plants and the majority of the adult-size categories were absent. It is possible that the season of our observation was exceptional, causing low overall mortality on the trunk, or maybe that other factors could explain our contradictory results (low seedling mortality and low abundance of plants in the trunk), for example, the mortality rate of larger plants in the trunk or seed dispersal in the crown.
The spatial distribution of Tillandsia first-year seedlings on B. fagaroides suggests that Tillandsia establishment is not homogeneous within the crown. Tillandsia are wind-dispersed plants, and the greatest concentration of seeds has been observed to remain near the mother plant (García-Franco and Rico-Gray 1988; Winkler 2005; Cascante-Marin 2006). Thus, the distribution pattern seems to reflect the proportion of seeds that reach each canopy zone. Furthermore, Zotz (1997) suggested that exterior twigs and branches could form an umbrella that can capture most seeds, and he found that twigs ≤1 cm in diameter constitute 29% of the substrate offered by the tree Annona glabra (Zotz 1997). We expected that in canopy zones high peeling rate should be the major mortality, but contrarily, we observed the lowest mortality in the trunk. A smaller number of seeds arriving to the trunk plus the highest peeling rate could explain the low abundance of plants on the trunk. However those seeds that colonized the trunk had the lowest probability of dying despite the high peeling rate, probably because of better microclimatic conditions on the trunk.
Our data suggest that Tillandisa species colonizing B. fagaroides could face rigorous conditions, either because of high rates of bark peeling or difficult microclimatic conditions in the crown. In the exterior crown of B. fagaroides peeling rate is low but humidity could be lower, while the peeling rate of the trunk is higher but microclimatic conditions are likely to be less rigorous. Differences in microclimatic conditions have been shown in tall canopy species (Madigosky 2004). In the habitat studied, deciduous B. fagaroides trees are small and exposed to high levels of sunlight and seasonal strong winds (80 km/h, Martínez and García-Franco 2004). It is therefore not surprising that the two bromeliad species we studied are among the few epiphytes that can survive, as these species are well adapted to drought (Benzing 1978a; Martin 1994). Tillandsia paucifolia, the more abundant at the study site, has been reported as a sun-loving epiphyte (Benzing 1978b; Benzing and Davidson 1979). This suggests that the two Tillandsia species colonizing B. fagaroides are adapted to rigorous microclimatic conditions, in areas of the canopy having the slowest peeling rate.
The low species richness usually found in host with peeling bark (Todzia 1986; Kiew and Anthonysamy 1987; ter Steege and Cornelissen 1989; Brown 1990; Talley et al. 1996; Zimmerman and Olmsted 1992) is probably associated with the high peeling rate of the trunk and interior branches. In non-peeling trees, the interior branches and the trunk could be inhabited by a rich epiphyte community (e.g. Lyons et al. 2000; Mehltreter et al. 2005). It is possible that the high peeling rate of these zones in B. fagarioides excludes many epiphytic species. In the study area, two epiphytic orchid species that inhabit on B. fagaroides preferentially colonize trunks and branches >6 cm in diameter (Flores-Palacios and Ortiz-Pulido 2005). But the presence of termite carton structures facilitates the establishment of these orchids (Flores-Palacios and Ortiz-Pulido 2005). Other organisms may facilitate the presence of adult-size plants on the trunks of B. fagarioides. For example, non-vascular epiphytes have been shown to play a key role in the establishment success and abundance of bromeliads (Bennett 1987; Callaway et al. 2001; Zotz and Vollrath 2003), and on B. fagaroides we observed non-vascular epiphytes (Flores-Palacios and Ortiz-Pulido 2005). Another possibility is that this adult plant could develop roots fast enough to counteract exfoliation, or that variability in trunk peeling provides certain areas where plants can prosper.
Despite its peeling bark, B. fagaroides features an abundance of bromeliads similar to that found on trees of similar size (<5 m in height). For example, with samples of 10–15 Taxodium ascendens Brongn. (Taxodiaceae) dwarf trees, Benzing (1981) found between 62 and 92 T. paucifolia plants in Florida. On Barro Colorado Island, Zotz (1997) found 762 Guzmania monostachia plants, 238 T. fasciculata plants, and 3,552 Werauhia sanguinolenta plants on eight dwarf Annona glabra trees; and on the same host species, another 20 epiphyte species could develop populations ranging from 7 to 276 adult plants per tree (Zotz et al. 1999). In other settings, bromeliad abundance may be quite low; for example, Bennett (1987) found 0.7–15 bromeliads per tree in Florida. This suggests that the bromeliad species studied are invading B. fagaroides as successfully as other epiphyte species do on non-peeling bark trees.
Plant–plant interactions are important for understanding how biological diversity is maintained. Epiphytes comprise 10% of the vascular flora worldwide (Kress 1986) and are wholly dependent on other plants (trees), but in forests dominated by trees with peeling bark they could be limited by the peeling behavior of the trees. We found, for the first time, that peeling rate varies inside the crown, and this variability could explain, the presence of epiphytes on them. Peeling rate of B. fagaroides depends on branch size, but the Tillandsia distribution depends not only on peeling rate but also on seed dispersion and the microclimatic conditions inside the crown. We suggest that to colonize B. fagaroides the epiphytes should be adapted to counteract the peeling rate or should be able to inhabit the areas of lowest peeling rate located in the exterior crown of trees.
References
Ackerman JD, Montalvo AM, Vera AM (1989) Epiphyte host specificity of Encyclia krugii, a Puerto Rican endemic orchid. Lindleyana 4:74–77
Ackerman JD, Sabat A, Zimmerman JK (1996) Seedling establishment in an epiphytic orchid: an experimental study of seed limitation. Oecologia 106:192–196
Bennett BC (1987) Spatial distribution of Catopsis and Guzmania (Bromeliaceae) in Southern Florida. Bull Torrey Bot Club 114:265–271
Benzing DH (1978a) Germination and early establishment of Tillandsia circinnata Schlecht (Bromeliaceae) on some of its host and other supports in the southern Florida. Selbyana 5:95–106
Benzing DH (1978b) The life history profile of Tillandsia circinnata (Bromeliaceae) and the rarity of extreme epiphytism among the angiosperms. Selbyana 2:325–337
Benzing DH (1981) The population dynamics of Tillandsia circinnata (Bromeliaceae): Cypress crown colonies in southern Florida. Selbyana 5:256–263
Benzing DH (1990) Vascular epiphytes. General biology and related biota. Cambridge University Press, Cambridge
Benzing DH, Davidson EA (1979) Oligotrophic Tillandsia circinnata Schlecht. (Bromeliaceae): an assessment of its patterns of mineral allocation and reproduction. Am J Bot 66:386–397
Brown DA (1990) El epifitismo en las selvas montanas del Parque Nacional “El Rey” Argentina: Composición florística y patrón de distribución. Rev Biol Trop 38:155–166
Callaway RM, Reihnart KO, Tucker SC, Pennings SC (2001) Effects of epiphytic lichens on host preference of the vascular epiphyte Tillandsia usneoides. Oikos 94:433–441
Callaway RM, Reihnart KO, Moore GW, Moore DJ, Pennings SC (2002) Epiphyte host preferences and host traits: mechanisms for species-specific interactions. Oecologia 132:221–230
Cascante-Marin A (2006) Establishment, reproduction and genetics of epiphytic bromeliad communities during premontane forest succession in Costa Rica. Ph.D. thesis, University of Amsterdam, Amsterdam
Castillo-Campos G, Medina-Abreo ME (2002) Árboles y arbustos de la reserva natural de La Mancha, Veracruz. Instituto de Ecología A. C., Xalapa
Castro-Hernández JC, Wolf JHD, García-Franco JG, González-Espinosa M (1997) The influence of humidity, nutrients and light on the establishment of the epiphytic bromeliad Tillandsia guatemalensis in the highlands of Chiapas, Mexico. Rev Biol Trop 47:763–773
Catling PM, Lefkovitch LP (1989) Associations of vascular epiphytes in a Guatemalan cloud forest. Biotropica 21:35–40
Flores-Palacios A, Ortiz-Pulido R (2005) Epiphyte orchid establishment on termite carton trails. Biotropica 37:457–461
Frei JK (1974) The ecology of epiphytic orchids in relation to their substrates. In: Szwat HH, Wemple J (eds) Proceedings of the Firths symposium on the scientific aspects of orchids, University of Detroit, Detroit, pp 46–62
Freiberg M (1996) Spatial distribution of vascular epiphytes on three emergent canopy trees in French Guiana. Biotropica 28:345–355
García-Franco JG (1996) Distribución de Epifitas Vasculares en Matorrales Costeros de Veracruz, México. Acta Bot Mex 37:1–9
García-Franco JG, Rico-Gray V (1988) Experiments on seed dispersal and deposition patterns of epiphytes. The case of Tillandsia deppeana Steudel (Bromeliaceae). Phytologia 65:73–78
Haberman SJ (1973) The analysis of residuals in cross-classified tables. Biometrics 29:205–220
Kiew R, Anthonysamy S (1987) A comparative study of vascular epiphytes in three epiphyte-rich habitats at Ulu Endau, Johore, Malaysia. Malay Nat J 41:303–315
Kress WJ (1986) The systematic distribution of vascular epiphytes: an update. Selbyana 9:2–22
Lyons B, Nadkarni NM, North MR (2000) Spatial distribution and succession of epiphytes on Tsuga heterophylla (western hemlock) in an old-grow Douglas-fir forest. Can J Bot 78:957–968
Madigosky SR (2004) Tropical microclimatic considerations. In: Lowman MD, Rinker HB (eds) Forest canopies. Elsevier Academic Press, London, pp 24–48
Martin CE (1994) Physiological ecology of the Bromeliaceae. Bot Rev 60:1–82
Martínez ML, García-Franco JG (2004) Plant–plant interactions in coastal dunes. In: Martínez ML, Pussy NP (eds) Coastal dunes, ecology and conservation. Spring-Verlag, Berlin, pp 205–220
Martínez-García E (1999) Estudio ecológico de las bromelias epífitas y sus hospederos en Selva Caducifolia de la Sierra de Huautla, Morelos. B.Sc. thesis. Facultad de Ciencias Biológicas, Universidad Autónoma del Estado de Morelos, Cuernavaca, Mexico
Mehltreter K, Flores-Palacios A, García-Franco JG (2005) Host preferences of low-trunk vascular epiphytes in a cloud forest of Veracruz, Mexico. J Trop Ecol 21:651–660
Mondragon D, Durán R, Ramírez I, Olmsted I (1999) Population dynamics of Tillandsia brachycaulos Schltdl. (Bromeliaceae) in Dzbilchaltun National Park, Yucatán. Selbyana 20:250–255
Mondragon D, Durán R, Ramírez I, Valverde T (2004) Temporal variation in the demography of the clonal epiphyte Tillandsia brachycaulos (Bromeliaceae) in the Yucatán Peninsula, Mexico. J Trop Ecol 20:189–200
Nadkarni NM (2000) Colonization of stripped branch surfaces by epiphytes in a lower montane cloud forest, Monteverde, Costa Rica. Biotropica 32:358–363
Rico-Gray V, García-Franco JG, Puch A, Sima P (1988) Composition and structure of a tropical dry forest in Yucatan, Mexico. Int J Ecol Environ Sci 14:21–29
Stevens GC (1987) Lianas as structural parasites: the Bursera simaruba example. Ecology 68:77–81
Talley SM, Setzer WN, Jackes BR (1996) Host associations of two adventitious-root-climbing vines in a north Queensland tropical rain forest. Biotropica 28:356–366
ter Steege H, Cornelissen JHC (1989) Distribution and ecology of vascular epiphytes in lowland rain forest of Guyana. Biotropica 21:331–339
Todzia C (1986) Growth habits, host tree species and density of hemiepiphytes on Barro Colorado island, Panama. Biotropica 18:22–27
Tremblay RL, Zimmerman JK, Lebrón L, Bayman P, Sastre I, Axelrod F, Alers-García L (1998) Host specificity and low reproductive success in the rare endemic Puerto Rican orchid Lepanthes caritensis. Biol Conserv 85:297–304
Valencia-Díaz S (1995) Estudio cuantitativo de la vegetación perenne asociada al pitayo (Stenocereus queretaroensis (Web.) Buxb.) en la cuenca de Sayula, Jalisco. B.Sc. thesis. Facultad de Biología, Universidad de Guadalajara, Guadalajara, Jalisco
Winkler M (2005) Population dynamics of epiphytes related to canopy structure in a Mexican humid montane forest. Ph.D. thesis, University of Natural Resources and Applied Life Sciences, Vienna
Zar JH (1996) Biostatistical analysis. Prentice-Hall, New Jersey
Zimmerman JK, Olmsted IC (1992) Host tree utilization by vascular epiphytes in a seasonally inundated forest (Tintal) in México. Biotropica 24:402–427
Zotz G (1997) Substrate use of three epiphytic bromeliads. Ecography 20:264–270
Zotz G (2004) Growth and survival of the early stages of the heteroblastic bromeliad Vriesia sanguinolenta. Ecotropica 10:51–57
Zotz G, Andrade JL (1998) Water relations of two co-occurring epiphyte bromeliads. J Plant Physiol 152:542–554
Zotz G, Tyree T (1996) Water stress in the epiphyte orchid Dimerandra emarginata (G. Meyer) Hoehne. Oecologia 107:151–159
Zotz G, Vollrath B (2002) Substrate preferences of epiphytic bromeliads: an experimental approach. Acta Oecol 23:99–102
Zotz G, Vollrath B (2003) The epiphyte vegetation of the palm Socratea exorrhiza – correlations with tree size, tree age and bryophyte cover. J Trop Ecol 19:81–90
Zotz G, Bermejo P, Dietz H (1999) The epiphyte vegetation of Annona glabra on Barro Colorado Island, Panama. J Biogeogr 26:761–776
Zotz G, Laube S, Scmidt G (2005) Long-term population dynamics of the epiphyte bromeliad Werahuia sanguinolenta. Ecography 28:806–814
Acknowledgments
M. L. Villalobos Arana and L. Solis-Montero helped during field work. Adolfo Espejo-Serna helped with species identification. Comments and suggestions by D. Mondragon, J. Gonzalez-Astorga, J. G. García-Franco, S. Valencia-Díaz, C. Ibarra Cerdeño, Steve Weller, and two anonymous reviewers improved the manuscript. This study was supported by a TELMEX scholarship grant to ALV. A preliminary version was presented as bachelor thesis by ALV at the Facultad de Química y Biología, Universidad de las Américas-Puebla.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
López-Villalobos, A., Flores-Palacios, A. & Ortiz-Pulido, R. The relationship between bark peeling rate and the distribution and mortality of two epiphyte species. Plant Ecol 198, 265–274 (2008). https://doi.org/10.1007/s11258-008-9402-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-008-9402-5